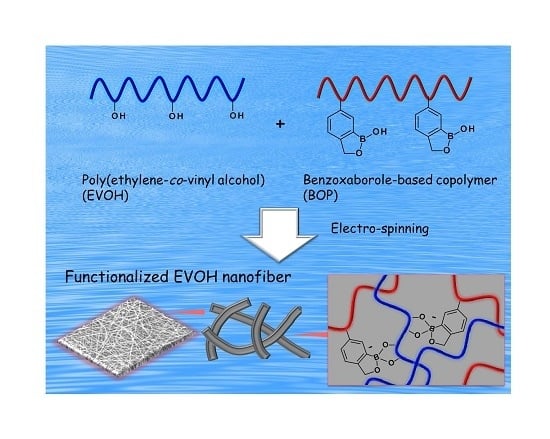
Facile Functionalization of Electrospun Poly(ethylene-co-vinyl alcohol) Nanofibers via the Benzoxaborole-Diol Interaction
Abstract
:1. Introduction
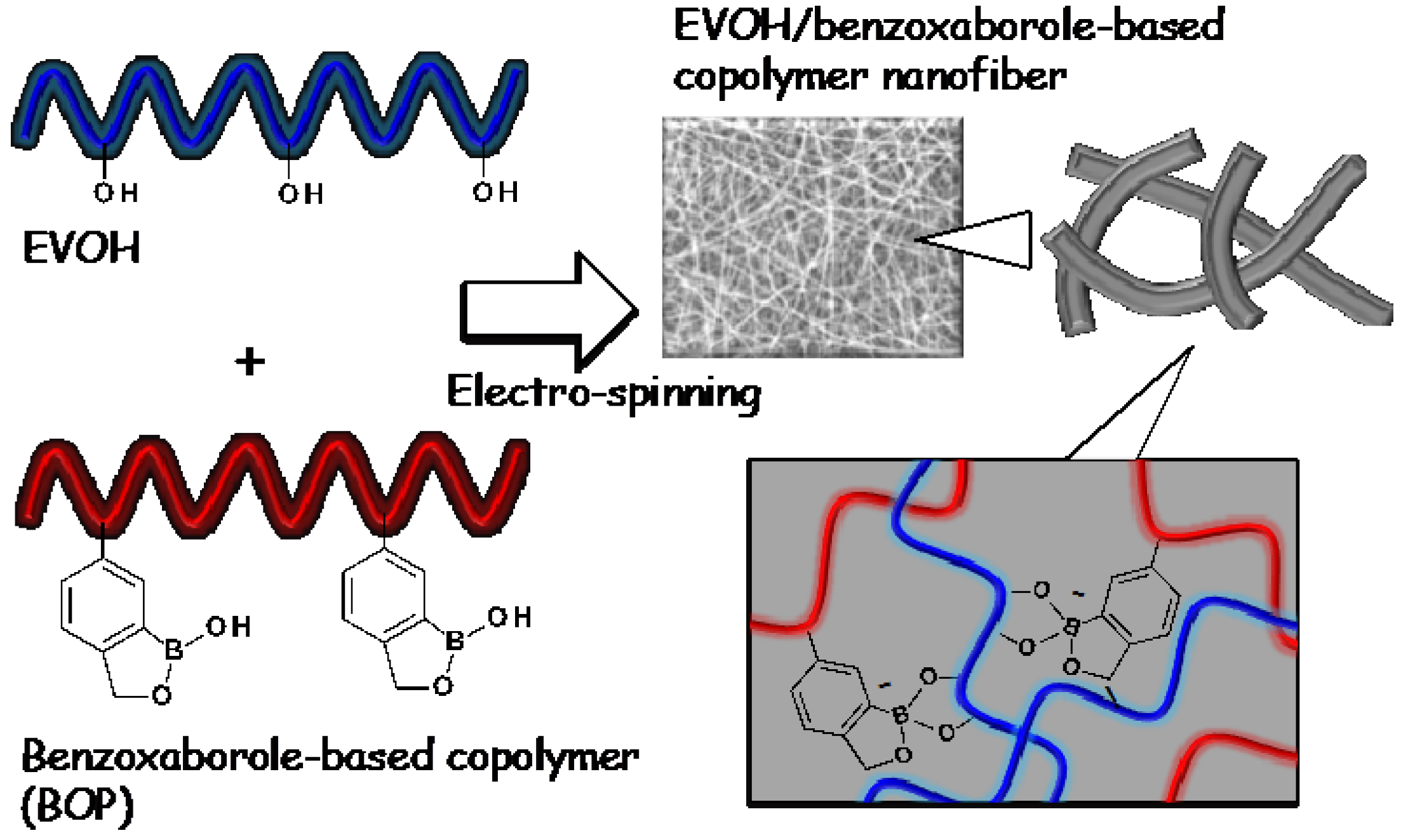
2. Experimental Section
2.1. Material
2.2. Preparation of Benzoxaborole-Based Copolymers
2.3. Preparation of EVOH/BOP Nanofiber Meshes
2.4. Stability Tests
2.5. Characterizations
3. Results and Discussion
3.1. Preparation of Benzoxaborole-Based Copolymers
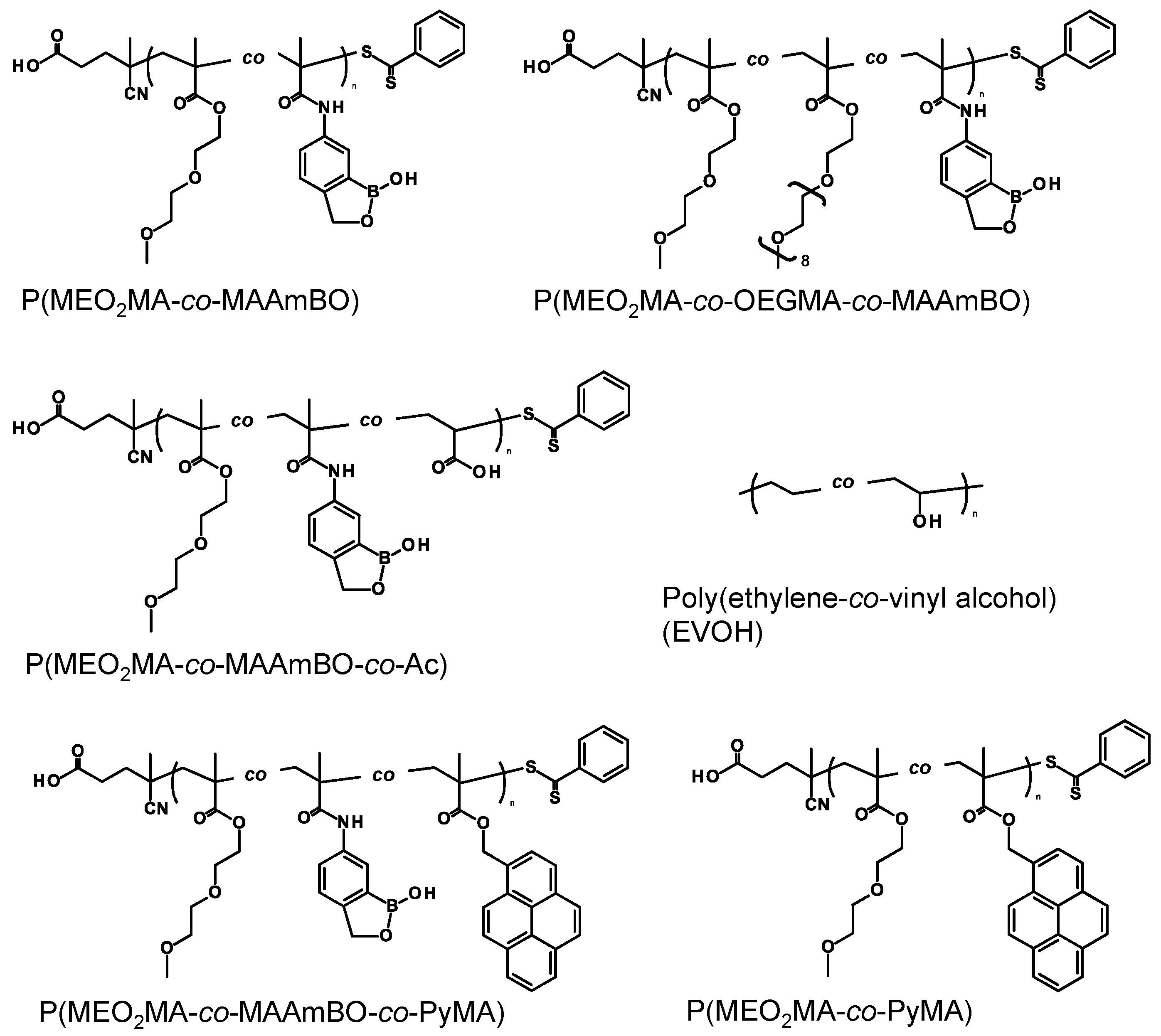
| Copolymer | In Copolymer (mol%) a | Mn b (g/mol) | Mw/Mn b (–) | LCST (°C) c | |||||
|---|---|---|---|---|---|---|---|---|---|
| MEO2MA | MAAmBO | OEGMA | Ac | PyMA | pH 2 d | pH 12 e | |||
| P(MEO2MA91.0-co-MAAmBO9.0) | 91.0 | 9.0 | – | – | – | 3000 | 1.26 | 18.3 | no LCST f |
| P(MEO2MA56.5-co-OEGMA38.2-co-MAAmBO5.3) | 56.5 | 5.3 | 38.2 | – | – | 6000 | 1.80 | 63.5 | no LCST f |
| P(MEO2MA86.7-co-MAAmBO4.8-co-Ac8.5) | 86.7 | 4.8 | – | 8.5 | – | 2300 | 1.32 | 17.6 | no LCST g |
| P(MEO2MA93.9-co-MAAmBO5.2-co-PyMA0.9) | 93.9 | 5.3 | – | – | 0.9 | 2600 | 1.59 | 15.2 | no LCST f |
| P(MEO2MA99.0-co-PyMA1.0) | 99.0 | – | – | – | 1.0 | 12,500 | 1.31 | 21.3 (pH 7.4 PBS) | |
3.2. Preparation of EVOH/BOP Nanofiber Meshes by Electro-Spinning

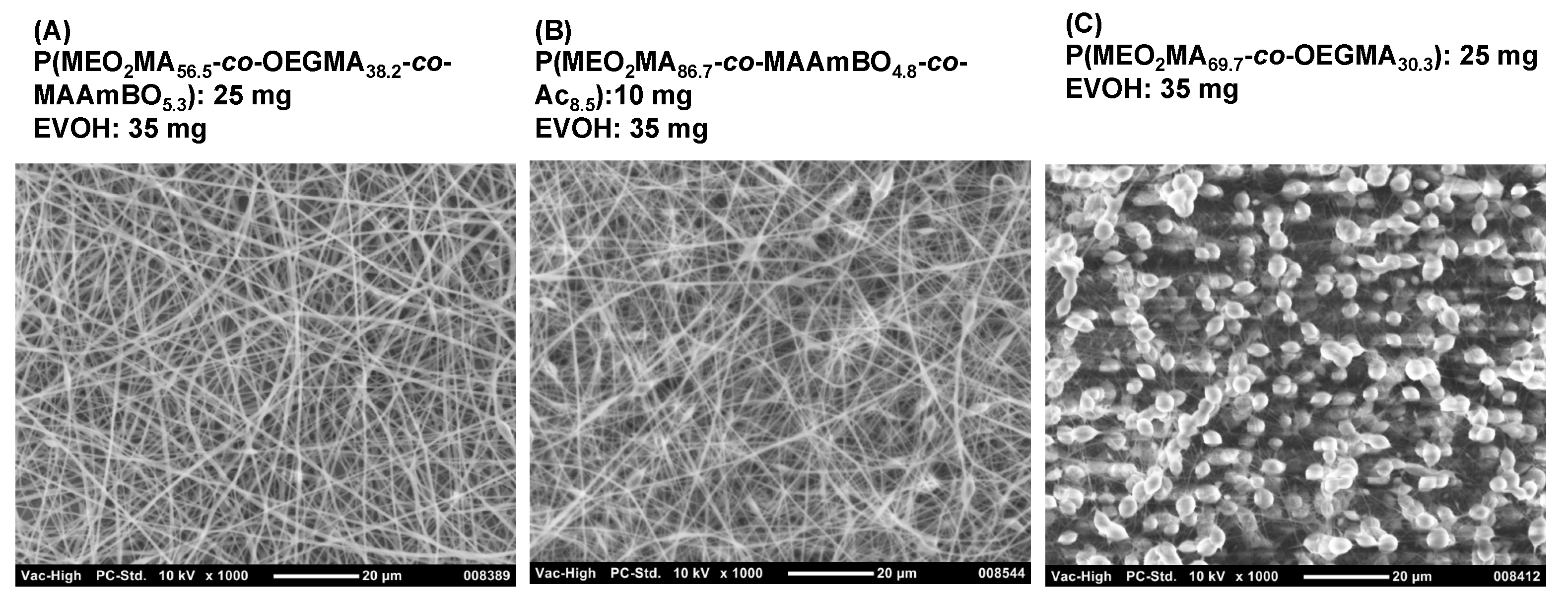
3.3. Stability Test of EVOH/BOP Nanofiber Meshes
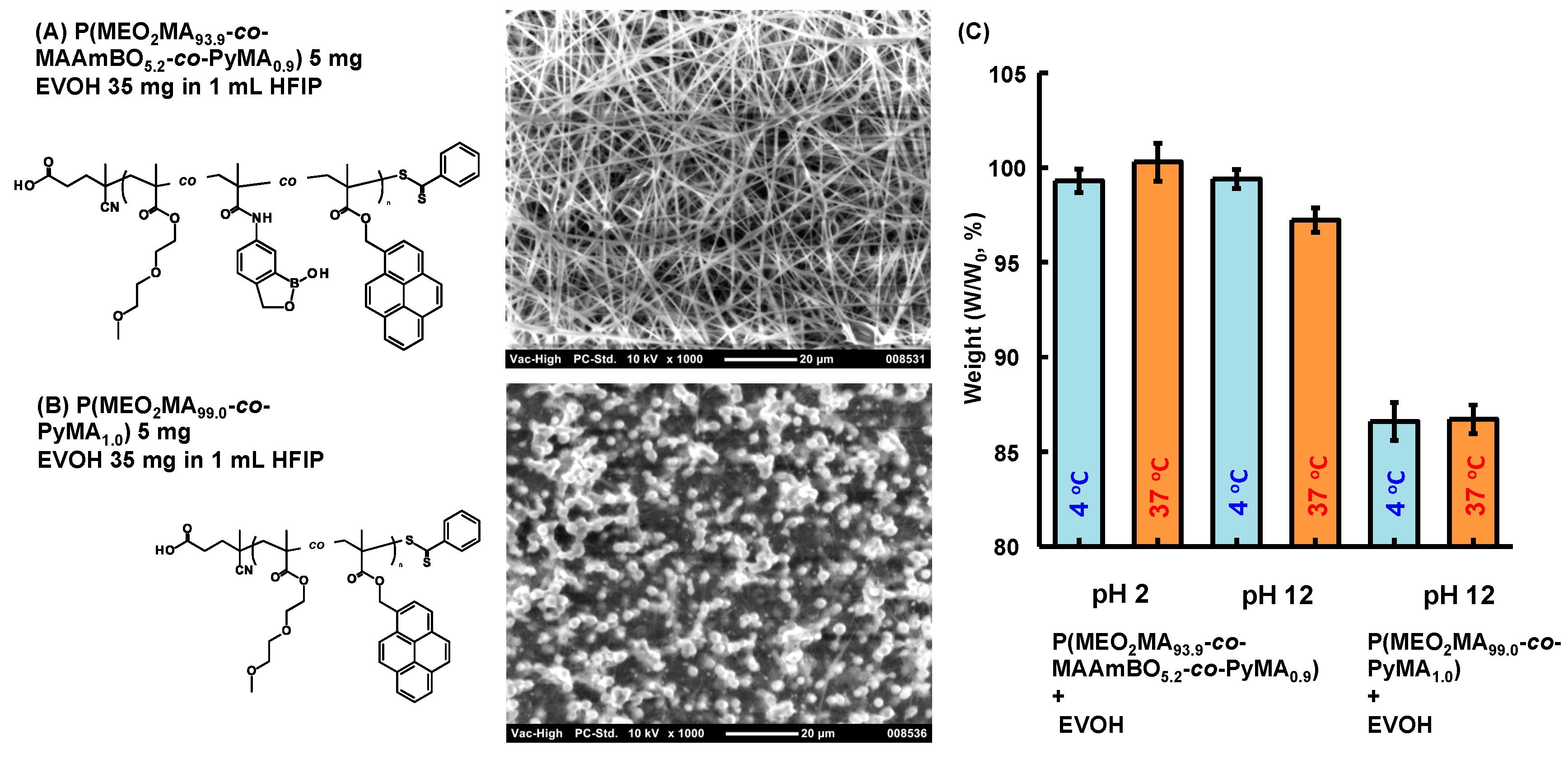
