Structure–Property Studies on a New Family of Halogen Free Flame Retardants Based on Sulfenamide and Related Structures
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
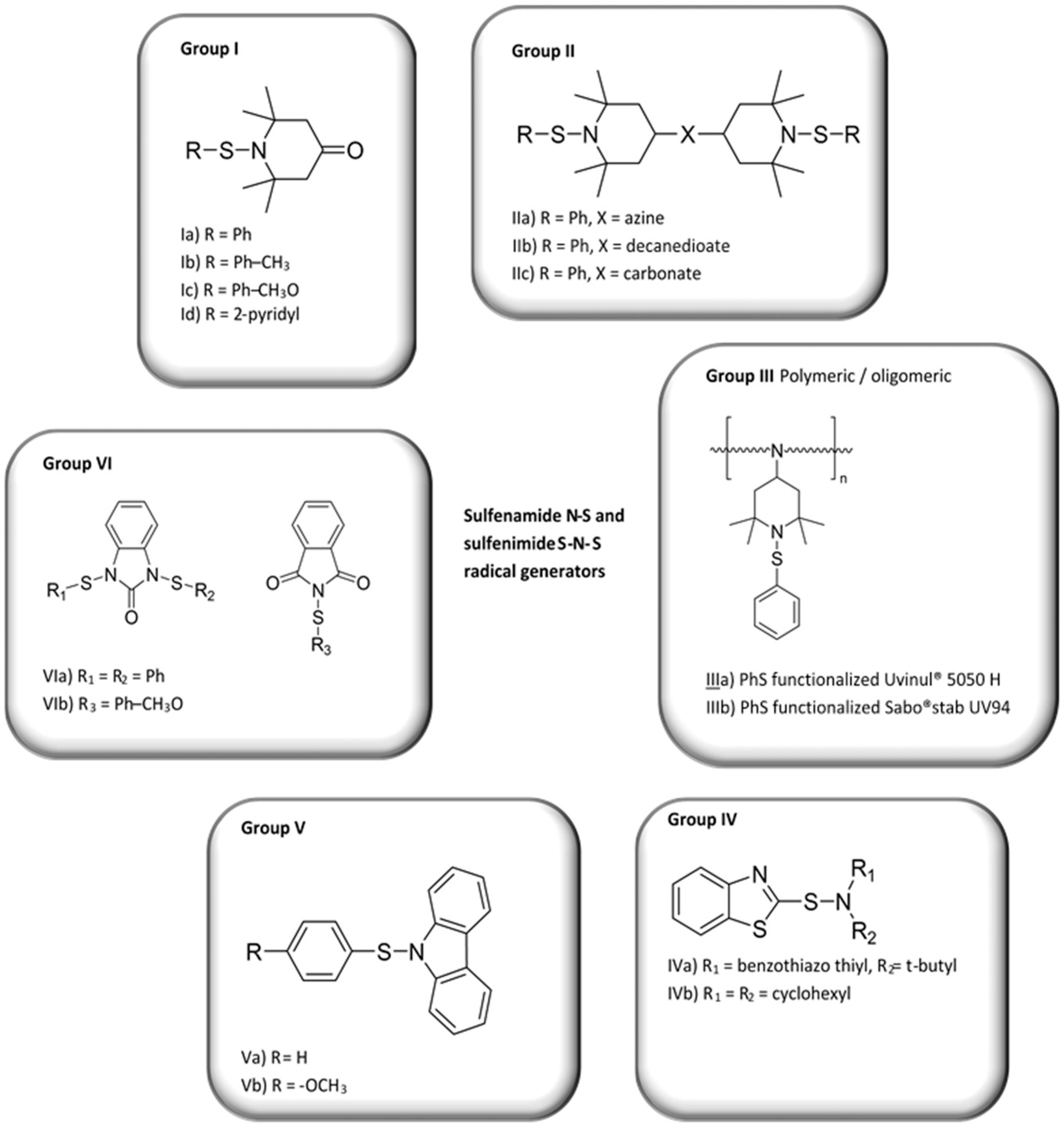
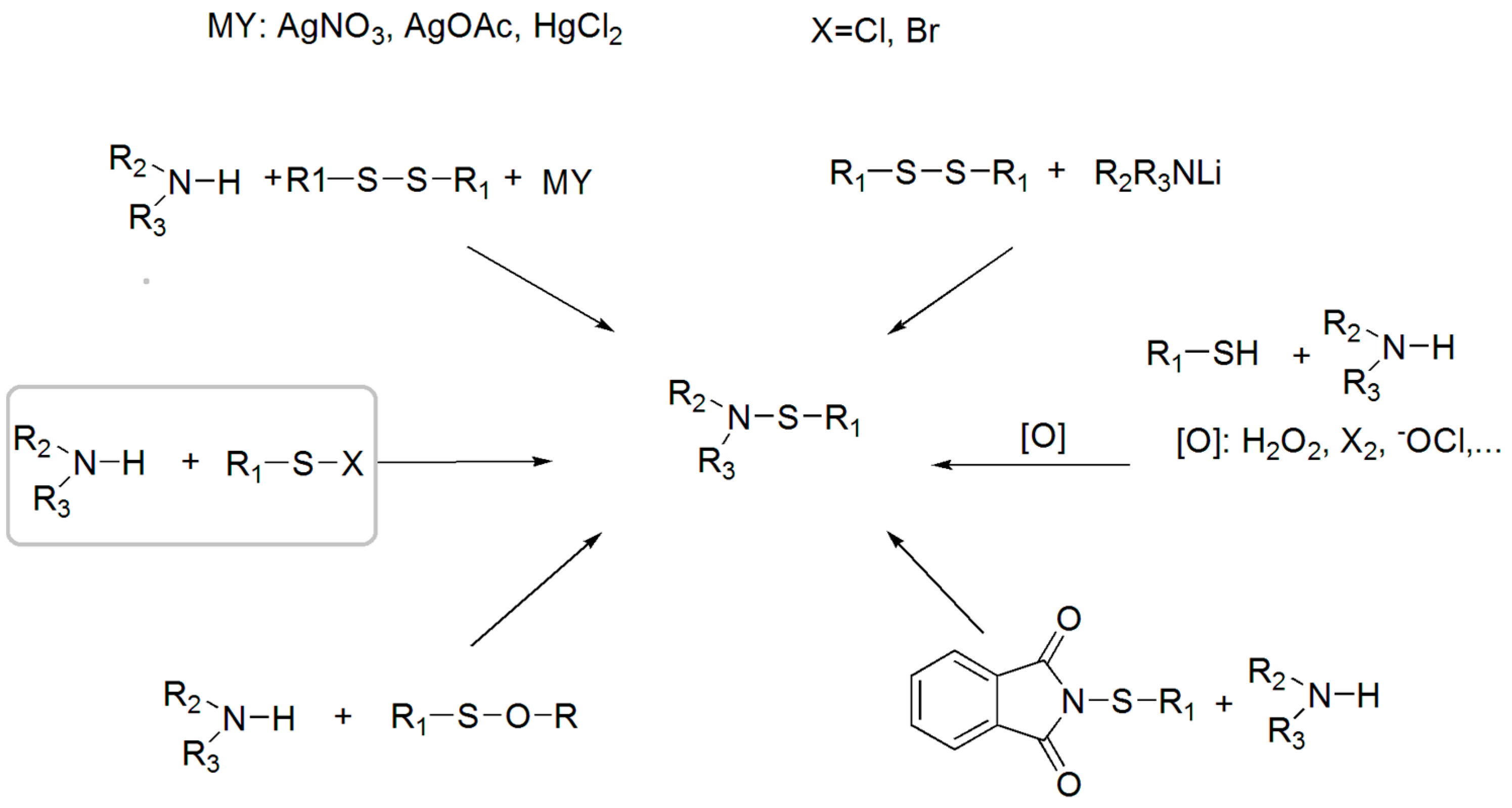
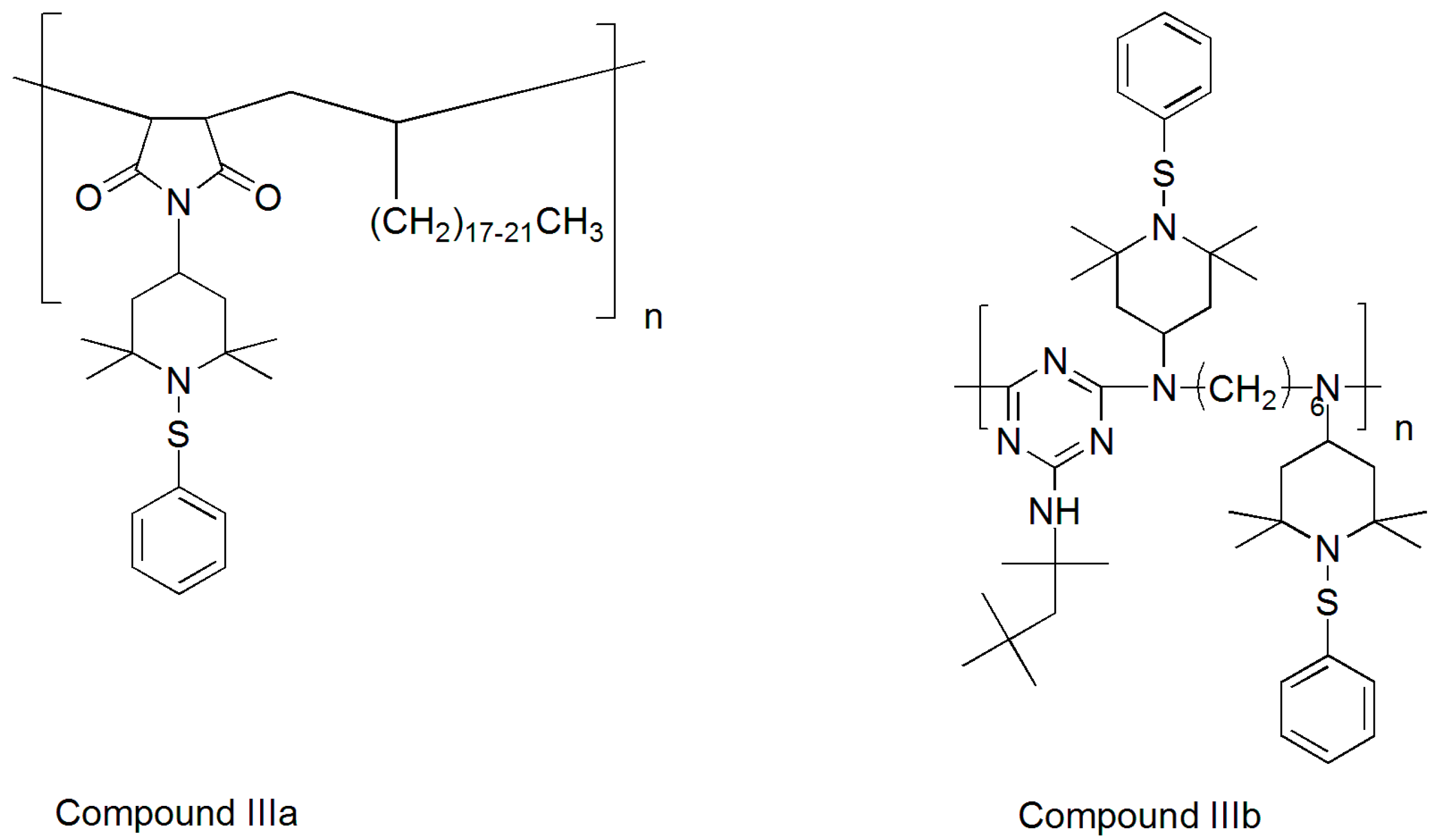
3.1. Synthesis of Sulfenamides
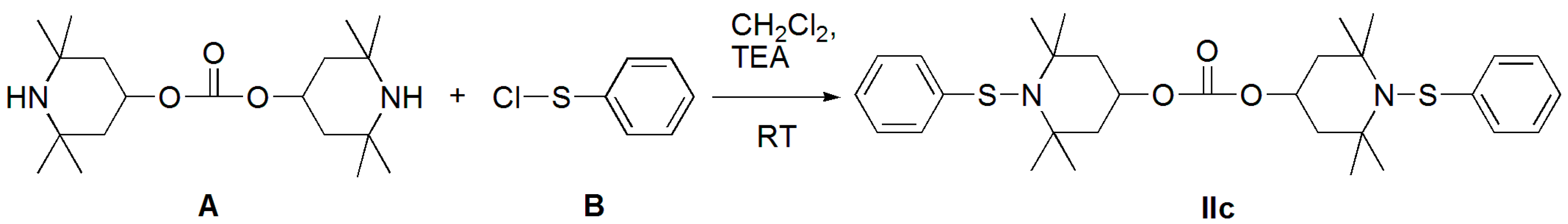
3.1.1. Bis(2,2,6,6-tetramethylpiperidin-4-yl) Carbonate (Diamine A)
3.1.2. Benzenesulfenyl Chloride (B)
3.1.3. Bis(2,2,6,6-tetramethyl-1-(phenylthio)piperidin-4-yl) Carbonate (Structure IIc)
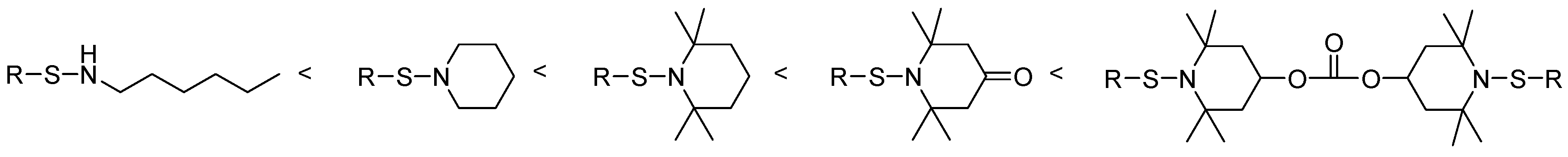
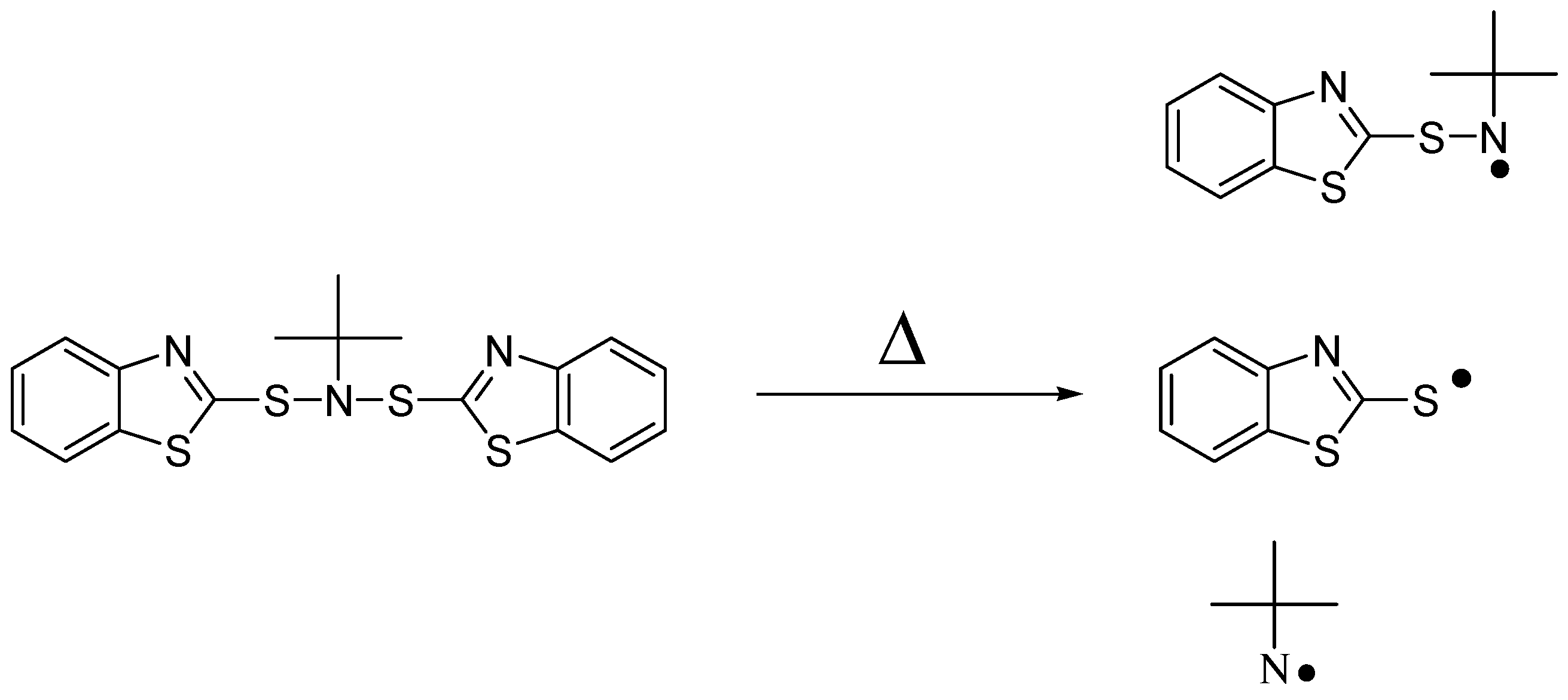
3.2. Thermal Degradation Behaviour and Tuning of Sulfenamide Structure
3.3. Flame Retardancy Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Wit, C.A. An Overview of Brominated Flame Retardants in the Environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Levchik, S. Phosphorus-Based FRs. In Non-Halogenated Flame Retardant Handbook; Morgan, A.B., Wilkie, C.A., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; pp. 17–74. [Google Scholar]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Klatt, M. Nitrogen-Based Flame Retardants. In Non-Halogenated Flame Retardant Handbook; Morgan, A.B., Wilkie, C.A., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; pp. 143–168. [Google Scholar]
