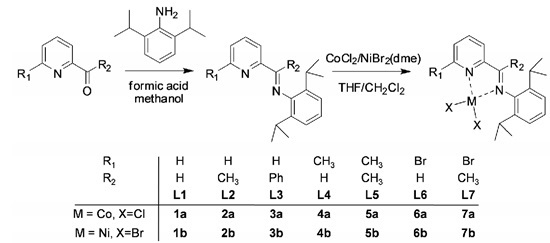
Iminopyridine-Based Cobalt(II) and Nickel(II) Complexes: Synthesis, Characterization, and Their Catalytic Behaviors for 1,3-Butadiene Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations and Materials
2.2. Procedure for 1,3-Butadiene Polymerization
2.3. Synthesis of Ligand (L1–L7)
2.3.1. 2,6-Bis(1-methylethyl)-N-(2-pyridinylmethylene)phenylamine (L1)
2.3.2. 2,6-Bis(1-methylethyl)-N-[1-(2-pyridinyl)-ethylidene]phenylamine (L2)
2.3.3. 2,6-Bis(1-methylethyl)-N-(phenyl-2-pyridinylmethylene)phenylamine (L3)
2.3.4. 2,6-Bis(1-methylethyl)-N-[(6-methyl-2-pyridinyl)-methylene]phenylamine (L4)
2.3.5. 2,6-Bis(1-methylethyl)-N-[1-(6-methyl-2-pyridinyl)-ethylidene]phenylamine (L5)
2.3.6. 2,6-Bis(1-methylethyl)-N-[(6-bromo-2-pyridinyl)-methylene]phenylamine (L6)
2.3.7. 2,6-Bis(1-methylethyl)-N-[1-(6-bromo-2-pyridinyl)-ethylidene]phenylamine (L7)
2.4. Preparation of Cobalt and Nickel Complexes
2.4.1. (2,6-Bis(1-methylethyl)-N-(2-pyridinylmethylene)phenylamine)cobalt(II) dichloride, 1a
2.4.2. (2,6-Bis(1-methylethyl)-N-[1-(2-pyridinyl)-ethylidene]phenylamine)cobalt(II) dichloride, 2a
2.4.3. (2,6-Bis(1-methylethyl)-N-(phenyl-2-pyridinylmethylene)phenylamine) cobalt(II) dichloride, 3a
2.4.4. (2,6-Bis(1-methylethyl)-N-[(6-methyl-2-pyridinyl)-methylene]phenylamine) cobalt(II) dichloride, 4a
2.4.5. (2,6-Bis(1-methylethyl)-N-[1-(6-methyl-2-pyridinyl)-ethylidene]phenylamine) cobalt(II) dichloride, 5a
2.4.6. (2,6-Bis(1-methylethyl)-N-[(6-bromo-2-pyridinyl)-methylene]phenylamine) cobalt(II) dichloride, 6a
2.4.7. (2,6-Bis(1-methylethyl)-N-[1-(6-bromo-2-pyridinyl)-ethylidene]phenylamine) cobalt(II) dichloride, 7a
2.4.8. (2,6-Bis(1-methylethyl)-N-(2-pyridinylmethylene)phenylamine)nickel(II) dibromide, 1b
2.4.9. (2,6-Bis(1-methylethyl)-N-[1-(2-pyridinyl)-ethylidene]phenylamine)nickel(II) dibromide, 2b
2.4.10. (2,6-Bis(1-methylethyl)-N-(phenyl-2-pyridinylmethylene)phenylamine) nickel(II) dibromide, 3b
2.4.11. (2,6-Bis(1-methylethyl)-N-[(6-methyl-2-pyridinyl)-methylene]phenylamine) nickel(II) dibromide, 4b
2.4.12. (2,6-Bis(1-methylethyl)-N-[1-(6-methyl-2-pyridinyl)-ethylidene]phenylamine) nickel(II) dibromide, 5b