3.4. Controlled Adsorption and Desorption of Cationic Dye on Anionic EVOH/Benzoxaborole Nanofiber Meshes
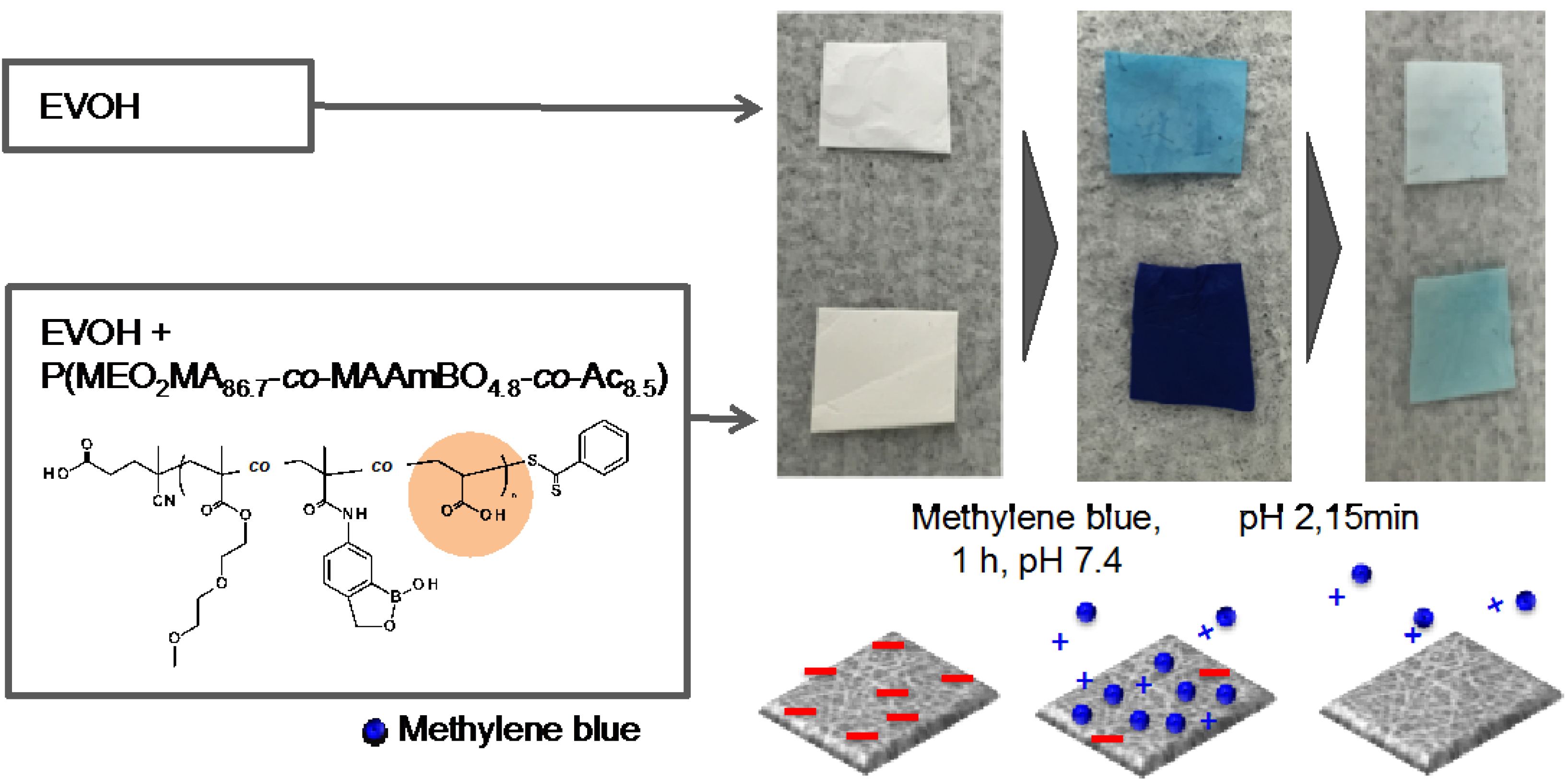
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mokwena, K.K.; Tang, J. Ethylene vinyl alcohol: A review of barrier properties for packaging shelf stable foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Suzuki, C.; Tsuji, T. Platelet activation through interaction with hemodialysis membranes induces neutrophils to produce reactive oxygen species. J. Biomed. Mater. Res. A 2006, 77, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Sirolli, V.; Ballone, E.; Stante, S.D.; Amoroso, L.; Bonomini, M. Cell activation and cellular-cellular interactions during hemodialysis: Effect of dialyzer membrane. Int. J. Artif. Organs 2002, 25, 529–537. [Google Scholar] [PubMed]
- Shang, M.; Matsuyama, H.; Teramoto, M.; Lloyd, D.R.; Kubota, N. Preparation and membrane performance of poly(ethylene-co-vinylalcohol) hollow fiber membrane via thermally induced phase separation. Polymer 2003, 44, 7441–7447. [Google Scholar]
- Lee, M.-H.; Thomas, J.L.; Ho, M.-H.; Yuan, C.; Lin, H.-Y. Synthesis of magnetic molecularly imprinted poly(ethylene-co-vinyl alcohol) nanoparticles and their uses in the extraction and sensing of target molecules in urine. ACS Appl. Mater. Interfaces 2010, 2, 1729–1736. [Google Scholar] [PubMed]
