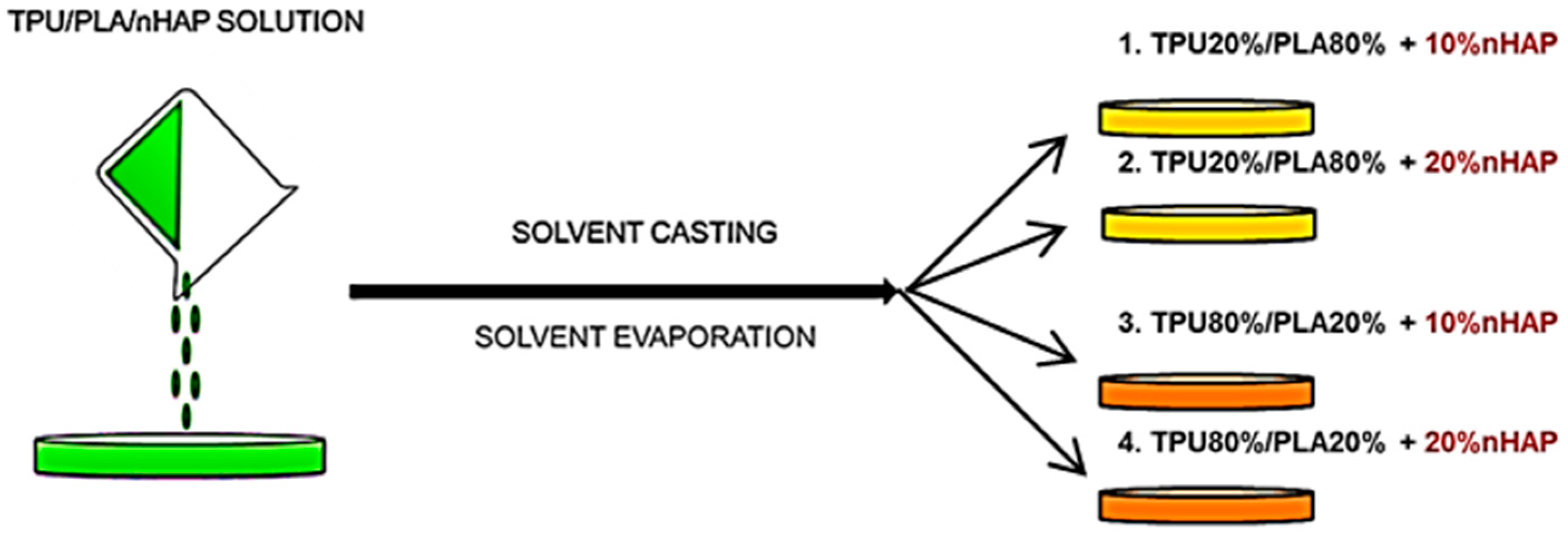
2.1. Materials
The following reactants were used: synthetic hydroxyapatite nanoparticles (nHAp) (Ca5(OH)(PO4)3, <100 nm. NanoSynHAp, Poznan, Poland), sodium chloride as porogen (NaCl particle size: 300 ÷ 600 µm, POCH S.A., Gliwice, Poland), thermoplastic polyurethane (TPU) (Elastollan® A12P000-INTiBS PAN, Wroclaw, Poland), inelastic polylactide PURASORB® (PLA) (PURACbiochembv, Gorinchem, The Netherlands), dimethyloformamide (DMF) as a solvent in molecular biology (≥99.0% purity, Sigma Aldrich, Poznan, Poland), and sterile filtered water for molecular biology methods and cell culture (Sigma Aldrich).
2.3. Characterization of Biomaterials
2.3.1. SEM/EDX Analysis
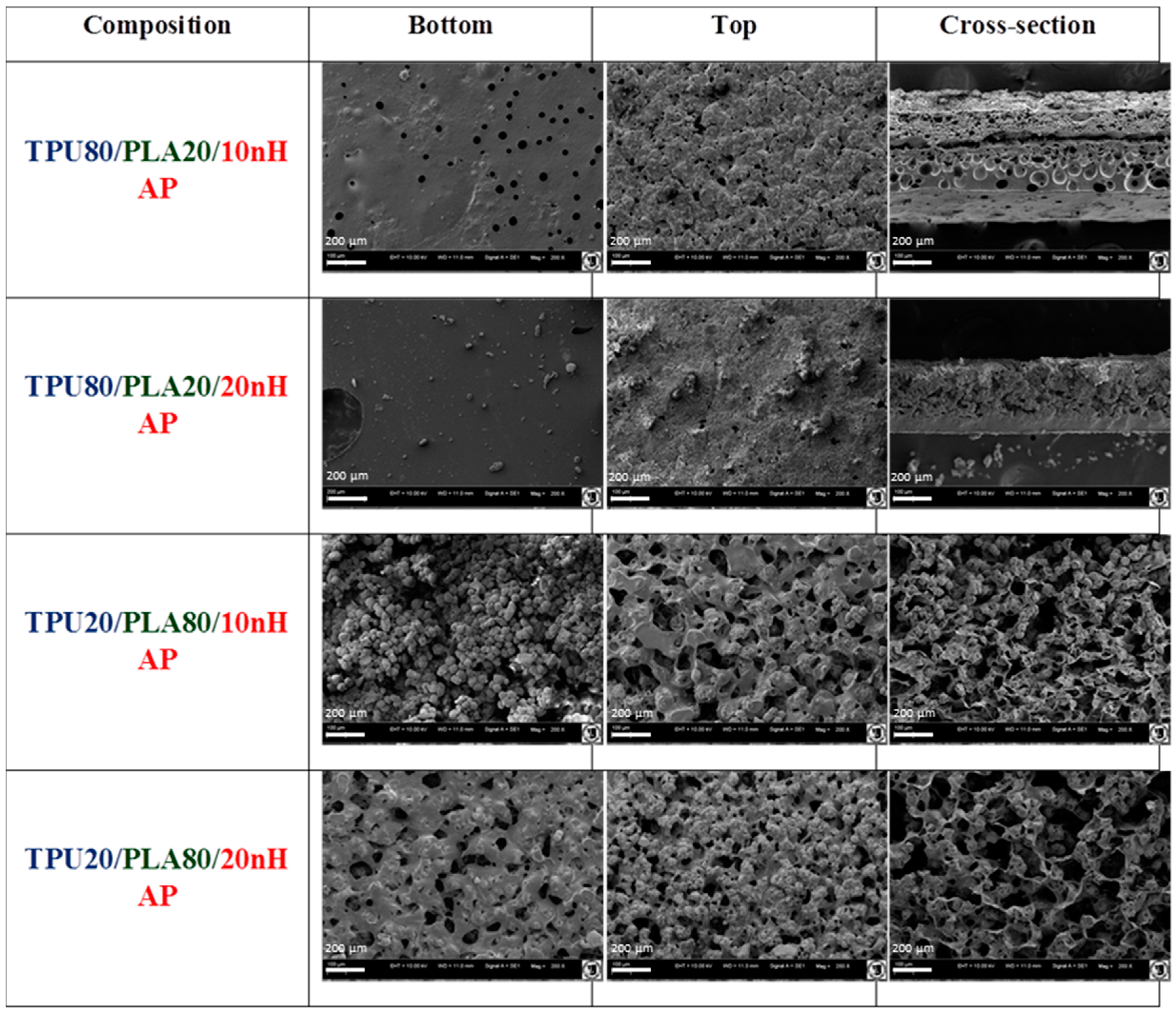
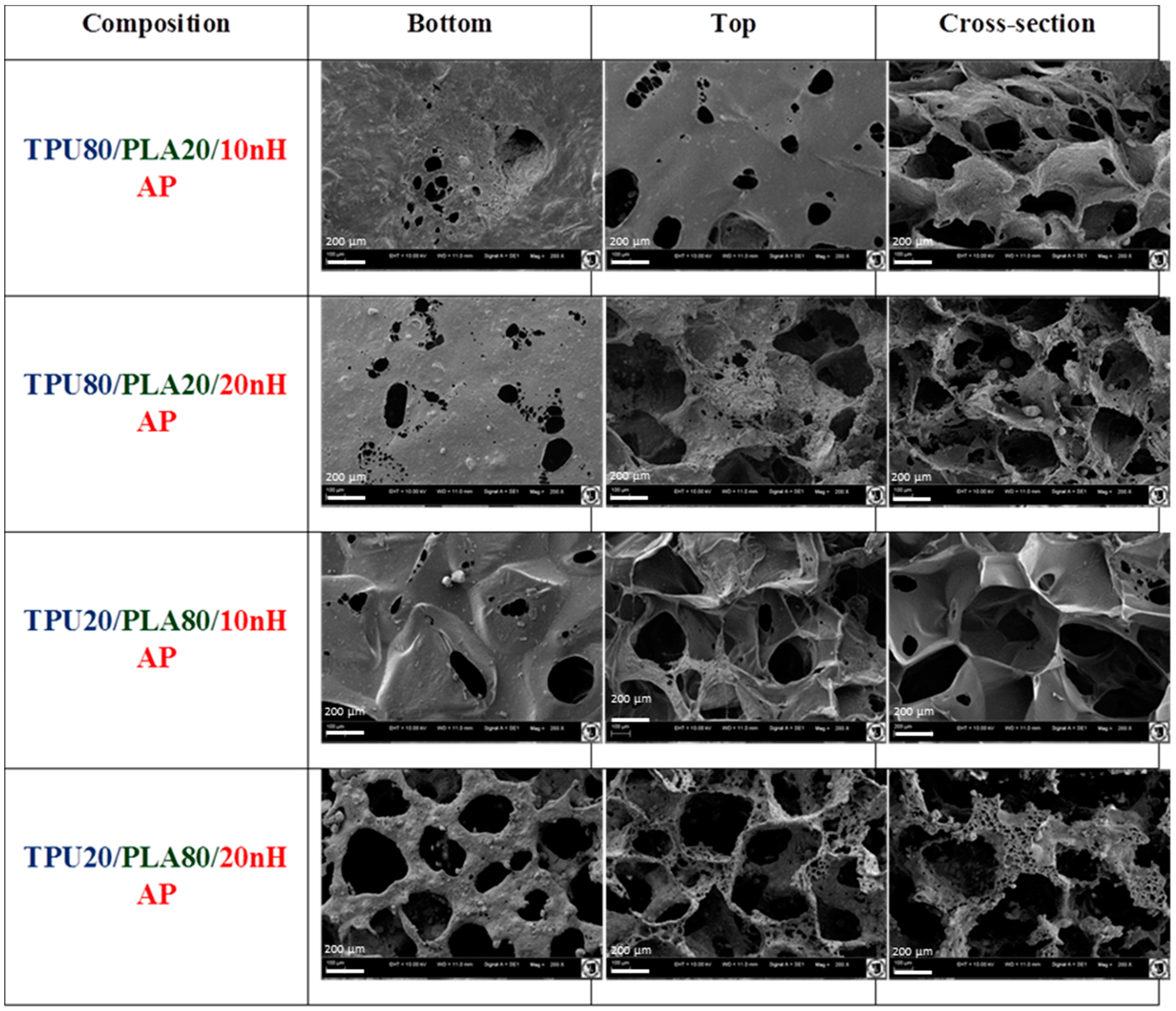
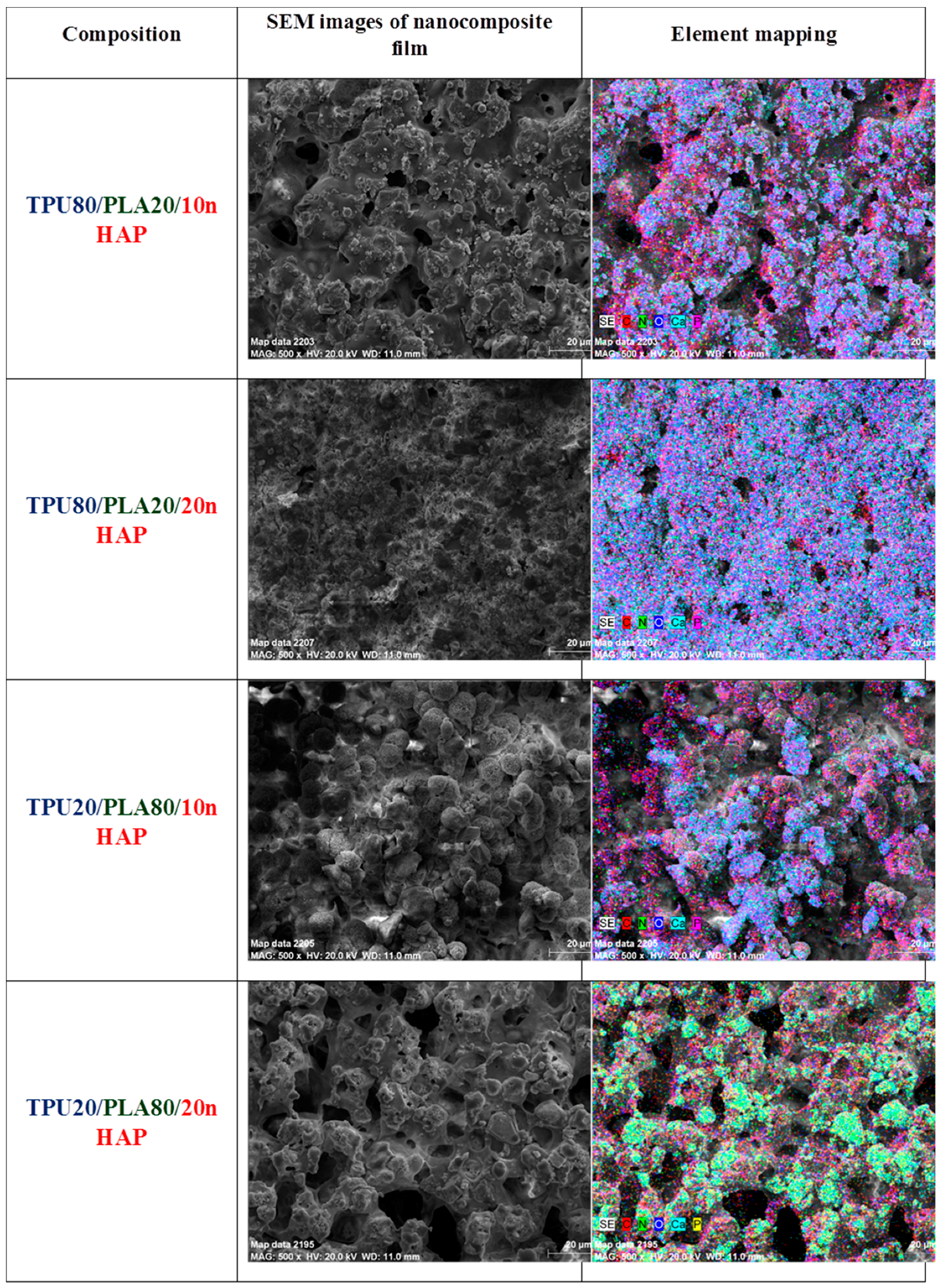
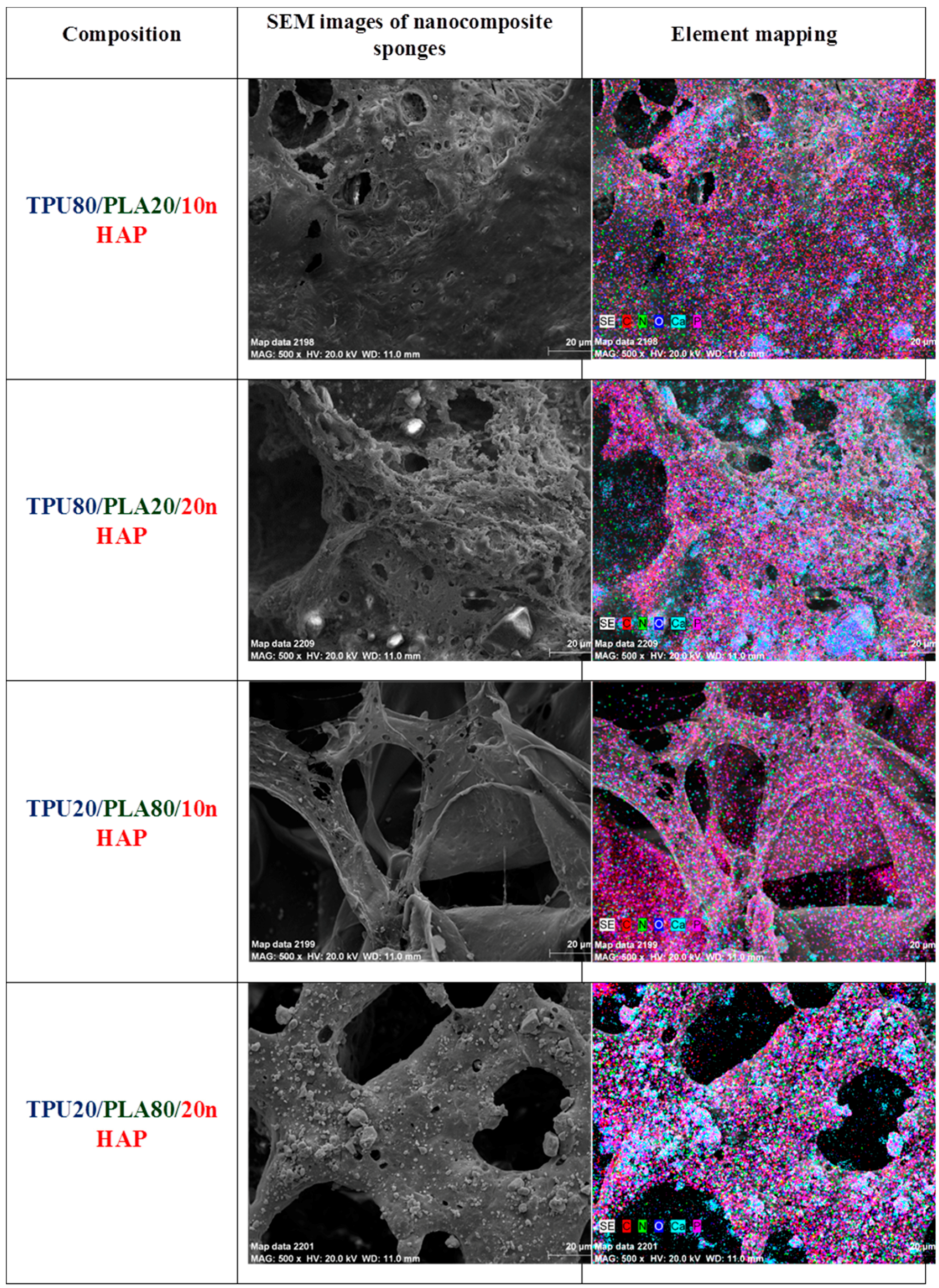
Characterization of the morphology and chemical elements of the TPU/PLA/HAp composite films and sponges was examined on a scanning electron microscope (SEM, Zeiss EVO LS15, Oberkochen, Germany). For SEM investigation, nanocomposite samples were coated with gold (ScanCoat6, Edwards, Warszawa, Poland). Cross-section and external top and bottom surfaces of the nanocomposite samples were fixed on SEM sample holders using conductive carbon tape.