2.4.13. (2,6-Bis(1-methylethyl)-N-[(6-bromo-2-pyridinyl)-methylene]phenylamine) nickel(II) dibromide, 6b
2.4.14. (2,6-Bis(1-methylethyl)-N-[1-(6-bromo-2-pyridinyl)-ethylidene]phenylamine) nickel(II) dibromide, 7b
3. Results and Discussions
3.1. Synthesis and Characterization of Iminopyridine Ligands (L1–L7) and Their Cobalt(II) and Nickel(II) Complexes
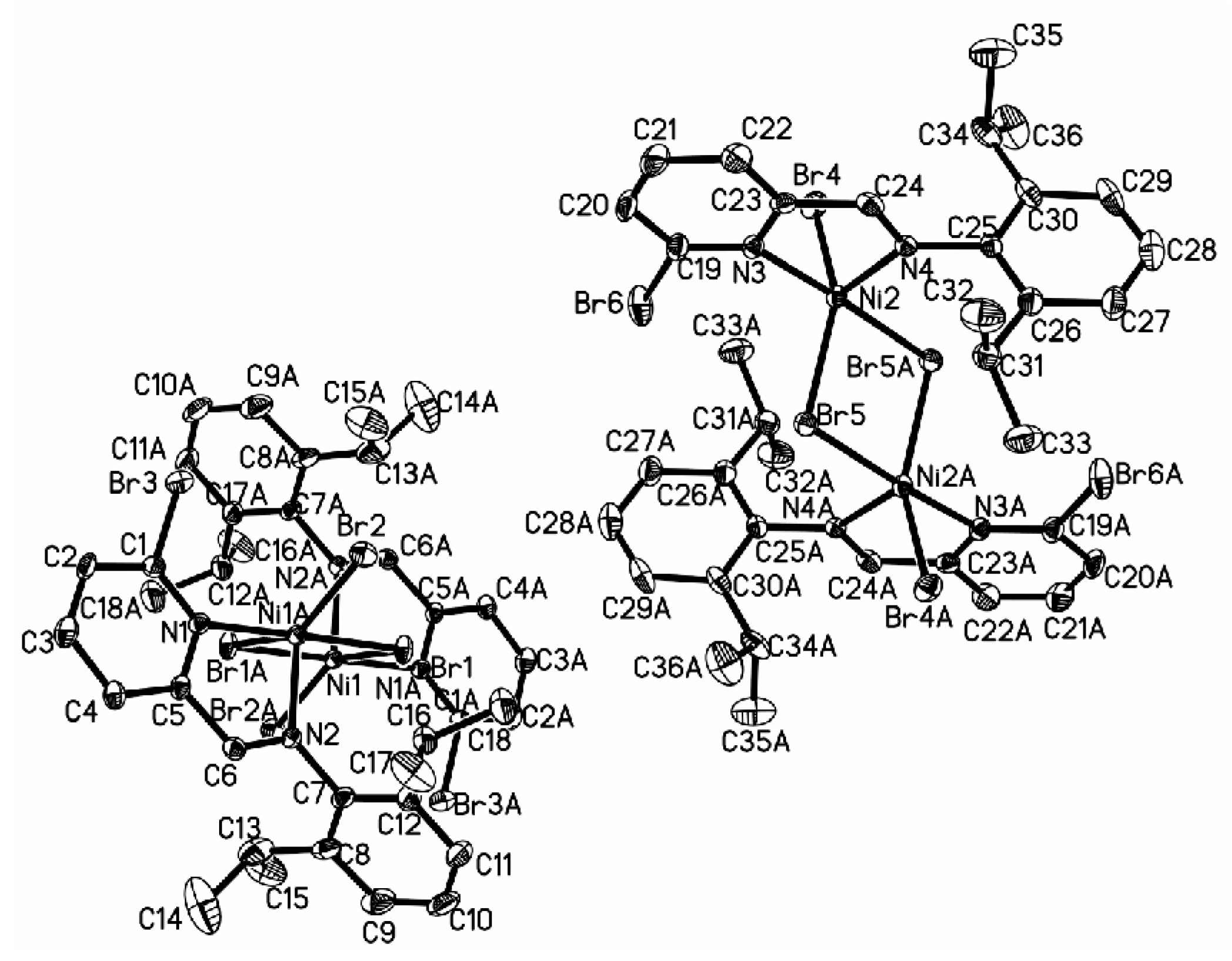
3.2. Crystal Structure of Complexes
| 3a·Et2O | 4a | 5a | 7a | 5b·THF | 6b | |
|---|---|---|---|---|---|---|
| Formula | C52H62Cl4Co2N4O | C19H24Cl2CoN2 | C20H26Cl2CoN2 | C19H23BrCl2CoN2 | C24H34Br2N2NiO | C36H42Br6N4Ni2 |
| Molecular weight | 1,018.72 | 410.23 | 424.26 | 489.13 | 585.06 | 1,127.62 |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/n | P21/c | P21/c | P21/c |
| a (Å) | 9.1341 (8) | 10.1638 (6) | 8.9107 (8) | 16.6693 (12) | 10.6823 (5) | 10.336 (2) |
| b (Å) | 16.4268 (15) | 19.5791 (11) | 16.0155 (14) | 10.4115 (7) | 18.6756 (8) | 19.755 (4) |
| c (Å) | 18.2838 (16) | 20.5215 (11) | 14.5532 (13) | 12.4751 (9) | 14.7019 (7) | 20.644 (4) |
| α (deg) | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 |
| β (deg) | 103.371 (1) | 95.071 (1) | 90.034 (1) | 106.366 (1) | 109.092 (1) | 93.835 (4) |
| γ (deg) | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 |
| V (Å3) | 2,669.0 (4) | 4067.8 (4) | 2,076.9 (3) | 2,077.4 (3) | 2,771.7 (2) | 4,205.8 (14) |
| Z | 2 | 8 | 4 | 4 | 4 | 4 |
| Dcalcd (Mg/m3) | 1.268 | 1.340 | 1.357 | 1.564 | 1.402 | 1.781 |
| Absorp coeff (mm−1) | 0.86 | 1.11 | 1.09 | 3.01 | 3.60 | 6.625 |
| F (000) | 1,064 | 1704 | 884 | 988 | 1192 | 2208 |
| Crystal size (mm) | 0.28 × 0.13 × 0.09 | 0.25 × 0.21 × 0.09 | 0.28×0.17 × 0.10 | 0.21×0.12 × 0.07 | 0.27 ×0.13 × 0.10 | 0.25×0.16 × 0.11 |
| θ Range (deg) | 2.3–26.0 | 2.3–24.9 | 2.5–24.5 | 2.6–26.0 | 2.3–26.0 | 2.2–24.5 |
| No. of reflns collected | 15,743 | 23,659 | 15,347 | 14,498 | 20,676 | 26,672 |
| No. of indep reflns | 5243 | 7,178 | 4,071 | 4,034 | 5,455 | 8,310 |
| No. of data/restraints/params | 5,243/7/313 (Rint = 0.026) | 7,178/0/443 (Rint = 0.042) | 4,071/0/232 (Rint = 0.038) | 4,034/0/231 (Rint = 0.023) | 5,455/0/277 (Rint = 0.024) | 8,310/0/441 (Rint = 0.055) |
