The Effect of Crystallinity on Compressive Properties of Al-PTFE
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Sample Preparation
2.2. X-ray Diffraction Analysis (XRD)
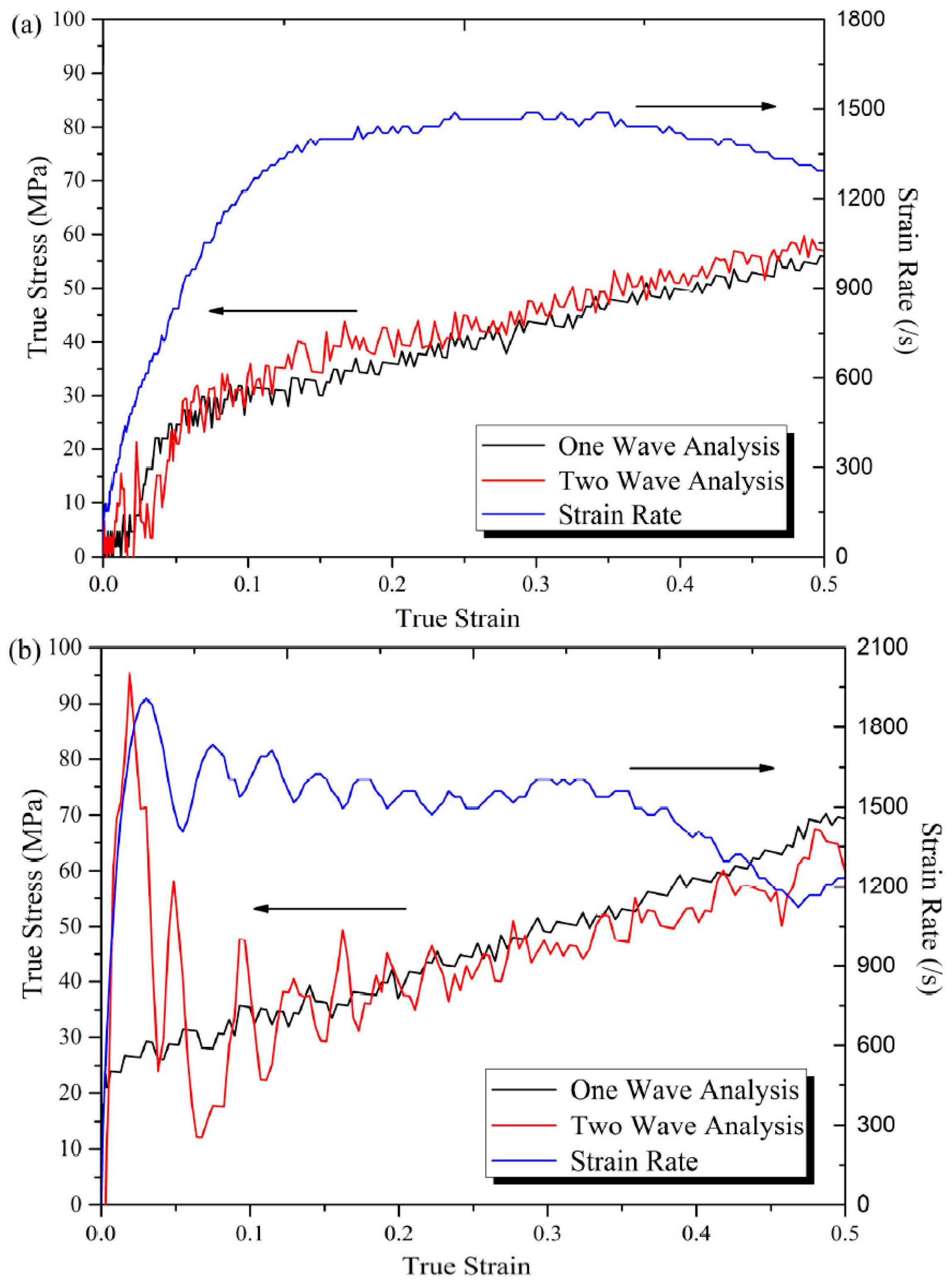
2.3. Compression Experiments at Different Strain Rates
3. Results and Discussion
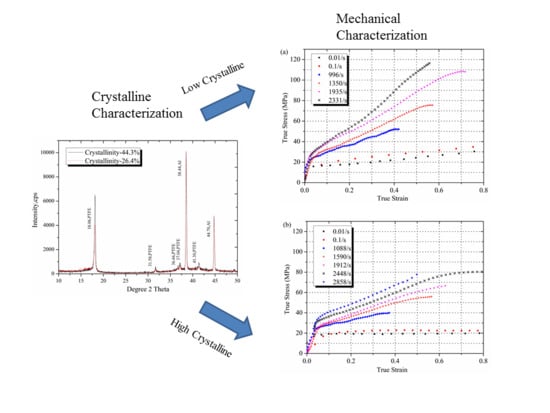
3.1. The Cystallinity of PTFE
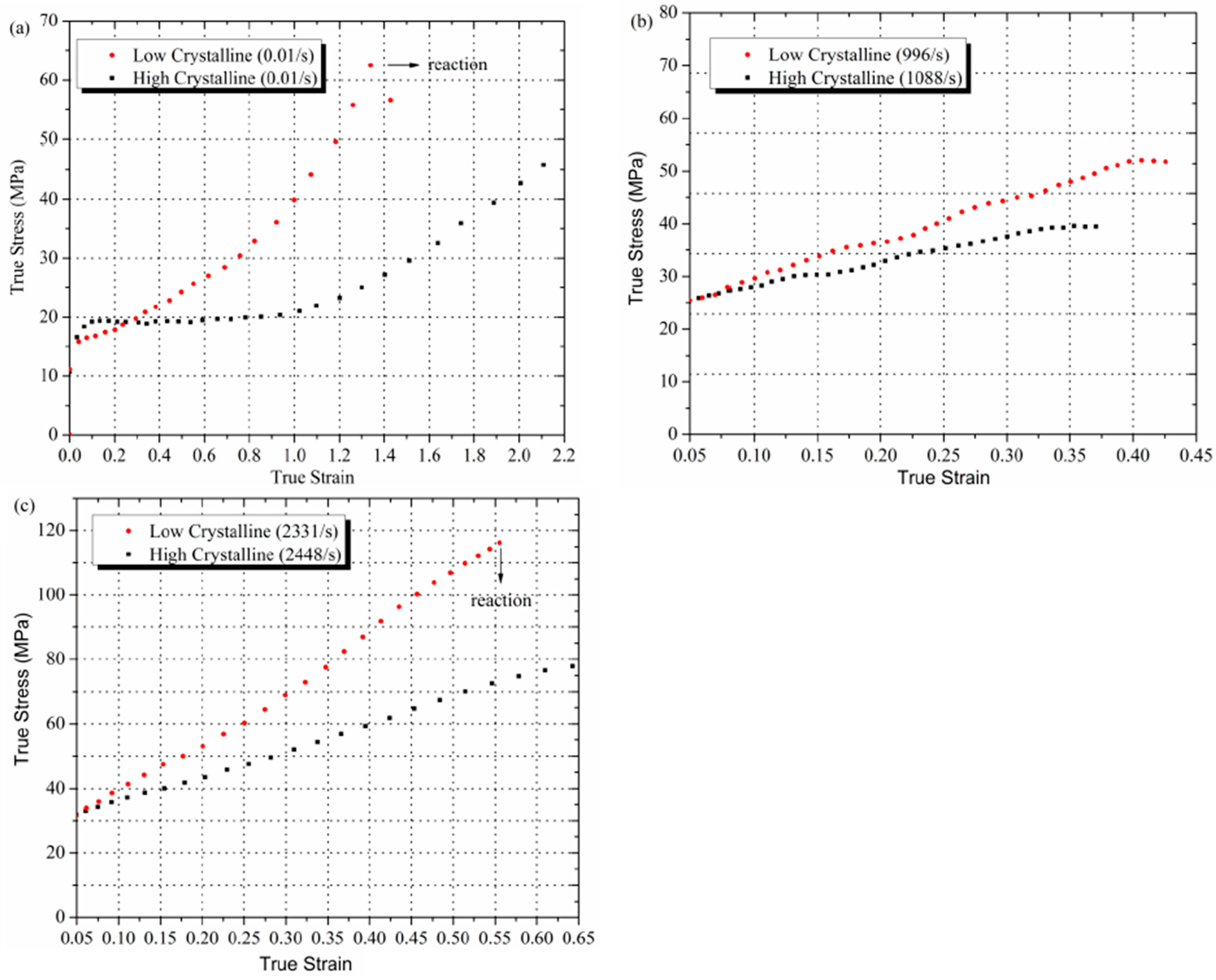
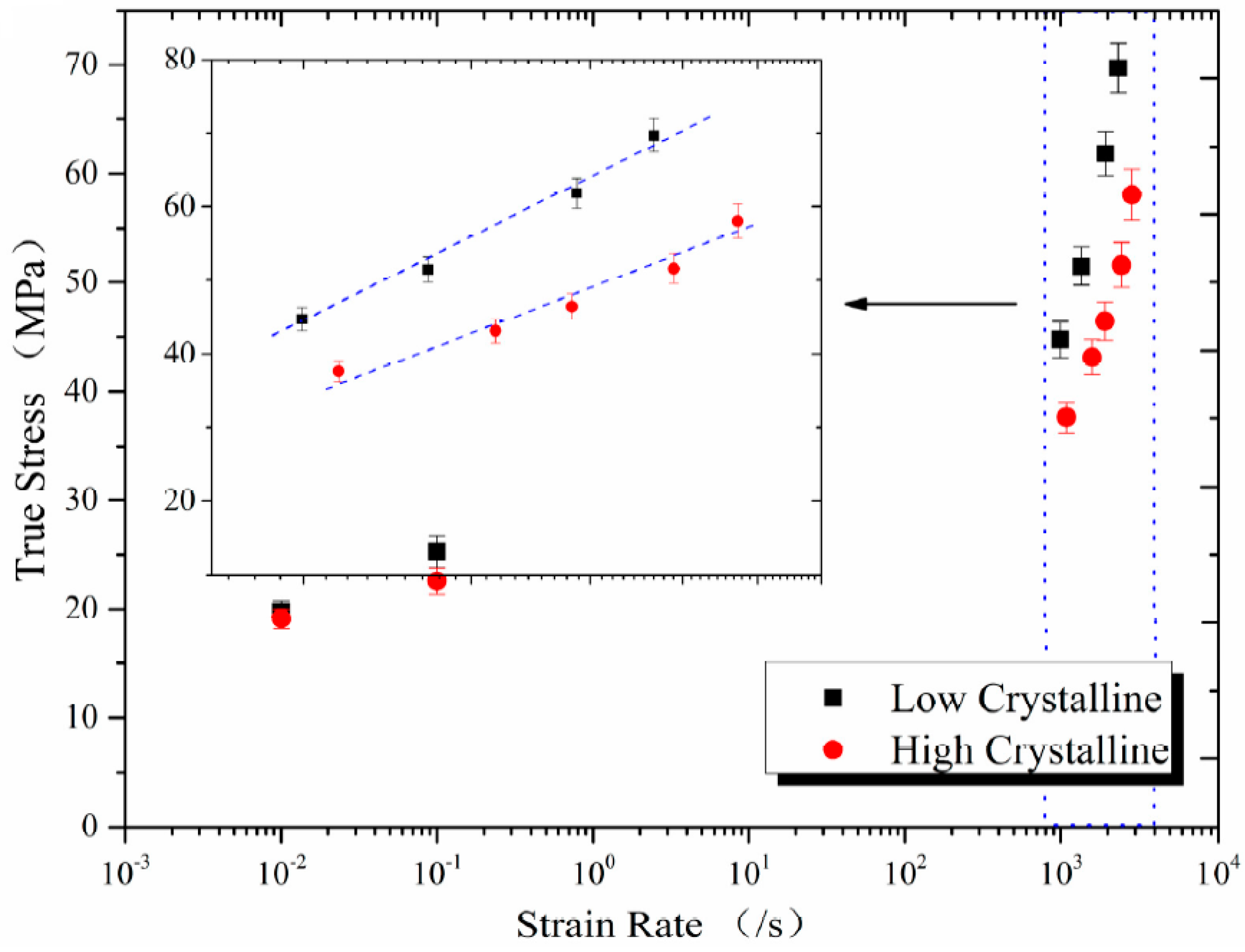
3.2. Mechanical Characterization
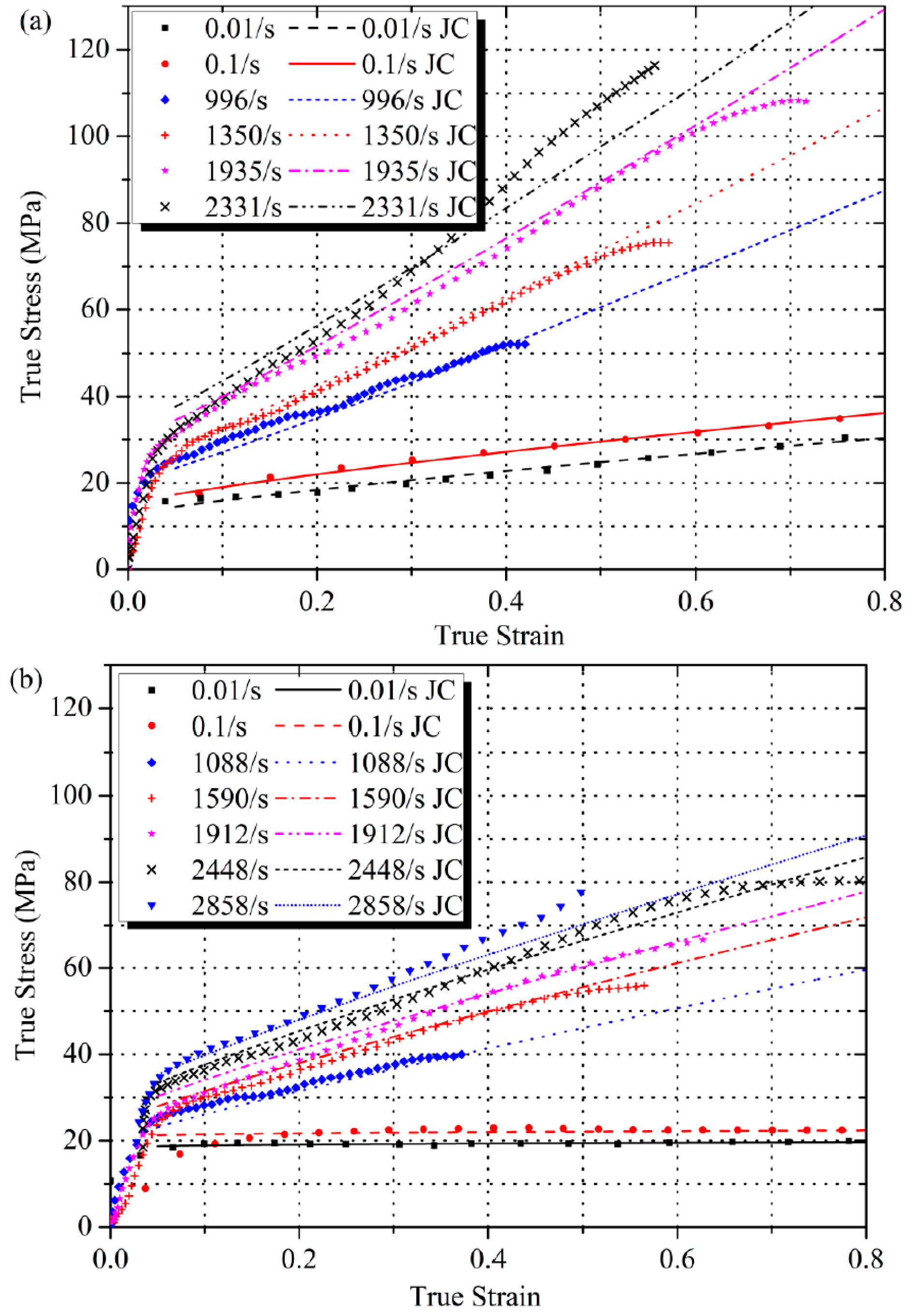
3.3. Application of the Johnson-Cook Model
3.4. Reactions of Al-PTFE in Compression
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.; Zheng, Y.; Yu, Q.; Liu, Z.; Yu, W. Impact-induced initiation and energy release behavior of reactive materials. J. Appl. Phys. 2011, 110, 074904. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducéré, J.-M.; Baijot, V.; Pinon, S.; Calais, T.; Estève, A.; Rouhani, M.D.