- Lv, R.; Zhou, J.; Du, Q.; Wang, H.; Zhong, W. Effect of posttreatment on morphology and properties of poly(ethylene-co-vinyl alcohol) microporous hollow fiber via thermally induced phase separation. J. Appl. Polym. Sci. 2007, 104, 4106–4112. [Google Scholar]
- Kenawy, E.-R.; Layman, J.M.; Watkins, J.R.; Bowlin, G.L.; Matthews, J.A.; Simpson, D.G.; Wnek, G.E. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials 2003, 24, 907–913. [Google Scholar]
- Namekawa, K.; Tokoro, M.S.; Aoyagi, T.; Ebara, M. Fabrication of zeolite–polymer composite nanofibers for removal of uremic toxins from kidney failure patients. Biomater. Sci. 2014, 2, 674–679. [Google Scholar]
- Zhou, J.; Meng, S.; Guo, Z.; Du, Q.; Zhong, W. Phosphorylcholine-modified poly(ethylene-co-vinyl alcohol) microporous membranes with improved protein-adsorption-resistance property. J. Membrane Sci. 2007, 305, 279–286. [Google Scholar]
- Zhu, J.; Sun, G. Facile fabrication of hydrophilic nanofibrous membranes with an immobilized metal-chelate affinity complex for selective protein separation. ACS Appl. Mater. Interfaces 2014, 6, 925–932. [Google Scholar] [PubMed]
- Hong, S.I.; Kim, K.B.; Lee, Y.; Cho, S.Y.; Ko, J.A.; Hong, S.K.; Park, H.J. Surface modification of ethylene-vinyl alcohol copolymer treated with plasma source ion implantation. J. Appl. Polym. Sci. 2009, 113, 2988–2996. [Google Scholar]
- Buntinx, M.; Willems, G.; Knockaert, G.; Adons, D.; Yperman, J.; Carleer, R.; Peeters, R. Evaluation of the thickness and oxygen transmission rate before and after thermoforming mono- and multi-layer sheets into trays with variable depth. Polymers 2014, 6, 3019–3043. [Google Scholar]