2.3.2. Profilometry
The roughness of the obtained TPU/PLA/nHAp composite films was analysed by a profilometer (Hommelwerke, Schwenningen, Germany). The roughness of 10 different spots on both sides of each sample was determined and the average surface roughness (Ra) was evaluated. All values are presented as means of ten measurements (±standard deviation (SD)).
2.3.3. 3D Analysis, BET Analysis—Porosity
Total porosity was determined using direct methods (determining the bulk volume of the porous sample, and then determining the volume of skeletal material with no pores (pore volume = total volume − material volume).
2.3.4. Water Adsorption Capacity and Wettability
Contact angles of the films to water were measured on the air-surface of the samples using a DSA10 contact angle meter (Krüss, Hamburg, Germany). Water absorption of the polymeric composite sponge were measured by immersing the sponges in distilled water for a predetermined time period, then the films were taken out with filter paper vacuum-drier and finally weighed. To evaluate the hydrophilicity of the TPU/PLA blend doped with nHAp, their surface contact angle to water and water absorption were measured and compared to PLA and TPU. Drops of filtered distilled water of 2 µL volume were added using a syringe. The values of the water contact angle were obtained by averaging the values of at least ten measurements for each nanocomposite film. The wetting behaviour of the TPU/PLA/nHAp sponge was analysed by measuring the water adsorption capacity of the composite sponge in the air. The polymer sponges were immersed in distilled water for seven days. Wettability measurements were conducted six times (after 2, 24, 48, 72, 96 and 168 h). The water volume was 20 mL.
2.3.5. Mechanical Properties—Tensile and Compressive Strength
Mechanical properties of the composite films (rectangular samples: 60 mm × 5 mm × 1 mm) were determined by measuring their tensile strength (σ) on a ZWICK Universal Test Machine with a tensile speed of 10 mm/min at room temperature.
2.3.6. Mechanical Compressive (Sponge) and Tensile Test (Film)
A universal machine (1435, Zwick & Roell, Wroclaw, Poland) was used for the measurement of the compression and tensile strength of the nanocomposite materials. Cylindrically (d and h = 10 mm) shaped samples were prepared for mechanical testing. Cylindrical samples were compressed between parallel plates using fully dried samples. Compressive strain-stress curves of all sponge series were determined with a 10 N load cell and a cross-head speed of 1 mm/min. The compressive Young’s modulus (MPa) was calculated between 0.5–1 N based on the stress-strain curve. All values are means of ten measurements (±standard deviation).
2.3.7. Thermal Analysis of TPU/PLA/nHAp Composites
The thermal degradation behaviour of TPU, PLA, TPU/PLA blends and their hydroxyapatite nanocomposites was investigated by termogravimetry (TGA) and differential scanning calorimetry (DSC). Thermal analyses of filler-free TPU/PLA blends and TPU/PLA/nHAP composites were carried out using a NETZSCH STA (449 F3 Jupiter, Selb, Germany) instrument under nitrogen. Initial sample weight was set as 5 mg for each operation. The specimen was heated from 20 to 600 °C at a heating rate of 10 °C/min.