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Casem, D.T. Mechanical Response of an Al-PTFE Composite to Uniaxial Compression over a Range of Strain Rates and Temperatures; DTIC Document; Army Research Laboratory: Adelphi, MD, USA, 2008. [Google Scholar]
- Raftenberg, M.; Mock, W., Jr.; Kirby, G. Modeling the impact deformation of rods of a pressed PTFE/Al composite mixture. Int. J. Impact Eng. 2008, 35, 1735–1744. [Google Scholar] [CrossRef]
- Rae, P.; Dattelbaum, D. The properties of poly (tetrafluoroethylene) (PTFE) in compression. Polymer 2004, 45, 7615–7625. [Google Scholar] [CrossRef]
- Rae, P.; Brown, E. The properties of poly (tetrafluoroethylene) (PTFE) in tension. Polymer 2005, 46, 8128–8140. [Google Scholar] [CrossRef]
- Brown, E.N.; Rae, P.J.; Bruce Orler, E.; Gray, G.T.; Dattelbaum, D.M. The effect of crystallinity on the fracture of polytetrafluoroethylene (PTFE). Mater. Sci. Eng. C 2006, 26, 1338–1343. [Google Scholar] [CrossRef]
- Feng, B.; Fang, X.; Li, Y.C.; Wang, H.X.; Mao, Y.M.; Wu, S.Z. An initiation phenomenon of Al-PTFE under quasi-static compression. Chem. Phys. Lett. 2015, 637, 38–41. [Google Scholar] [CrossRef]