- Son, T.W.; Lim, S.K.; Lee, D.W.; Lee, E.W. Physical modification of polypropylene. III. Novel morphology of polypropylene and poly(ethylene-co-vinyl alcohol) with epoxy blend fibers. J. Appl. Polym. Sci. 1999, 73, 1049–1057. [Google Scholar]
- Neppalli, R.; Causin, V.; Marigo, A.; Meincken, M.; Hartmann, P.; Reenen, A.J. Effect of electrospun ethylene vinyl alcohol copolymer (EVOH) fibres on the structure, morphology, and properties of poly(lactic acid) (PLA). Polymer 2013, 54, 5909–5919. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Olsson, R.T.; Lopez-Rubio, A.; Lagaron, J.M. Development of bacterial cellulose nanowhiskers reinforced EVOH composites by electrospinning. J. Appl. Polym. Sci. 2012, 124, 1398–1408. [Google Scholar] [CrossRef]
- Shimada, N.; Tsutsumi, H.; Nakane, K.; Ogihara, T.; Ogata, N. Poly(ethylene-co-vinyl alcohol) and nylon 6/12 nanofibers produced by melt electrospinning system equipped with a line-like laser beam melting device. J. Appl. Polym. Sci. 2010, 116, 2998–3004. [Google Scholar] [CrossRef]
- Wang, D.; Xu, W.; Sun, G.; Chiou, B. Radical graft polymerization of an allyl monomer onto hydrophilic polymers and their antibacterial nanofibrous membranes. ACS Appl. Mater. Interfaces 2011, 3, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhu, K.; Zheng, X.; Xiao, R. Poly(ethylene-co-vinyl alcohol) functional nanofiber membranes for the removal of Cr(VI) from water. Ind. Eng. Chem. Res. 2015, 54, 6836–6844. [Google Scholar] [CrossRef]