- Schartel, B. Phosphorus-Based Flame Retardancy Mechanisms-Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Horsey, D.W.; Andrews, S.M.; Davis, L.H.; Dyas, D.D.; Gray, R.L.; Gupta, A.; Hein, B.V.; Puglisi, J.S.; Ravichandran, R.; Shields, P.; et al. Flame Retardant Compositions. WO 1999000450 A1, 7 January 1999. [Google Scholar]
- Tirri, T.; Aubert, M.; Pawelec, W.; Wilen, C.; Pfaendner, R.; Hoppe, H.; Roth, M.; Sinkkonen, J. Preparation and Characterization of Bis-[1,3,5]Triazinyl Diazenes and their Utilization as Flame Retardants in Polypropylene Films. J. Appl. Polym. Sci. 2014, 131, 40413/1–40413/9. [Google Scholar] [CrossRef]
- Aubert, M.; Tirri, T.; Wilen, C.; Francois-Heude, A.; Pfaendner, R.; Hoppe, H.; Roth, M. Versatile Bis(1-Alkoxy-2,2,6,6-Tetramethylpiperidin-4-Yl)-Diazenes (AZONORs) and Related Structures and their Utilization as Flame Retardants in Polypropylene, Low Density Polyethylene and High-Impact Polystyrene. Polym. Degrad. Stab. 2012, 97, 1438–1446. [Google Scholar] [CrossRef]
- Aubert, M.; Wilen, C.; Pfaendner, R.; Kniesel, S.; Hoppe, H.; Roth, M. Bis(1-Propyloxy-2,2,6,6-Tetramethylpiperidin-4-Yl)-Diazene-an Innovative Multifunctional Radical Generator Providing Flame Retardancy to Polypropylene Even after Extended Artificial Weathering. Polym. Degrad. Stab. 2011, 96, 328–333. [Google Scholar] [CrossRef]