- Osborne, D.T.; Pantoya, M.L. Effect of al particle size on the thermal degradation of Al/teflon mixtures. Combust. Sci. Technol. 2007, 179, 1467–1480. [Google Scholar] [CrossRef]
- Brown, E.N.; Dattelbaum, D.M. The role of crystalline phase on fracture and microstructure evolution of polytetrafluoroethylene (PTFE). Polymer 2005, 46, 3056–3068. [Google Scholar] [CrossRef]
- Speerschneider, C.J.; Li, C.H. A correlation of mechanical properties and microstructure of polytetrafluoroethylene at various temperatures. J. Appl. Phys. 1963, 34, 3004. [Google Scholar] [CrossRef]
- Martin, J.; Ponçot, M.; Hiver, J.M.; Bourson, P.; Dahoun, A. Real-time Raman spectroscopy measurements to study the uniaxial tension of isotactic polypropylene: A global overview of microstructural deformation mechanisms. J. Raman Spectrosc. 2013, 44, 776–784. [Google Scholar] [CrossRef]
- Matsuba, G.; Ito, C.; Zhao, Y.; Inoue, R.; Nishida, K.; Kanaya, T. In situ small-angle X-ray and neutron scattering measurements on a blend of deuterated and hydrogenated polyethylenes during uniaxial drawing. Polym. J. 2013, 45, 293–299. [Google Scholar] [CrossRef]
- Koo, G.P.; Andrews, R.D. Mechanical behavior of polytetrafluoroethylene around the room-temperature first-order transition. Polym. Eng. Sci. 1969, 9, 268–276. [Google Scholar] [CrossRef]
- Jordan, J.L.; Siviour, C.R.; Foley, J.R.; Brown, E.N. Compressive properties of extruded polytetrafluoroethylene. Polymer 2007, 48, 4184–4195. [Google Scholar] [CrossRef]
- Lee, C.S.; Caddell, R.M.; Yeh, G.S.Y. Cold extrusion and cold drawing of polymeric rod: The influence on subsequent tensile and compressive mechanical properties. Mater. Sci. Eng. 1972, 10, 241–248. [Google Scholar] [CrossRef]
- Sperati, C.; McPherson, J. The effect of crystallinity and molecular weight on physical properties of polytetrafluoroethylene. In Proceedings of the Meeting of the American Chemical Society, Atlantiv City, NJ, USA, 16–21 September 1956.