- Kouzu, T.; Hirata, Y.; Hamada, K. Sorption of water vapor of EVAL/PAA blend nano-nonwovens modified by layer-by-layer technique. Desalin. Water Treat. 2010, 17, 2–9. [Google Scholar]
- Benaglia, M.; Chiefari, J.; Chong, Y.K.; Moad, G.; Rizzardo, E.; Thang, S.H. Universal (switchable) RAFT agents. J. Am. Chem. Soc. 2009, 131, 6914–6915. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar]
- Otsuka, H. Reorganization of polymer structures based on dynamic covalent chemistry: Polymer reactions by dynamic covalent exchanges of alkoxyamine units. Polym. J. 2013, 45, 879–891. [Google Scholar]
- Kotsuchibashi, Y.; Agustin, R.V.C.; Lu, J.-Y.; Hall, D.G.; Narain, R. Temperature, pH, and glucose responsive gels via simple mixing of boroxole- and glyco-based polymers. ACS Macro. Lett. 2013, 2, 260–264. [Google Scholar]
- Kotsuchibashi, Y.; Ebara, M.; Sato, T.; Wang, Y.; Rajender, R.; Hall, D.G.; Narain, R.; Aoyagi, T. Spatiotemporal control of synergistic gel disintegration consisting of boroxole- and glyco-based polymers via photoinduced proton transfer. J. Phys. Chem. B 2015, 119, 2323–2329. [Google Scholar] [PubMed]