| GOF on F2 | 1.05 | 0.98 | 1.01 | 1.04 | 1.04 | 0.97 |
| R1 (1 > 2sigma(1)) | 0.045 | 0.038 | 0.036 | 0.030 | 0.025 | 0.039 |
| wR2 | 0.145 | 0.090 | 0.090 | 0.083 | 0.068 | 0.083 |
| 3a | 4a | 5a | 7a | ||||
|---|---|---|---|---|---|---|---|
| Bond lengths | |||||||
| Co1–N1 | 2.099 (2) | Co1–N1 | 2.181 (2) | Co1–N1 | 2.0439 (18) | Co1–N1 | 2.0667 (18) |
| Co1–N2 | 2.128 (2) | Co1–N2 | 2.089 (2) | Co1–N2 | 2.0498 (18) | Co1–N2 | 2.0514 (17) |
| Co1–Cl1 | 2.2830 (9) | Co1–Cl1 | 2.2769 (8) | Co1–Cl1 | 2.2071 (7) | Co1–Cl1 | 2.2079 (7) |
| Co1–Cl2 | 2.3607 (8) | Co1–Cl2 | 2.4428 (8) | Co1–Cl2 | 2.2148 (7) | Co1–Cl2 | 2.2235 (7) |
| Co1–Cl2A | 2.4372 (8) | Co2–N4 | 2.097 (2) | N2–C7 | 1.290 (3) | N2–C8 | 1.451 (3) |
| Co1A–Cl2 | 2.4371 (8) | Co2–N3 | 2.182 (2) | N2–C9 | 1.448 (3) | N2–C6 | 1.285 (3) |
| C5–C6 | 1.493 (4) | Co2–Cl3 | 2.2709 (8) | C5–C7 | 1.492 (3) | C5–C6 | 1.492 (3) |
| N2–C6 | 1.301 (4) | Co2–Cl4 | 2.4433 (8) | C7–C8 | 1.490 (3) | C6–C7 | 1.489 (3) |
| N2–C13 | 1.454 (4) | N2–C8 | 1.442 (3) | C1–C6 | 1.491 (3) | Br1–C1 | 1.888 (3) |
| C6–C7 | 1.489 (4) | N2–C6 | 1.271 (3) | ||||
| N4–C27 | 1.438 (3) | ||||||
| N4–C25 | 1.272 (3) | ||||||
| Bond angles | |||||||
| N1–Co1–N2 | 76.36 (9) | N1–Co1–N2 | 77.69 (8) | N1–Co1–N2 | 80.68 (7) | N2–Co1–N1 | 80.14 (7) |
| N1–Co1–Cl1 | 92.08 (7) | N1–Co1–Cl1 | 91.41 (6) | N1–Co1–Cl1 | 118.90 (6) | N1–Co1–Cl1 | 118.64 (6) |
| N2–Co1–Cl1 | 117.17 (7) | N2–Co1–Cl1 | 111.97 (6) | N2–Co1–Cl1 | 115.04 (6) | N2–Co1–Cl1 | 111.66 (5) |
| N1–Co1–Cl2 | 164.04 (8) | N1–Co1–Cl2 | 171.60 (6) | N1–Co1–Cl2 | 107.53 (5) | N2–Co1–Cl2 | 117.93 (5) |
| N2–Co1–Cl2 | 98.55 (7) | N2–Co1–Cl2 | 96.91 (6) | N2–Co1–Cl2 | 114.54 (6) | N1–Co1–Cl2 | 110.67 (5) |
| Cl1–Co1–Cl2 | 103.60 (3) | Cl1–Co1–Cl2 | 96.61 (3) | Cl1–Co1–Cl2 | 115.44 (3) | Cl1–Co1–Cl2 | 113.88 (3) |
| N1–Co1–Cl2A | 85.08 (7) | N4–Co2–N3 | 77.66 (8) | C7–N2–C9 | 120.43 (19) | N1–C1–Br1 | 116.75 (17) |
| N2–Co1–Cl2A | 125.21 (7) | N4–Co2–Cl3 | 114.12 (6) | N1–C5–C7 | 115.52 (19) | C2–C1–Br1 | 119.39 (19) |
| Cl1–Co1–Cl2A | 114.55 (3) | N3–Co2–Cl3 | 94.45 (6) | C4–C5–C7 | 122.7 (2) | C7–C6–C5 | 118.39 (19) |