- Sperati, C.A.; Starkweather, H.W. Fluorine-containing polymers. II. Polytetrafluoroethylene. Adv. Polym. Sci. 2006, 2, 465–495. [Google Scholar]
- Nielson, D.B.; Tanner, R.L.; Lund, G.K. High Strength Reactive Materials. U.S. Patent US20030096897 A1, 22 May 2003. [Google Scholar]
- Gray, G.; Kuhn, H.; Medlin, D. Asm Handbook Volume 8: Mechanical Testing and Evaluation; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Lee, T.H.; Boey, F.Y.C.; Khor, K.A. X-ray diffraction analysis technique for determining the polymer crystallinity in a polyphenylene sulfide composite. Polym. Compos. 1995, 16, 481–488. [Google Scholar] [CrossRef]
- Lehnert, R.J.; Hendra, P.J.; Everall, N.; Clayden, N.J. Comparative quantitative study on the crystallinity of poly(tetrafluoroethylene) including raman, infra-red and 19F nuclear magnetic resonance spectroscopy. Polymer 1997, 38, 1521–1535. [Google Scholar] [CrossRef]
- Hu, T.Y. Characterization of the crystallinity of polytetrafluoroethylene by X-ray and IR spectroscopy, differential scanning calorimetry, viscoelastic spectroscopy and the use of a density gradient tube. Wear 1982, 82, 369–376. [Google Scholar] [CrossRef]
- Cai, J.; Walley, S.; Hunt, R.; Proud, W.; Nesterenko, V.; Meyers, M. High-strain, high-strain-rate flow and failure in PTFE/Al/W granular composites. Mater. Sci. Eng. A 2008, 472, 308–315. [Google Scholar] [CrossRef]
- Gray, G.T.I.; Blumenthal, W.R.; Trujillo, C.P.; Carpenter, R.W.I. Influence of temperature and strain rate on the mechanical behavior of adiprene L-100. J. Phys. IV 1997, 07, 523–528. [Google Scholar] [CrossRef]