- Aubert, M.; Nicolas, R.C.; Pawelec, W.; Wilen, C.; Roth, M.; Pfaendner, R. AzoalkaneS–Novel Flame Retardants and their Structure-Property Relationship. Polym. Adv. Technol. 2011, 22, 1529–1538. [Google Scholar] [CrossRef]
- Pawelec, W.; Aubert, M.; Pfaendner, R.; Hoppe, H.; Wilen, C. Triazene Compounds as a Novel and Effective Class of Flame Retardants for Polypropylene. Polym. Degrad. Stab. 2012, 97, 948–954. [Google Scholar] [CrossRef]
- Pawelec, W.; Holappa, A.; Tirri, T.; Aubert, M.; Hoppe, H.; Pfaendner, R.; Wilen, C. Disulfides-Effective Radical Generators for Flame Retardancy of Polypropylene. Polym. Degrad. Stab. 2014, 110, 447–456. [Google Scholar] [CrossRef]
- Pfaendner, R.; Metzsch-Zilligen, E.; Stec, M. Use of Organic Oxy Imides as Flame Retardants for Plastics and Flame-Retardant Plastics Composition and Mouldings Produced Therefrom. WO 2014154636A1, 2 October 2014. [Google Scholar]
- Wagner, J.; Deglmann, P.; Fuchs, S.; Ciesielski, M.; Fleckenstein, C.A.; Doering, M. A Flame Retardant Synergism of Organic Disulfides and Phosphorous Compounds. Polym. Degrad. Stab. 2016, 129, 63–76. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Ciesielski, M.; Zevaco, T.; Doering, M. New Phosphacyclic Molecules and their Application as Flame Retardants for Epoxy Resins. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 989–996. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, H.; Li, J.; Xia, Z.; Tan, H. A Novel Flame Retardant Containing Phosphorus, Nitrogen, and Sulfur. J. Therm. Anal. Calorim. 2014, 115, 1639–1649. [Google Scholar] [CrossRef]
- Wilen, C.E.; Aubert, M.; Tirri, T.; Pawelec, W. Sulfenamides as Flame Retardants. WO 2015067736A1, 14 May 2015. [Google Scholar]
- Zincke, T. New Series of Aromatic Sulfur Compounds. Ber. Dtsch. Chem. Ges. 1911, 44, 769–771. [Google Scholar] [CrossRef]
- Koval, I.V. Synthesis and Application of Sulfenamides. Russ. Chem. Rev. 1996, 65, 421–440. [Google Scholar] [CrossRef]
- Nti-Addae, K.W. Synthesis and Physiochemical Characterization of Sulfenamide Prodrugs of Antimicrobial Oxazolidinones. Ph.D. Thesis, University of Kansas, Lawrence, KS, USA, December 2008. [Google Scholar]
- Cohen, M.P.; D’Sidocky, R.M. Rubber Chemicals. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Yoo, J. Synthesis of New Biodegradable Polysulfenamides for Applications in Medicine. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2011. [Google Scholar]