| Cl2–Co1–Cl2A | 85.73 (3) | N4–Co2–Cl4 | 96.58 (6) | C8–C7–C5 | 118.6 (2) | C6–N2–C8 | 120.24 (18) |
| N2–C6–C7 | 125.8 (3) | N3–Co2–Cl4 | 169.32 (6) | N2–C7–C8 | 124.9 (2) | N2–C6–C7 | 125.0 (2) |
| C6–N2–C13 | 118.9 (2) | Cl3–Co2–Cl4 | 96.12 (3) | N1–C1–C6 | 116.7 (2) | N2–C6–C5 | 116.58 (19) |
| N2–C6–C5 | 115.7 (3) | N2–C6–C5 | 120.4 (2) | N2–C7–C5 | 116.4 (2) | ||
| 5b | 6b | ||||
|---|---|---|---|---|---|
| Bond lengths | |||||
| Ni1–N2 | 2.0383 (16) | Ni1–N2 | 2.049 (4) | Br1–Ni1A | 2.4770 (8) |
| Ni1–N1 | 2.0677 (17) | Ni1–N1 | 2.092 (3) | Br3–C1 | 1.887 (5) |
| Ni1–O1 | 2.1478 (15) | Ni1–Br2 | 2.4153 (8) | Br5–Ni2A | 2.5076 (8) |
| Ni1–Br1 | 2.4376 (3) | Ni1–Br1A | 2.4770 (8) | Br6–C19 | 1.875 (5) |
| Ni1–Br2 | 2.4602 (3) | Ni1–Br1 | 2.5230 (8) | ||
| C5–C7 | 1.492 (3) | Ni2–N4 | 2.038 (3) | ||
| C7–C8 | 1.494 (3) | Ni2–N3 | 2.080 (3) | ||
| N2–C7 | 1.286 (3) | Ni2–Br4 | 2.4214 (9) | ||
| N2–C9 | 1.445 (2) | Ni2–Br5 | 2.4777 (8) | ||
| C1–C6 | 1.489 (3) | Ni2–Br5A | 2.5075 (8) | ||
| Bond angles | |||||
| N2–Ni1–N1 | 80.22 (6) | N2–Ni1–N1 | 80.27 (13) | N4–Ni2–Br4 | 107.02 (10) |
| N2–Ni1–Br1 | 106.94 (5) | N2–Ni1–Br2 | 107.62 (9) | N3–Ni2–Br4 | 90.38 (10) |
| N1–Ni1–Br1 | 91.16 (5) | N1–Ni1–Br2 | 90.87 (9) | N4–Ni2–Br5 | 104.32 (10) |
| N2–Ni1–Br2 | 111.61 (5) | N2–Ni1–Br1A | 104.84 (10) | N3–Ni2–Br5 | 90.36 (10) |
| N1–Ni1–Br2 | 90.48 (5) | N1–Ni1–Br1A | 90.92 (9) | Br4–Ni2–Br5 | 148.29 (3) |
| Br1–Ni1–Br2 | 141.124 (13) | Br2–Ni1–Br1A | 147.32 (3) | N4–Ni2–Br5A | 100.41 (9) |
| C5–C7–C8 | 118.23 (17) | N2–Ni1–Br1 | 100.45 (9) | N3–Ni2–Br5A | 176.73 (10) |
| N2–C7–C8 | 125.39 (19) | N1–Ni1–Br1 | 176.28 (9) | Br4–Ni2–Br5A | 92.55 (2) |
| N1–C1–C6 | 118.41 (19) | Br2–Ni1–Br1 | 92.38 (2) | Br5–Ni2–Br5A | 86.39 (2) |
| C2–C1–C6 | 120.9 (2) | Br1A–Ni1–Br1 | 85.37 (2) | N2–C6–C5 | 120.1 (4) |
| C7–N2–C9 | 119.55 (16) | N4–Ni2–N3 | 80.09 (13) | N1–C1–Br3 | 117.2 (3) |
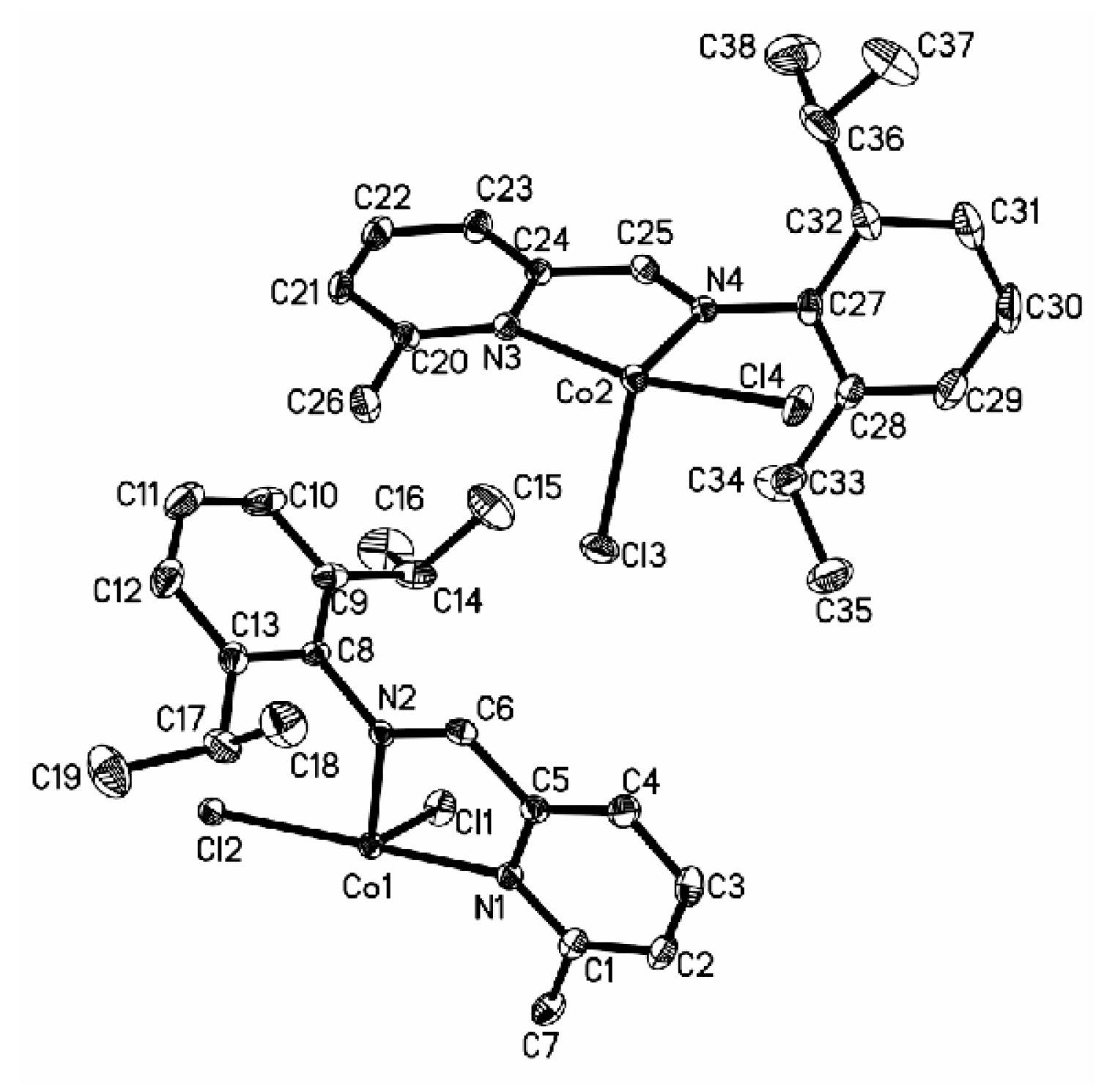


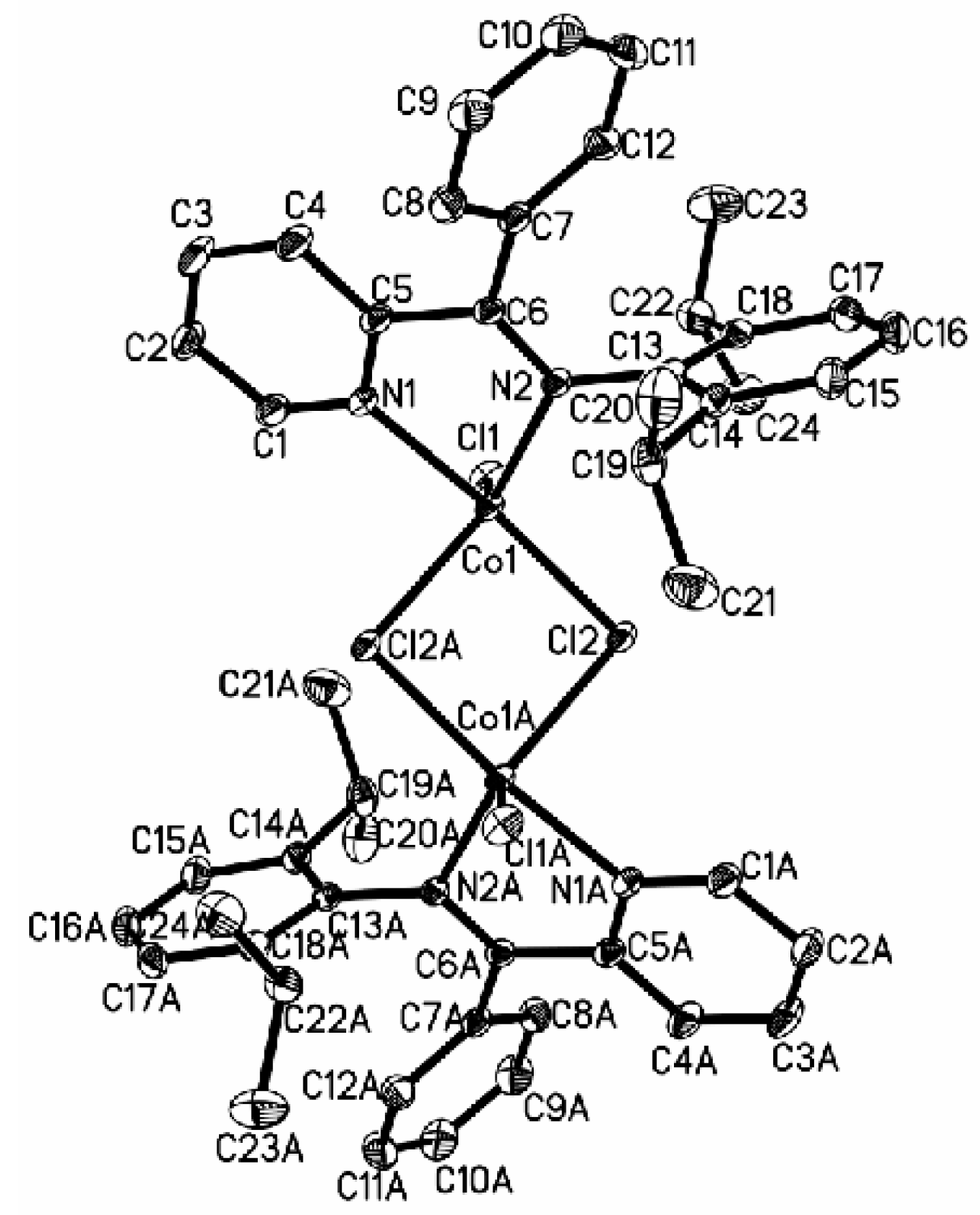

3.3. Solution Polymerization of 1,3-Butadiene
| Run | Complex | Yield (%) | Mn b × 10−4 | Mw/Mn b | Microstructure c (%) | ||