2.3.8. Focused Ion Beam—Scanning Electron Microscopy
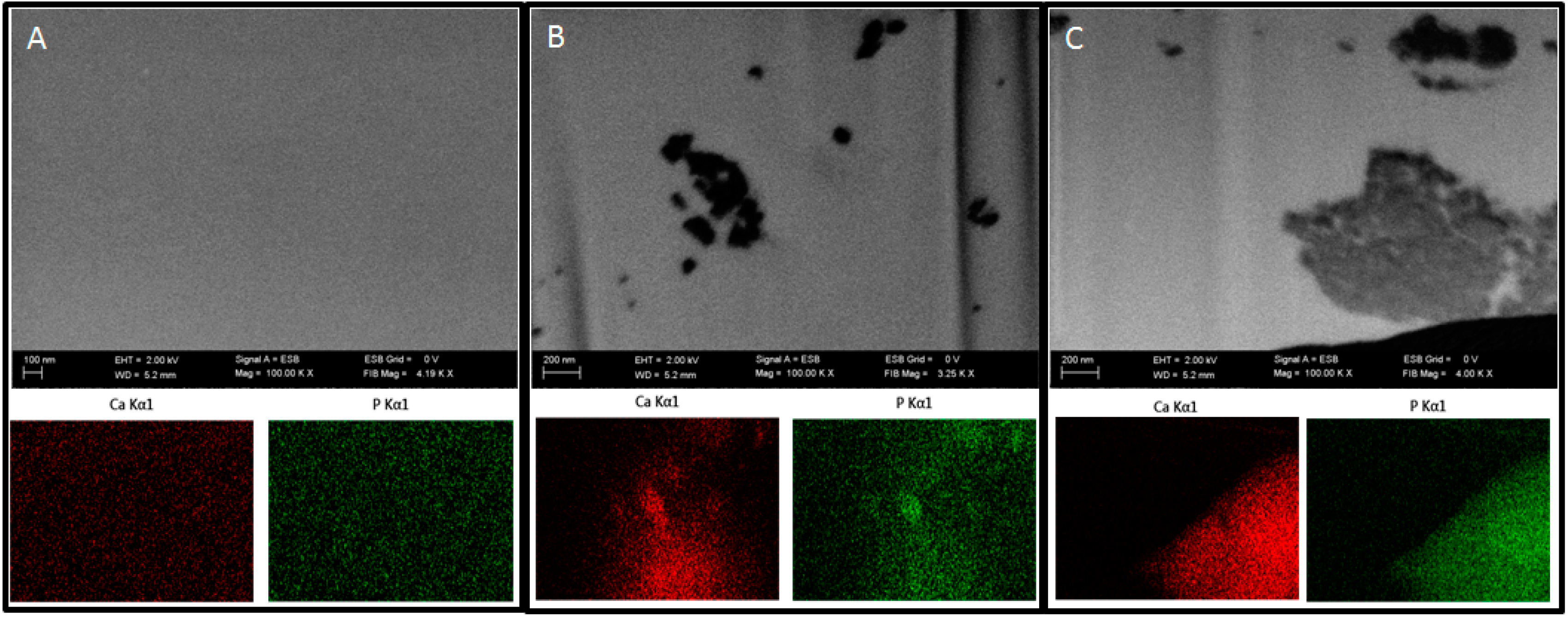
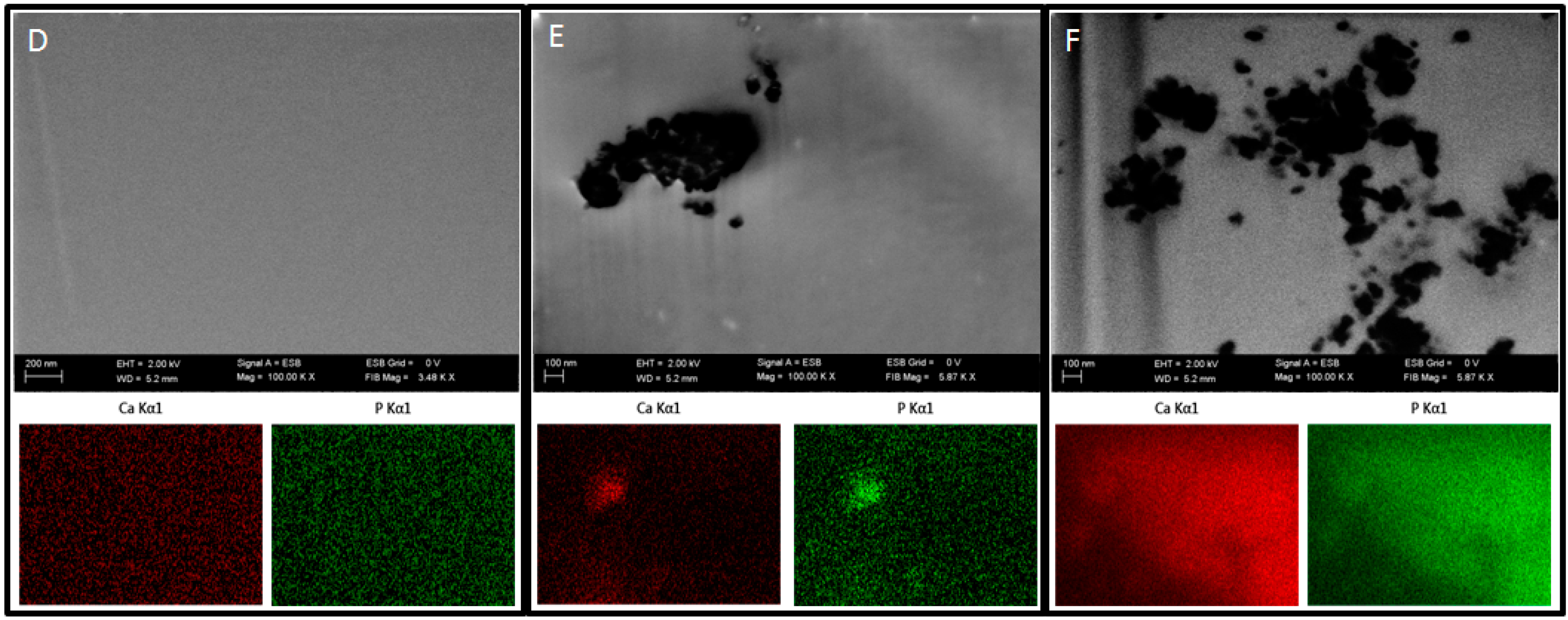
In order to examine the presence of nHAp in the prepared polymer blends, focused ion beam (FIB) milling and low voltage scanning electron microscopy with energy-selective backscattered detector (EsB, Auriga 60, Zeiss, Oberkochen, Germany) were used. The samples were embedded in a low viscosity resin (Agar, Cambridge, UK). Next, the sample blocks were trimmed using a glass knife to recover the samples on the block surfaces and initially polish the examined surface. The prepared blocks were mounted on microscope stubs using carbon tape, sputtered with a 30-nm-thick layer of gold and placed in a microscope. The region of interest was localized, and a groove was milled with FIB in the selected sites using a 30 kV/4 nA aperture. The recovered surface was polished using a 30 kV/600 pA aperture. Polished surfaces were imaged at 2 kV using an EsB detector, with detector’s grid set to 0 V. Nano-hydroxyapatite particles were imaged and additionally examined using EDX mapping at 20 kV (Oxford, UK) to confirm the elemental composition of the nanoparticles.