- Techawanitchai, P.; Ebara, M.; Idota, N.; Aoyagi, T. Light-induced spatial control of pH-jump reaction at smart gel interface. Colloids Surf. B 2012, 99, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Bérubé, M.; Hall, D.G. Design, synthesis, and screening of a library of peptidyl bis(boroxoles) as oligosaccharide receptors in water: Identification of a receptor for the tumor marker TFantigen disaccharide. Angew. Chem. Int. Ed. 2010, 49, 1492–1495. [Google Scholar]
- Ellis, G.A.; Palte, M.J.; Raines, R.T. Boronate-mediated biologic delivery. J. Am. Chem. Soc. 2012, 134, 3631–3634. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, A.; Geonnotti, A.R.; Balzarini, J.; Kiser, P.F. Activity and safety of synthetic lectins based on benzoboroxole-functionalized polymers for inhibition of HIV entry. Mol. Pharm. 2011, 8, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Wang, Q.; Zhang, F.; Wang, Z.; Bowling, T.; Nare, B.; Jacobs, R.T.; Zhang, J.; Ding, D.; Liu, Y.; et al. Chalcone-benzoxaborole hybrid molecules as potent antitrypanosomal agents. J. Med. Chem. 2012, 55, 3553–3557. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.I.; Lai, B.E.; Myszka, D.G.; Mahalingam, A.; Langheinrich, K.; Katz, D.F.; Kiser, P.F. Multivalent benzoboroxole functionalized polymers as gp120 glycan targeted microbicide entry inhibitors. Mol. Pharm. 2010, 7, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Böeseken, J. The use of boric acid for the determination of the configuration of carbohydrates. Adv. Carbohydr. Chem. 1949, 4, 189–210. [Google Scholar]