|---|---|---|---|---|---|---|---|
| Cis-1,4 | 1.2 | Trans-1,4 | |||||
| 1 | 1a | 56.9 | 23.0 | 2.4 | 98.1 | 1.0 | 0.9 |
| 2 | 2a | 51.6 | 27.7 | 2.1 | 98.2 | 1.0 | 0.8 |
| 3 | 3a | 92.3 | 9.0 | 3.3 | 96.5 | 1.6 | 1.9 |
| 4 | 4a | 42.9 | 31.5 | 1.9 | 98.3 | 0.9 | 0.8 |
| 5 | 5a | 31.9 | 33.8 | 1.7 | 98.5 | 0.9 | 0.6 |
| 6 | 6a | 92.8 | 10.9 | 3.0 | 97.2 | 1.4 | 1.4 |
| 7 | 7a | 90.8 | 12.0 | 3.2 | 97.0 | 1.4 | 1.6 |
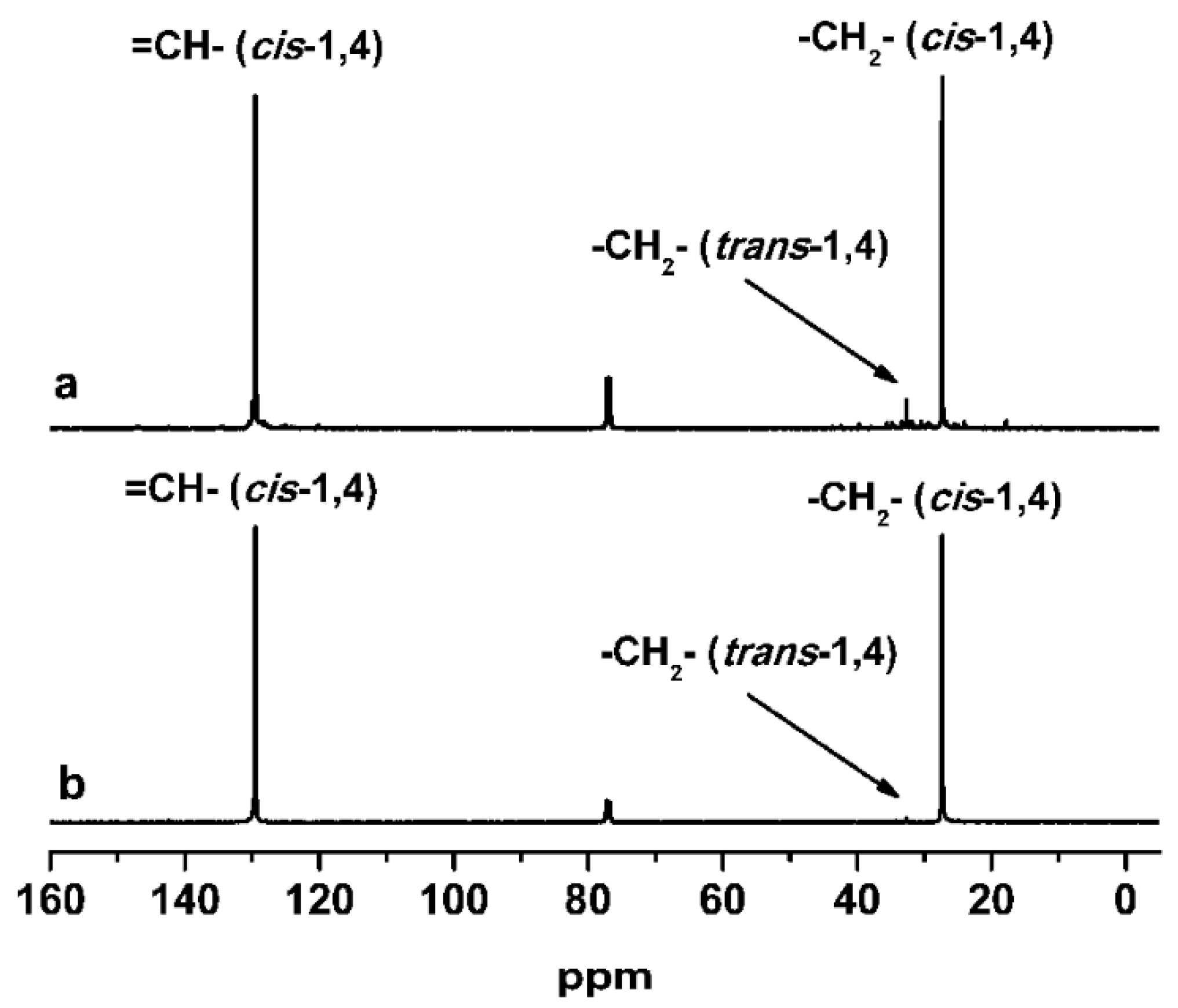
| Run | Complex | Yield (%) | Mn b | Mw/Mn b | Microstructure c (%) | ||
|---|---|---|---|---|---|---|---|
| Cis-1,4 | 1.2 | Trans-1,4 | |||||
| 8 | 1b | 69.7 | 8,100 | 2.40 | 87.1 | 3.4 | 9.5 |
| 9 | 2b | 35.8 | 8,700 | 1.87 | 91.6 | 3.5 | 4.9 |
| 10 | 3b | 29.2 | 9,900 | 1.72 | 90.7 | 3.8 | 5.5 |
| 11 | 4b | 75.4 | 7,100 | 2.38 | 84.1 | 1.7 | 14.2 |
| 12 | 5b | 47.1 | 8,500 | 1.87 | 90.7 | 3.3 | 6.0 |
| 13 | 6b | 79.3 | 7,200 | 2.67 | 79.3 | 1.3 | 19.4 |
| 14 | 7b | 72.8 | 7,000 | 2.17 | 87.1 | 2.3 | 10.6 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Thiele, S.K.H.; Wilson, D.R. Alternate transition metal complex based diene polymerization. J. Macromol. Sci. Polym. Rev. 2003, 43, 581–628. [Google Scholar] [CrossRef]
- Cooper, W. Aspects of mechanism of coordination polymerization of conjugated dienes. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 457–466. [Google Scholar] [CrossRef]
- Furukawa, J. Mechanism of diene polymerization. Pure Appl. Chem. 1975, 42, 495–508. [Google Scholar] [CrossRef]
- Oehme, A.; Gebauer, U.; Gehrke, K.; Lechner, M.D. The influence of ageing and polymerization conditions on the polymerization of butadiene using a neodymium catalyst system. Angew. Makromol. Chem. 1996, 235, 121–130. [Google Scholar] [CrossRef]
- Gong, D.R.; Dong, W.M.; Hu, Y.M.; Bi, J.F.; Zhang, X.Q.; Jiang, L.S. Syndiotactically enriched 1,2-selective polymerization of 1,3-butadiene initiated by iron catalysts based on a new class of donors. Polymer 2009, 50, 5980–5986. [Google Scholar] [CrossRef]
- Lu, J.; Hu, Y.M.; Zhang, X.; Bi, J.F.; Dong, W.M.; Jiang, L.S.; Huang, B.T. Fe(2-EHA)3/Al(i-Bu)3/hydrogen phosphite catalyst for preparing syndiotactic 1,2-polybutadiene. J. Appl. Polym. Sci. 2006, 100, 4265–4269. [Google Scholar] [CrossRef]