2.3.9. Isolation of Cells
All experiments were approved by the Second Local Ethics Committee at the Wroclaw University of Environmental and Life Sciences (registry number KB-177/2014, March 2014).
Adipose stem cells were isolated from subcutaneous fat tissue donated by patients aged 44–77 years during surgical procedures at the Jagiellonian University Medical College. Isolation procedures were performed according to the method described previously [
19]. Removed tissue fragments were placed in sterile Hank’s balanced salt solution (HBSS, Sigma Aldrich) and transported to the laboratory. The material was minced with a surgical blade, washed extensively off of the blood traces in fresh HBSS, and placed in collagenase solution (5 mg/mL) in Dulbecco’s modified eagle medium/Nutrient mixture F12 Ham (DMEM F12, Sigma Aldrich) for 40 min at 37 °C/5% CO
2, with extensive shaking every 10 min. The digested tissue was then centrifuged at 1200×
g for 10 min, after which the supernatant with released oil and remaining tissue was discarded. Cell pellet was suspended in DMEM F12 containing 10% fetal bovine serum (FBS, Sigma Aldrich) and 1% antibiotics (penicillin/streptomycin/amphotericin b, PSA, Sigma Aldrich) and cultured in a humidified incubator at 37 °C/5% CO
2 until cells reached about 80% confluence. Prior to the experiment, the cells from the primary cultures were further propagated in the secondary cultures up to three passages in Dulbecco’s modified eagle’s medium (DMEM, Sigma Aldrich) containing 4500 mg/L of glucose, 10% FBS and 1% PSA.
2.3.10. Flow Cytometry
The immunophenotype analysis was performed by means of four-coloured cytofluorometry using a FACS Calibur flow cytometer (BD, Warszawa, Poland). For analysis, the following monoclonal, fluorophore-conjugated antibodies were used: CD34-PE, CD44-PE, CD45-PE, CD90-FITC, CD105-PerCP, CD73-APC (BD). The cells were detached from the culture vessels using accutase (BioWest, Nuaillé, France), followed by rinsing in PBS with 2% FBS at a concentration of 5 × 105/mL. The cells were centrifuged at 300× g for 5 min, and the cell pellet was resuspended in a fresh culture medium containing proper antibodies (1:1000 dilution) and incubated at 4 °C for 20 min. The samples were washed three times in PBS and measured. At least 1 × 104 of the cells were measured for each sample. The obtained results were analysed using the CellQuest Pro software (BD).
2.3.11. Biomaterial in Vitro Evaluation
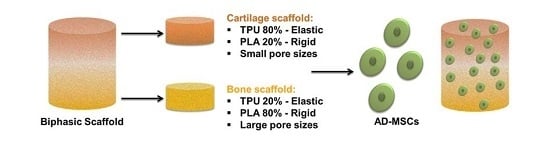
To evaluate the influence of biomaterials on adipose stem cells, the scaffolds were placed in a 24-well culture plate. Different types of biomaterials were used in this experiment, assigned according to
Table 3 below.
Cells were seeded on biomaterials at a concentration of 3 × 104 cells per well and cultured in DMEM F12 supplemented with 10% FBS and 1% PSA. After 48 h of culture, the media were replaced with differentiation-stimulating media specific for chondrogenic and osteogenic lineages (StemPro, Thermo Scientific, Waltham, MA, USA). The media were replaced every 72 h. Prior to the evaluation, the cells were maintained in culture conditions for 21 days. Control cells were seeded in a 24-well plate to empty wells. After the last day of the experiment, conditioned culture media were collected for further analysis. The cells for microscopic observations were fixed with biomaterials in 4% paraformaldehyde, while the cells for gene expression analysis were homogenized using TRIzol reagent (Thermo Scientific).
2.3.12. Viability Assay
The bromodeoxyuridine assay (BrdU Cell Proliferation ELISA Kit, Abcam, Cambridge, UK) was performed to evaluate the viability and proliferative potential of cells cultured on different biomaterials and in control conditions. The test based on quantitative measurements of DNA synthesis was performed on the last day of the experiment, according to the standard protocol delivered with the kit. The intensity of immunoenzymatic reaction was measured with a spectrophotometric microplate reader (BMG Labtech, Ortenberg, Germany) at 450 and 550 nm wavelengths.