- Yoo, J.; Kuruvilla, D.J.; D’Mello, S.R.; Salem, A.K.; Bowden, N.B. New Class of Biodegradable Polymers Formed from Reactions of an Inorganic Functional Group. Macromolecules 2012, 45, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kuruvilla, D.J.; Salem, A.K.; Bowden, N.B. New Biodegradable Polymers with Sulfenamide Bonds for Drug Delivery Applications. WO 2012115806A1, 30 August 2012. [Google Scholar]
- Eichhorn, J. Synergism of Free Radical Initiators with Self-Extinguishing Additives in Vinyl Aromatic Polymers. J. Appl. Polym. Sci. 1964, 8, 2497–2524. [Google Scholar] [CrossRef]
- Eichhorn, J. Self-Extinguishing Resins, especially Polystyrene. FR1391298, 5 March 1391. [Google Scholar]
- Underwriters Laboratories. UL 94: Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances; Underwriters Laboratories Inc.: Northbrook, IL, USA, 1998. [Google Scholar]
- Craine, L.; Raban, M. The Chemistry of Sulfenamides. Chem. Rev. 1989, 89, 689–712. [Google Scholar] [CrossRef]
- Davis, F.A. Adventures in Sulfur–Nitrogen Chemistry. J. Org. Chem. 2006, 71, 8993–9003. [Google Scholar] [CrossRef] [PubMed]
- Koval, I.V. Sylfenyl Chlorides in Organic Synthesis. Russ. Chem. Rev. 1995, 64, 731–751. [Google Scholar] [CrossRef]
- Koval, I.V. Advances in the Chemistry of Sulphenic Acid Amides. Russ. Chem. Rev. 1990, 59, 396–403. [Google Scholar] [CrossRef]