- Endo, K.; Kitagawa, T.; Nakatani, K. Effect of an alkyl substituted in salen ligands on 1,4-cis selectivity and molecular weight control in the polymerization of 1,3-butadiene with (salen)Co(II). J. Polym. Sci. Polym. Chem. 2006, 44, 4088–4094. [Google Scholar] [CrossRef]
- Chandran, D.; Kwak, C.H.; Ha, C.S.; Kim, I. Polymerization of 1,3-butadiene by bis(salicylaldiminate)cobalt(II) catalysts combined with organoaluminium cocatalysts. Catal. Today 2008, 131, 505–512. [Google Scholar] [CrossRef]
- Gong, D.R.; Wang, B.L.; Cai, H.G.; Zhang, X.Q.; Jiang, L.S. Synthesis, characterization and butadiene polymerization studies of cobalt(II) complexes bearing bisiminopyridine ligand. J. Organomet. Chem. 2011, 696, 1584–1590. [Google Scholar] [CrossRef]
- Cariou, R.; Chirinos, J.J.; Gibson, V.C.; Jacobsen, G.; Tomov, A.K.; Britovsek, G.J.P.; Whitea, A.J.P. The effect of the central donor in bis(benzimidazole)-based cobalt catalysts for the selective cis-1,4-polymerisation of butadiene. Dalton Trans. 2010, 39, 9039–9045. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.R.; Jia, X.Y.; Wang, B.L.; Zhang, X.Q.; Jiang, L.S. Synthesis, characterization, and butadiene polymerization of iron(III), iron(II) and cobalt(II) chlorides bearing 2,6-bis(2-benzimidazolyl)pyridyl or 2,6-bis(pyrazol)pyridine ligand. J. Organomet. Chem. 2012, 702, 10–18. [Google Scholar] [CrossRef]
- Appukuttan, V.; Zhang, L.; Ha, J.Y.; Chandran, D.; Bahuleyan, B.K.; Ha, C.S.; Kim, I. Stereospecific polymerizations of 1,3-butadiene catalyzed by Co(II) complexes ligated by 2,6-bis(benzimidazolyl)pyridines. J. Mol. Catal. A Chem. 2010, 325, 84–90. [Google Scholar] [CrossRef]
- Nobbs, J.D.; Tomov, A.K.; Cariou, R.; Gibson, V.C.; White, A.J.P.; Britovsek, G.J.P. Thio-Pybox and Thio-Phebox complexes of chromium, iron, cobalt and nickel and their application in ethylene and butadiene polymerisation catalysis. Dalton Trans. 2012, 41, 5949–5964. [Google Scholar] [CrossRef] [PubMed]