- Matsumoto, A.; Yamamoto, K.; Yoshida, R.; Kataoka, K.; Aoyagi, T.; Miyahara, Y. A totally synthetic glucose responsive gel operating in physiological aqueous conditions. Chem. Commun. 2010, 46, 2203–2205. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Cheng, G.; Sundararaman, A.; Jäkle, F. Well-defined boron-containing polymeric lewis acids. J. Am. Chem. Soc. 2002, 124, 12672–12673. [Google Scholar] [CrossRef] [PubMed]
- Dowlut, M.; Hall, D.G. An improved class of sugar-binding boronic acids, soluble and capable of complexing glycosides in neutral water. J. Am. Chem. Soc. 2006, 128, 4226–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Tomsho, J.W.; Benkovic, S.J. The unique chemistry of benzoxaboroles: Current and emerging applications in biotechnology and therapeutic treatments. Bioorg. Med. Chem. 2014, 22, 4462–4473. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Borys, K.M.; Sporzyński, A. Recent developments in the chemistry and biological applications of benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Akdemir, Ö.; Hoth, A. Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: Is the age of Poly(NIPAM) over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kotsuchibashi, Y.; Uto, K.; Ebara, M.; Aoyagi, T.; Liu, Y.; Narain, R. pH and glucose responsive nanofibers for the reversible capture and release of lectins. Biomater. Sci. 2015, 3, 152–162. [Google Scholar] [CrossRef]