- Kharasch, N.; Potempa, S.J.; Wehrmeister, H.L. The Sulfenic Acids and their Derivatives. Chem. Rev. 1946, 39, 269–332. [Google Scholar] [CrossRef] [PubMed]
- Heimer, N.E.; Field, L. Organic Disulfides and Related Substances. XXIX. Sulfenamides. J. Org. Chem. 1970, 35, 3012–3022. [Google Scholar] [CrossRef]
- Sato, S.; Yoshioka, T.; Tamura, C. The Crystal Structures of 2,2,6,6-Tetramethyl-4-oxopiperidine Derivatives. Acta Crystallogr. B 1975, 31, 1385–1392. [Google Scholar] [CrossRef]
- Brito, I.; Lopez-Rodriguez, M.; Vargas, D.; Leon, Y. N,N-Dibenzyl-3-nitrobenzenesulfenamide. Acta Crystallogr. A 2006, 62, o914–o916. [Google Scholar] [CrossRef]
- Bharatam, P.V.; Kaur, A.; Kaur, D. Electronic Structure of N-Sulfenylimines. J. Phys. Org. Chem. 2003, 16, 183–188. [Google Scholar] [CrossRef]
- Ohtsuki, M.; Sato, Y. Rubber Composites with Good rubber/metal Adhesion and Rubber Compositions with Proper Vulcanization Reaction without the Vulcanization Retarder. WO 2010150419 A1, 29 December 2010. [Google Scholar]
- Hopkins, D.; Quintiere, J.G. Material Fire Properties and Predictions for Thermoplastics. Fire Saf. J. 1996, 26, 241–268. [Google Scholar] [CrossRef]
- Beach, M.W.; Rondan, N.G.; Froese, R.D.; Gerhart, B.B.; Green, J.G.; Stobby, B.G.; Shmakov, A.G.; Shvartsberg, V.M.; Korobeinichev, O.P. Studies of Degradation Enhancement of Polystyrene by Flame Retardant Additives. Polym. Degrad. Stab. 2008, 93, 1664–1673. [Google Scholar] [CrossRef]