2.3.13. Quantitative Analysis of Cell Oxidative Stress Factors
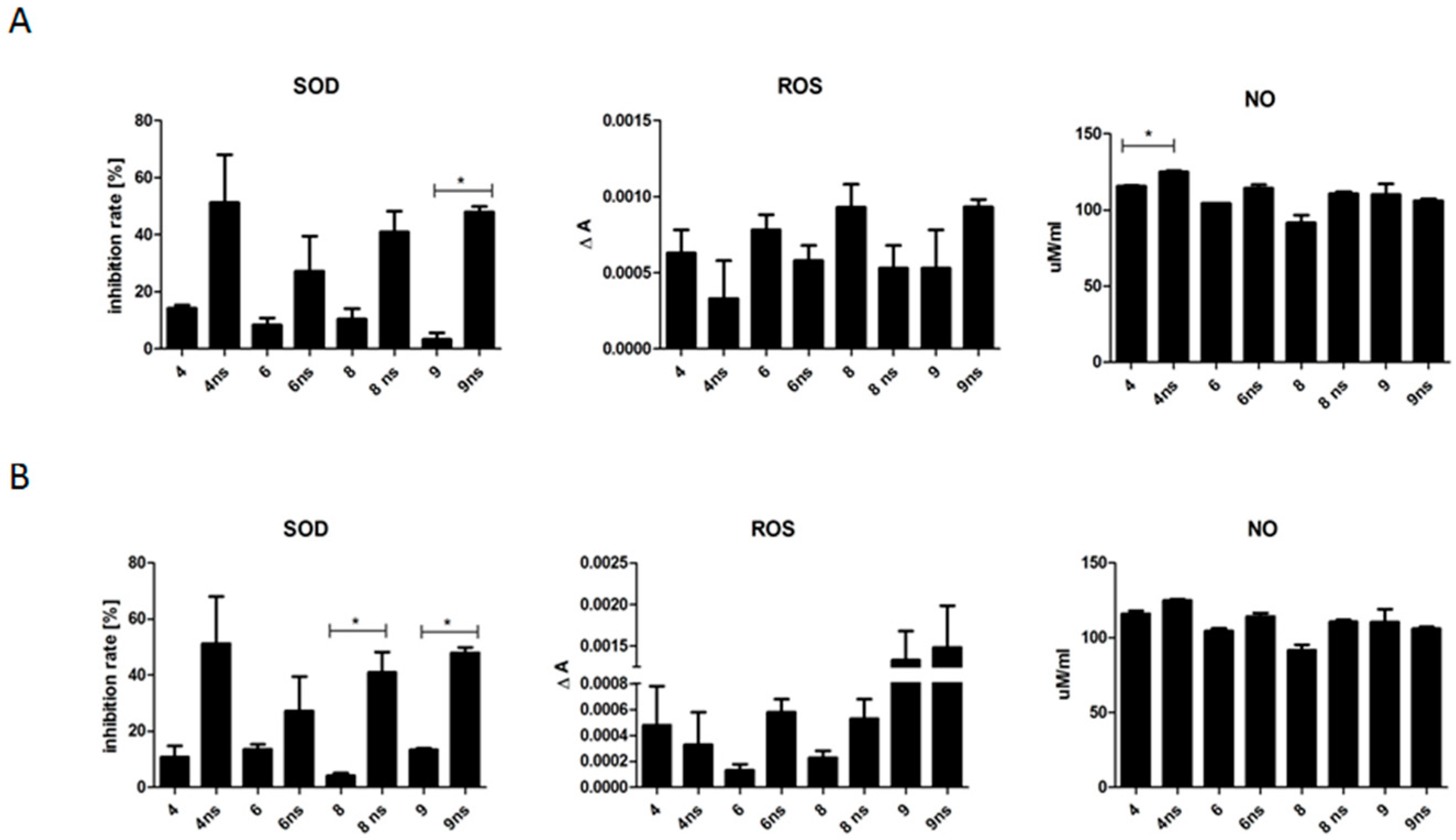
To evaluate the possible effect of biomaterials on the cellular stress, the levels of nitric oxide (NO, Griess Reagent Kit, Thermo Scientific), reactive oxygen species (ROS, CellROX, Thermo Scientific) and superoxide dismutase (SOD, SOD determination kit, Sigma Aldrich) in the conditioned culture media were determined according to the protocols provided by vendors. At least two replicates were performed for each experimental sample.
2.3.14. SEM-EDX Analysis
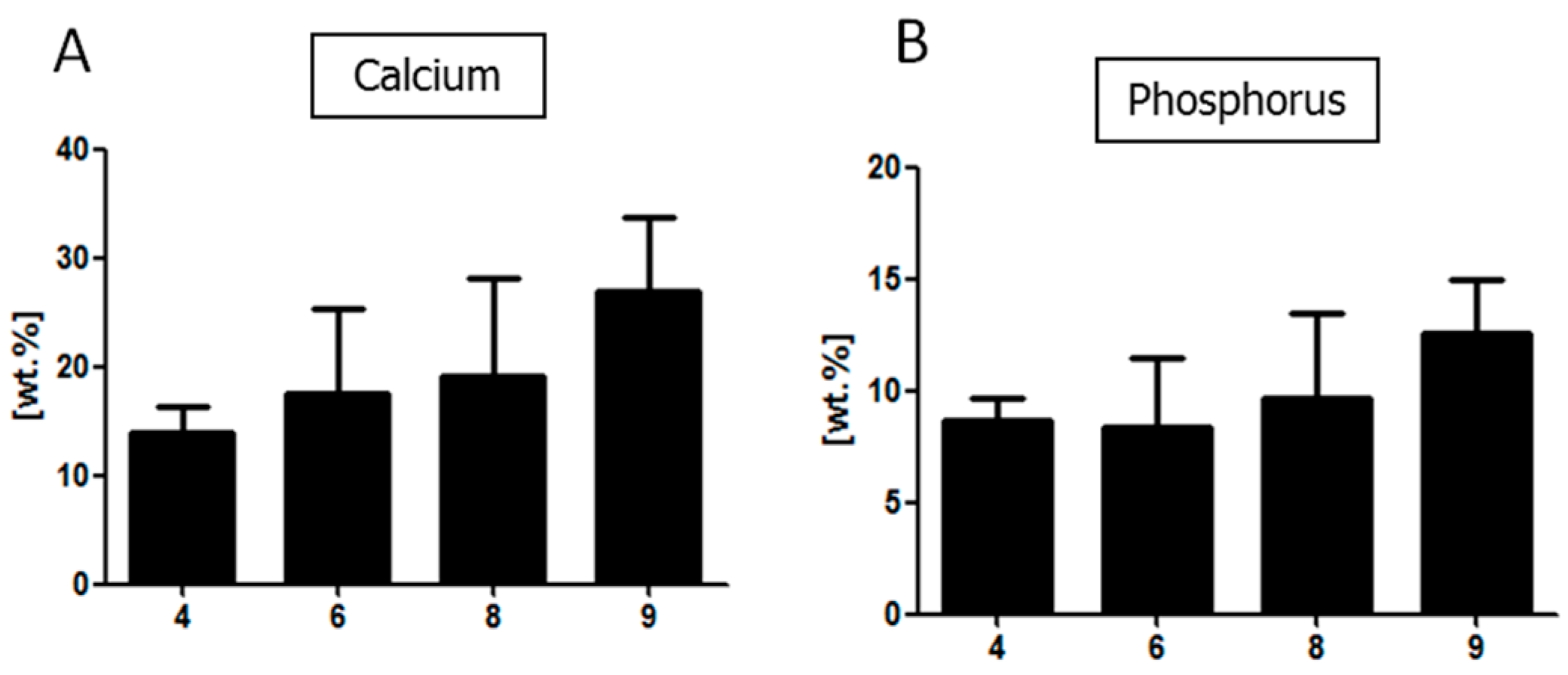
To evaluate the morphology of the cells cultured on each biomaterial, fixed samples were washed three times in PBS, followed by their dehydration in a graded ethanol series and drying in dessicator. The samples were mounted on microscope stubs, sputtered with gold using a ScanCoat6 coater (Edwards), and observed with a scanning electron microscope (EVO LS15, Zeiss, Oberkochen, Germany) at 10 kV of filament tension. Energy-dispersive X-ray spectrometry (EDX, Quantax Bruker, Billerica, MA, USA) was performed at 20 kV of filament tension.