- Gray, G.T. High-Strain-Rate Testing of Materials: The Split-Hopkinson Pressure Bar; John Wiley & Sons, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Schrauwen, B. Deformation and Failure of Semi-Crystalline Polymer Systems: Influence of Micro and Molecular Structure. Master’s Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2003. [Google Scholar]
- Peterlin, A. Chain folding in lamellar crystals. Macromolecules 1980, 13, 777–782. [Google Scholar] [CrossRef]
- Walley, S.; Field, J. Strain rate sensitivity of polymers in compression from low to high rates. DYMAT J. 1994, 1, 211–227. [Google Scholar]
- Resnyansky, A.D.; Bourne, N.K.; Millett, J.C.F.; Brown, E.N. Constitutive modeling of shock response of polytetrafluoroethylene. J. Appl. Phys. 2011, 110, 033530. [Google Scholar] [CrossRef]
- Resnyansky, A.D.; Bourne, N.K.; Brown, E.N.; Millett, J.C.F. Phase transition modeling of polytetrafluoroethylene during taylor impact. J. Appl. Phys. 2014, 116, 223502. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, A.; Qiao, L.; Zhang, J.; Zhang, Y.; Guan, Z. Experimental study on impact-initiated characters of multifunctional energetic structural materials. J. Appl. Phys. 2013, 113, 083508. [Google Scholar] [CrossRef]
- Sorensen, B. High-velocity impact of encased AL/PTFE projectiles on structural aluminum armor. Procedia Eng. 2015, 103, 569–576. [Google Scholar] [CrossRef]