- To, B.H.; Sikora, D.J. N-t-Butyl Benzothiazole Sulfenimide a New Long-Delay, Slow-Curing Primary Amine Based Accelerator. Nippon Gomu Kyokaishi 1994, 67, 132–141. [Google Scholar] [CrossRef]
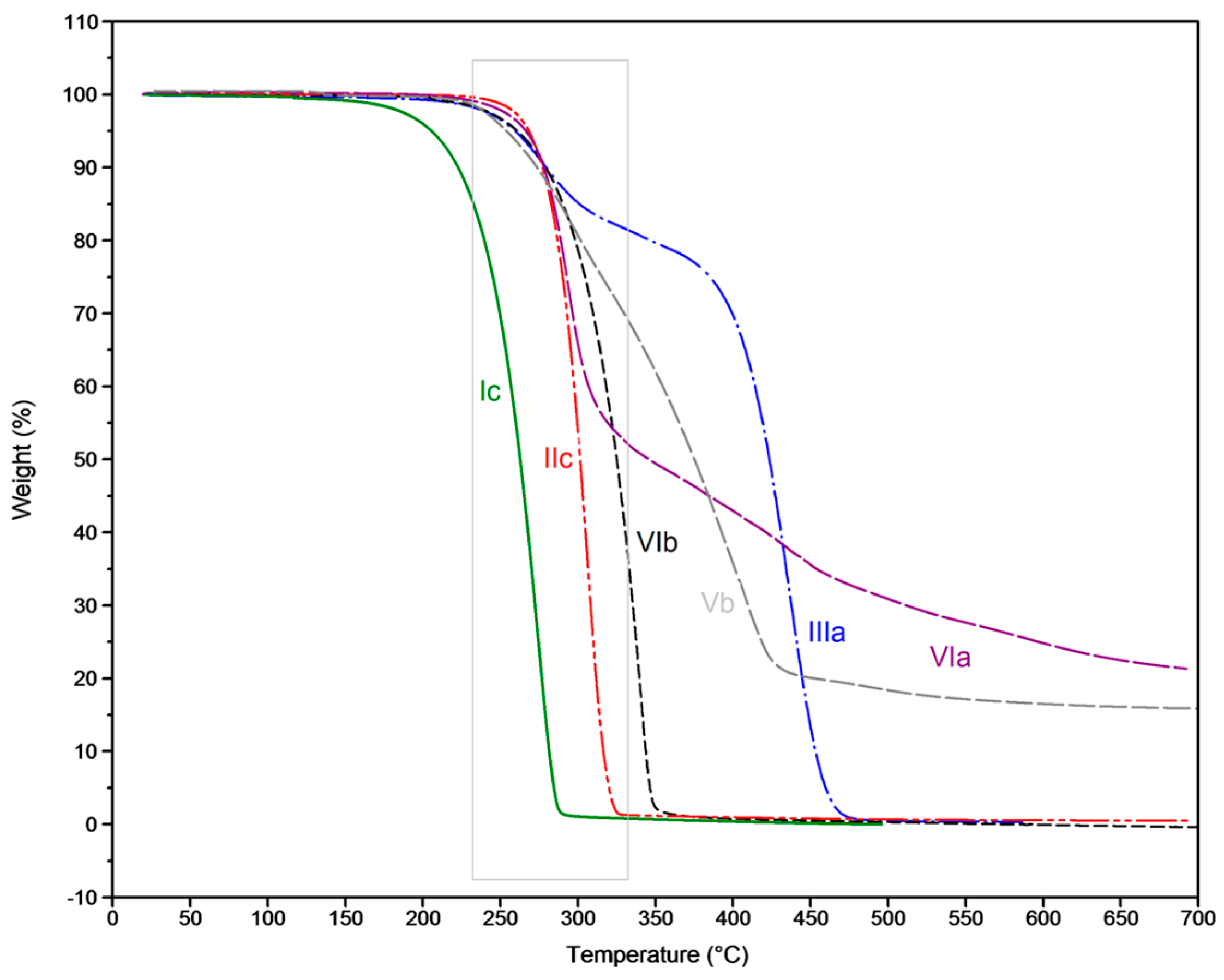
| Flame retardant | TGA weight loss T5% | DIN 4102-1 B2 1 | |||
|---|---|---|---|---|---|
| FR amount (wt %) | Average damage length (mm) | Average burning time (s) 2 | (Pass/Fail) | ||
| Reference PP | 0 | 190 | 39 | Fail | |
| Ia | ND 3 | 0.5 | 71 | 15 | Pass |
| Ib | ND | 0.5 | 56 | 8 | Pass |
| Ic | ND | 0.5 | 68 | 9 | Pass |
| Id | ND | 1.0 | 64 | 16 | Pass |
| IIa | 264 | 1.0 | 102 | 12 | Pass |
| IIb | 248 | 1.0 | 81 | 10 | Pass |
| IIc | 269 | 1.0 | 76 | 11 | Pass |
| IIIa | 254 | 1.0 | 80 | 12 | Pass |
| IIIb | 259 | 1.0 | 83 | 18 | Pass |
| IVb | 234 | 0.5 | 98 | 23 | Pass |
| Va | 238 | 1.0 | 75 | 12 | Pass |
| Vb | 254 | 1.0 | 61 | 7 | Pass |
| VIa | 266 | 0.5 | 67 | 14 | Pass |
| VIb | 265 | 1.0 | 69 | 18 | Pass |
| Flame retardant | DIN 4102-1 B2 | |||
|---|---|---|---|---|
| FR amount (wt %) | Average damage length (mm) | Average burning time (s) 1 | Pass/Fail | |
| Ic | 1.0 | 43 | 20 | Pass |
| IIb | 1.0 | 43 | 21 | Pass |
| IIc | 1.0 | 52 | 30 | Pass |