- Ai, P.F.; Chen, L.; Guo, Y.T.; Jie, S.Y.; Li, B.G. Polymerization of 1,3-butadiene catalyzed by cobalt(II) and nickel(II) complexes bearing imino- or amino-pyridyl alcohol ligands in combination with ethylaluminum sesquichloride. J. Organomet. Chem. 2012, 705, 51–58. [Google Scholar] [CrossRef]
- Jie, S.Y.; Ai, P.F.; Li, B.G. Highly active and stereospecific polymerization of 1,3-butadiene catalyzed by dinuclear cobalt(II) complexes bearing 3-aryliminomethyl-2-hydroxybenzaldehydes. Dalton Trans. 2011, 40, 10975–10982. [Google Scholar] [CrossRef] [PubMed]
- Kwag, G.; Jang, Y.C.; Lee, H. Ligand structure and cocatalyst effects on high 1,4-cis polymerization of 1,3-butadiene using Ni-based catalysts. Polym. J. 1999, 31, 1274–1276. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsumura, S.; Satoh, Y.; Sogoh, K.; Yasuda, H. Random and block copolymerizations of norbornene with conjugated 1,3-dienes catalyzed by novel Ni compounds involving N- or O-donated ligands. React. Funct. Polym. 2004, 59, 253–266. [Google Scholar] [CrossRef]
- Jang, Y.G.; Choi, D.S.; Han, S. Effects of tris(pentafluorophenyl)borane on the activation of a metal alkyl-free Ni-based catalyst in the polymerization of 1,3-butadiene. J. Polym. Sci. Polym. Chem. 2004, 42, 1164–1173. [Google Scholar] [CrossRef]
- Gong, D.R.; Wang, B.L.; Bai, C.X.; Bi, J.F.; Wang, F.; Dong, W.M.; Zhang, X.Q.; Jiang, L.S. Metal dependent control of cis-/trans-1,4 regioselectivity in 1,3-butadiene polymerization catalyzed by transition metal complexes supported by 2,6-bis[1-(iminophenyl)ethyl]pyridine. Polymer 2009, 50, 6259–6264. [Google Scholar] [CrossRef]
- Irrgang, T.; Keller, S.; Maisel, H.; Kretschmer, W.; Kempe, R. Sterically demanding iminopyridine ligands. Eur. J. Inorg. Chem. 2007, 2007, 4221–4228. [Google Scholar] [CrossRef]
- Laine, T.V.; Piironen, U.; Lappalainen, K.; Klinga, M.; Aitola, E.; Leskela, M. Pyridinylimine-based nickel(II) and palladium(II) complexes: Preparation, structural characterization and use as alkene polymerization catalysts. J. Organomet. Chem. 2000, 606, 112–124. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Rios, I.G.; Meli, A.; Oberhauser, W.; Sorace, L.; Toti, A. Synthesis of a new polydentate ligand obtained by coupling 2,6-bis(imino)pyridine and (imino)pyridine moieties and its use in ethylene oligomerization in conjunction with iron(II) and cobalt(II) bis-halides. Organometallics 2007, 26, 5066–5078. [Google Scholar] [CrossRef]