- Walley, S.M.; Field, J.E.; Greenaway, M.W. Crystal sensitivities of energetic materials. Mater. Sci. Technol. 2006, 22, 402–413. [Google Scholar] [CrossRef]
- Fuller, K.N.G.; Field, J.E. The temperature rise at the tip of fast-moving cracks in glassy polymers. Proc. R. Soc. A 1975, 341, 537–557. [Google Scholar] [CrossRef]
- Döll, W. Optical interference measurements and fracture mechanics analysis of crack tip craze zones. In Crazing in Polymers; Springer: Berlin, Germany, 1983. [Google Scholar]
- Rittel, D. Transient temperature measurement using embedded thermocouples. Exp. Mech. 1998, 38, 73–78. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Jiang, C.; Cheng, Q.; Kang, G. In-situ observation of temperature rise during scratch testing of poly (methylmethacrylate) and polycarbonate. Tribol. Int. 2016, 95, 1–4. [Google Scholar] [CrossRef]
- Swallowe, G.M.; Field, J.E. The ignition of a thin layer of explosive by impact; the effect of polymer particles. Proc. R. Soc. A Math. Phys. Eng. Sci. 1982, 379, 389–408. [Google Scholar] [CrossRef]
- Meyers, M.A.; Nesterenko, V.F.; Lasalvia, J.C.; Xue, Q. Shear localization in dynamic deformation of materials: Microstructural evolution and self-organization. Mater. Sci. Eng. A 2001, 317, 204–225. [Google Scholar] [CrossRef]
- Hoffmann, T. Viscoelastic Properties of Polymers; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Brown, E.N.; Rae, P.J.; Liu, C. Mixed-mode-I/II fracture of polytetrafluoroethylene. Mater. Sci. Eng. A 2007, 468–470, 253–258. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.