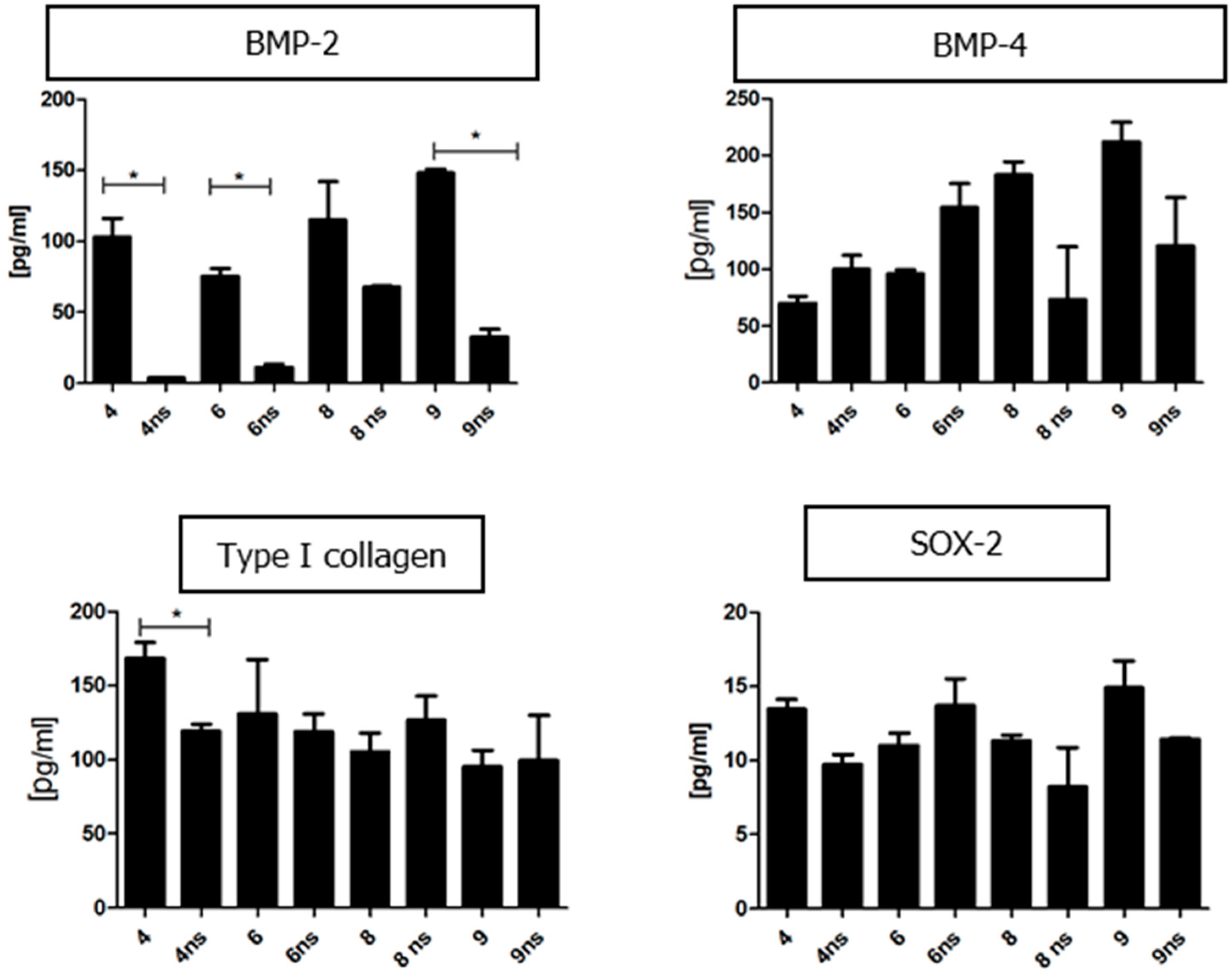
2.3.15. Quantitative Analysis of Proteins Present in the Conditioned Media
To evaluate the concentration of specific proteins in the collected conditioned media, enzyme-linked immunosorbent assay (ELISA) was used. A complete list of assayed proteins and kits used for the experiment is shown in
Table 4. The proteins were detected using monoclonal antibodies conjugated to horseradish peroxidase (HRP). The enzyme substrate consisted of 3,3,5,5-tetramethylbenzidine (TMB). Colorimetric reaction was stopped with 2N sulphuric acid (VI). The absorbance was measured at a single wavelength of 450 nm using a microplate reader.