- Rosa, V.; Carabineiro, S.A.; Aviles, T.; Gomes, P.T.; Welter, R.; Campos, J.M.; Ribeiro, M.R. Synthesis, characterisation and solid state structures of α-diimine cobalt(II) complexes: Ethylene polymerisation tests. J. Organomet. Chem. 2008, 693, 769–775. [Google Scholar] [CrossRef]
- Song, S.J.; Xiao, T.P.F.; Liang, T.L.; Wang, F.S.; Redshaw, C.; Sun, W.H. Synthesis, characterization and ethylene oligomerization behaviour of 8-(1-aryliminoethylidene)quinaldinylnickel dihalides. Catal. Sci. Technol. 2011, 1, 69–75. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Strömberg, S.; et al. Iron and cobalt ethylene polymerization catalysts bearing 2,6-bis(imino)pyridyl ligands: Synthesis, structures, and polymerization studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.H.; Zhang, S.; Hou, J.; Wedeking, K.; Schultz, S.; Fröhlich, R.; Song, H. Synthesis, characterization, and ethylene oligomerization and polymerization of [2,6-bis(2-benzimidazolyl)pyridyl] chromium chlorides. Organometallics 2006, 25, 1961–1969. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.; Jia, X.; Yang, F.; Bai, C.; Hu, Y.; Zhang, X. Iminopyridine-Based Cobalt(II) and Nickel(II) Complexes: Synthesis, Characterization, and Their Catalytic Behaviors for 1,3-Butadiene Polymerization. Polymers 2016, 8, 12. https://doi.org/10.3390/polym8010012
Dai Q, Jia X, Yang F, Bai C, Hu Y, Zhang X. Iminopyridine-Based Cobalt(II) and Nickel(II) Complexes: Synthesis, Characterization, and Their Catalytic Behaviors for 1,3-Butadiene Polymerization. Polymers. 2016; 8(1):12. https://doi.org/10.3390/polym8010012
Chicago/Turabian StyleDai, Quanquan, Xiangyu Jia, Feng Yang, Chenxi Bai, Yanming Hu, and Xuequan Zhang. 2016. "Iminopyridine-Based Cobalt(II) and Nickel(II) Complexes: Synthesis, Characterization, and Their Catalytic Behaviors for 1,3-Butadiene Polymerization" Polymers 8, no. 1: 12. https://doi.org/10.3390/polym8010012