-C.; Wu, S.-Z.; Wang, H.-X.; Tao, Z.-M.; Fang, X. A crack-induced initiation mechanism of AL-PTFE under quasi-static compression and the investigation of influencing factors. Mater. Des. 2016, 108, 411–417. [Google Scholar] [CrossRef]
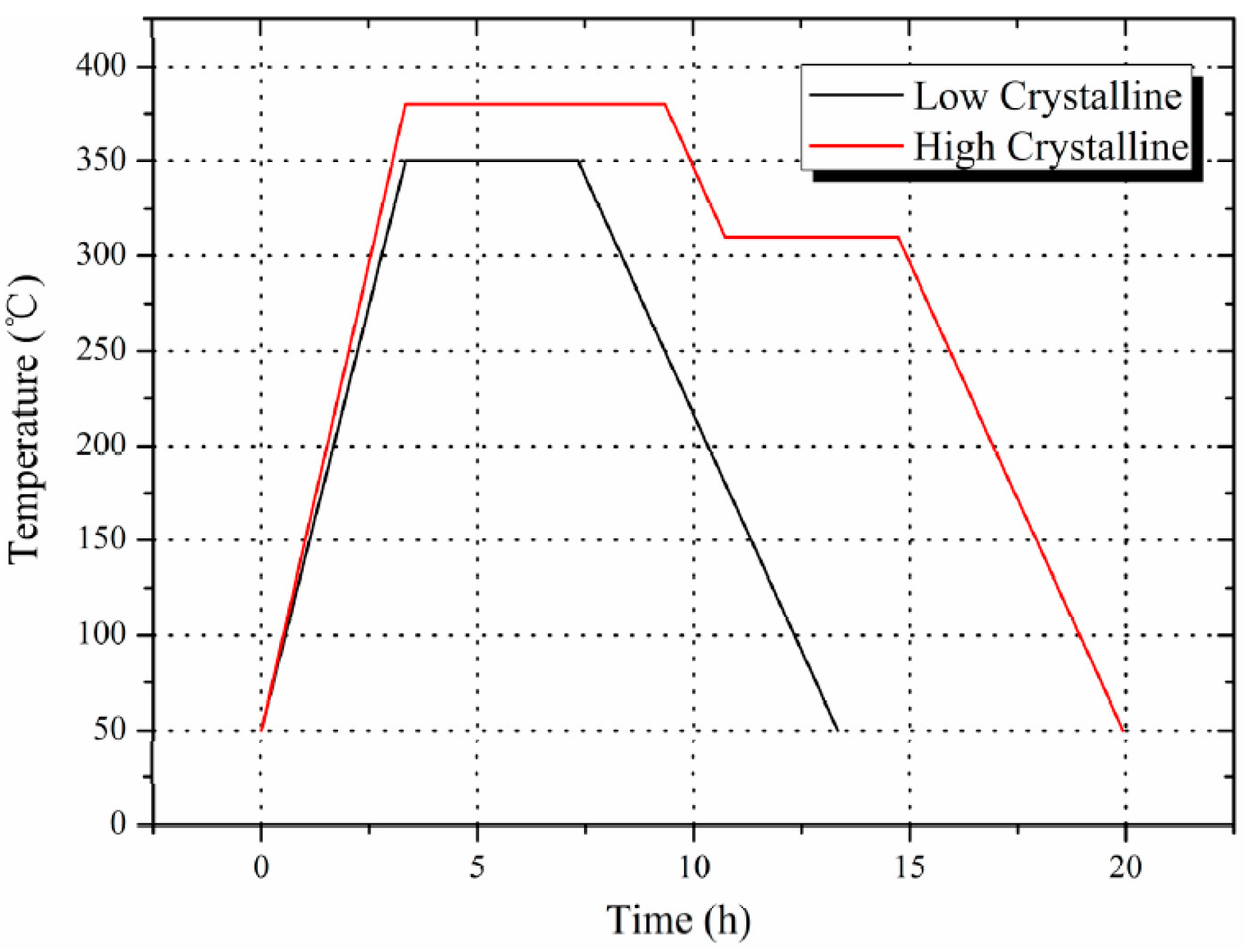
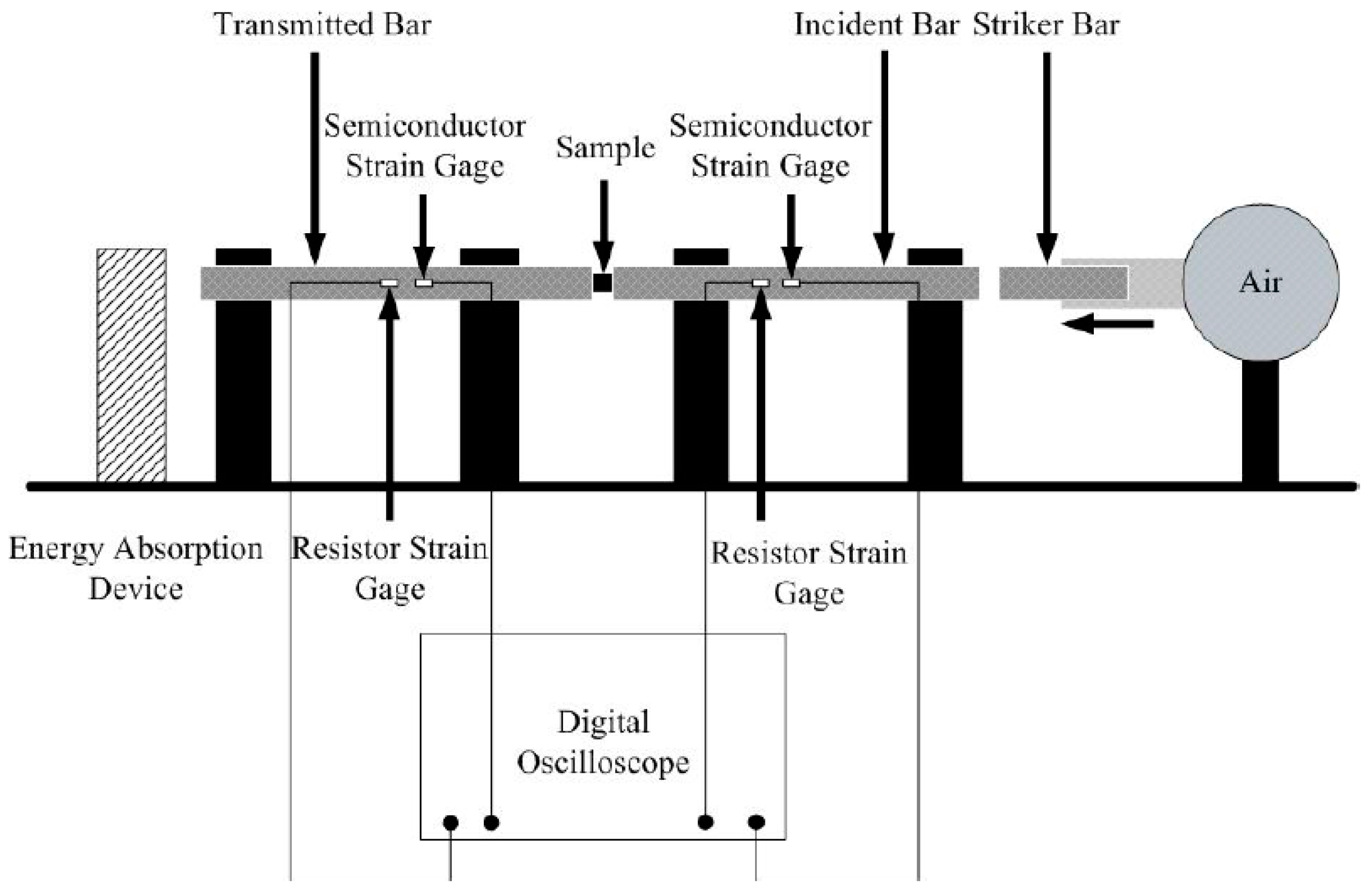
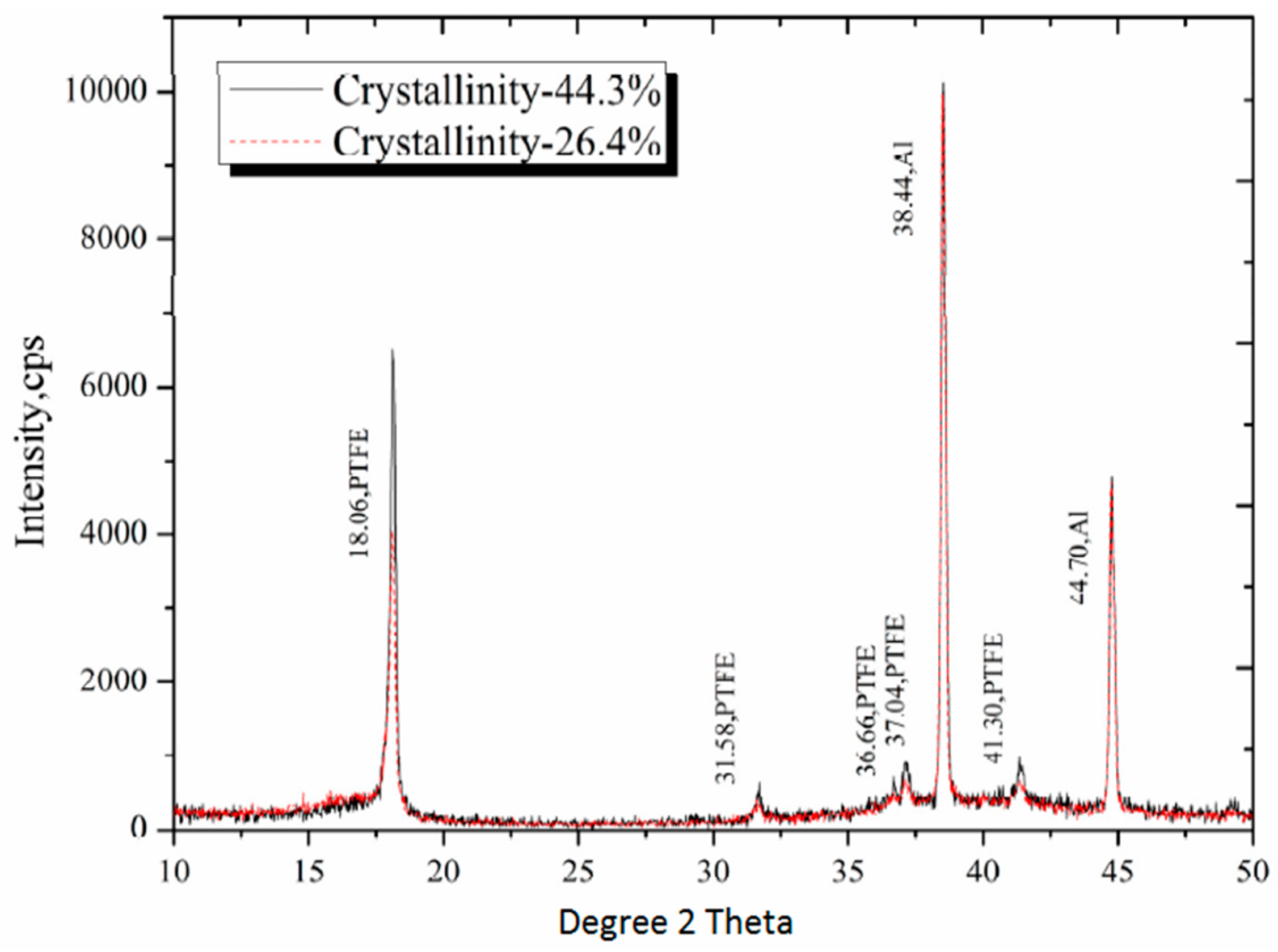


| Sintering temperature (°C) | Density (g/cm3) | Xc (Density method) | Xc (XRD method) | |
|---|---|---|---|---|
| Low Crystalline Sample | 350 | 2.25 | 43.2% | 26.4% |
| High Crystalline Sample | 380 | 2.30 | 63.7% | 44.3% |
| Parameter | Quasi-Static | Dynamic | ||
|---|---|---|---|---|
| Low crystalline | High crystalline | Low crystalline | High crystalline | |
| A | 20.41 | 18.00 | 24.51 | 23.80 |
| B | 22.19 | 1.79 | 104.82 | 58.52 |
| C | 0.07 | 0.06 | 0.59 | 0.45 |
| n | 1.35 | 0.30 | 1.09 | 0.88 |
| 0.01 | 0.01 | 1350 | 1590 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, B.; Fang, X.; Wang, H.-X.; Dong, W.; Li, Y.-C. The Effect of Crystallinity on Compressive Properties of Al-PTFE. Polymers 2016, 8, 356. https://doi.org/10.3390/polym8100356
Feng B, Fang X, Wang H-X, Dong W, Li Y-C. The Effect of Crystallinity on Compressive Properties of Al-PTFE. Polymers. 2016; 8(10):356. https://doi.org/10.3390/polym8100356
Chicago/Turabian StyleFeng, Bin, Xiang Fang, Huai-Xi Wang, Wen Dong, and Yu-Chun Li. 2016. "The Effect of Crystallinity on Compressive Properties of Al-PTFE" Polymers 8, no. 10: 356. https://doi.org/10.3390/polym8100356