- Kikuchi, A.; Suzuki, K.; Okabayashi, O.; Hoshino, H.; Kataoka, K.; Sakurai, Y.; Okano, T. Glucose-sensing electrode coated with polymer complex gel containing phenylboronic acid. Anal. Chem. 1996, 68, 823–828. [Google Scholar]
- Kotsuchibashi, Y.; Ebara, M.; Hoffman, A.S.; Narain, R.; Aoyagi, T. Temperature-responsive mixed core nanoparticle properties determined by the composition of statistical and block copolymers in the core. Polym. Chem. 2015, 6, 1693–1697. [Google Scholar]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsuchibashi, Y.; Ebara, M. Facile Functionalization of Electrospun Poly(ethylene-co-vinyl alcohol) Nanofibers via the Benzoxaborole-Diol Interaction. Polymers 2016, 8, 41. https://doi.org/10.3390/polym8020041
Kotsuchibashi Y, Ebara M. Facile Functionalization of Electrospun Poly(ethylene-co-vinyl alcohol) Nanofibers via the Benzoxaborole-Diol Interaction. Polymers. 2016; 8(2):41. https://doi.org/10.3390/polym8020041
Chicago/Turabian StyleKotsuchibashi, Yohei, and Mitsuhiro Ebara. 2016. "Facile Functionalization of Electrospun Poly(ethylene-co-vinyl alcohol) Nanofibers via the Benzoxaborole-Diol Interaction" Polymers 8, no. 2: 41. https://doi.org/10.3390/polym8020041
APA StyleKotsuchibashi, Y., & Ebara, M. (2016). Facile Functionalization of Electrospun Poly(ethylene-co-vinyl alcohol) Nanofibers via the Benzoxaborole-Diol Interaction. Polymers, 8(2), 41. https://doi.org/10.3390/polym8020041