| Flame retardant | DIN 4102-1 B2 | |||
|---|---|---|---|---|
| FR amount (wt %) | Average damage length (mm) | Average burning time (s) 1 | (Pass/Fail) | |
| Reference LDPE | 0 | 190 | 37 | Fail |
| Ia | 0.5 | 108 | 30 | Pass |
| IIa | 0.5 | 133 | 36 | Pass |
| 1.0 | 122 | 28 | Pass | |
| Flame retardant | TGA weight loss T5% | UL 94 V | ||
|---|---|---|---|---|
| FR amount (wt %) | Total burning time (s) 1 | Rating (V-0/Fail) | ||
| Reference PS | - | 0 | ND | Fail |
| Iva | 194 | 5 | 4 | V-0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirri, T.; Aubert, M.; Pawelec, W.; Holappa, A.; Wilén, C.-E. Structure–Property Studies on a New Family of Halogen Free Flame Retardants Based on Sulfenamide and Related Structures. Polymers 2016, 8, 360. https://doi.org/10.3390/polym8100360
Tirri T, Aubert M, Pawelec W, Holappa A, Wilén C-E. Structure–Property Studies on a New Family of Halogen Free Flame Retardants Based on Sulfenamide and Related Structures. Polymers. 2016; 8(10):360. https://doi.org/10.3390/polym8100360
Chicago/Turabian StyleTirri, Teija, Melanie Aubert, Weronika Pawelec, Anton Holappa, and Carl-Eric Wilén. 2016. "Structure–Property Studies on a New Family of Halogen Free Flame Retardants Based on Sulfenamide and Related Structures" Polymers 8, no. 10: 360. https://doi.org/10.3390/polym8100360






