Study on the Microstructure of Polyester Polyurethane Irradiated in Air and Water
Abstract
:1. Introduction
2. Experimental Section
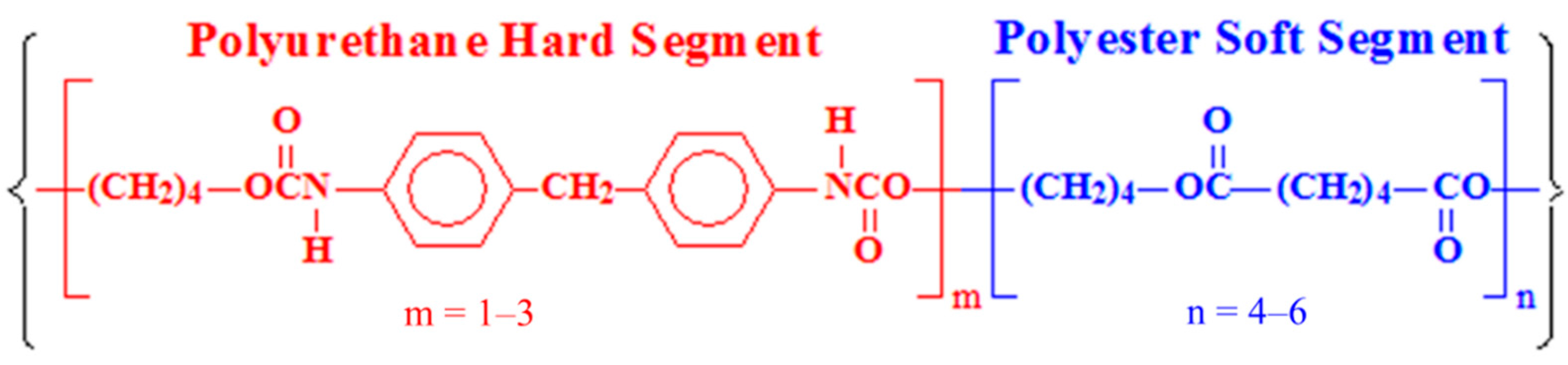
2.1. Materials
2.2. Characterization
3. Results
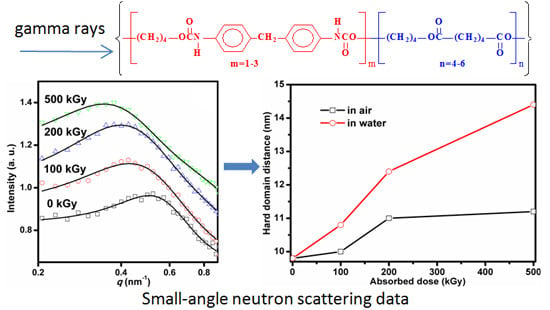
3.1. Small-Angle Neutron Scattering (SANS)

| Samples | RHS (nm) | acor (nm) | v | v/RHS3 (nm−3) |
|---|---|---|---|---|
| Original | 4.9 ± 0.1 | 1.8 ± 0.3 | 0.21 ± 0.03 | 1.8 × 10−3 |
| 100 kGy in air | 5.0 ± 0.1 | 1.7 ± 0.2 | 0.18 ± 0.02 | 1.4× 10−3 |
| 200 kGy in air | 5.5 ± 0.2 | 1.0 ± 0.2 | 0.10 ± 0.02 | 6.0× 10−4 |
| 500 kGy in air | 5.6 ± 0.5 | 0.9 ± 0.3 | 0.05 ± 0.02 | 2.8 × 10−4 |
| 100 kGy in water | 5.4 ± 0.1 | 1.1 ± 0.1 | 0.10 ± 0.02 | 6.4 × 10−4 |
| 200 kGy in water | 6.2 ± 0.1 | 0.8 ± 0.1 | 0.08 ± 0.01 | 3.4 × 10−4 |
| 500 kGy in water | 7.2 ± 0.2 | 1.0 ± 0.1 | 0.09 ± 0.01 | 2.4 × 10−4 |
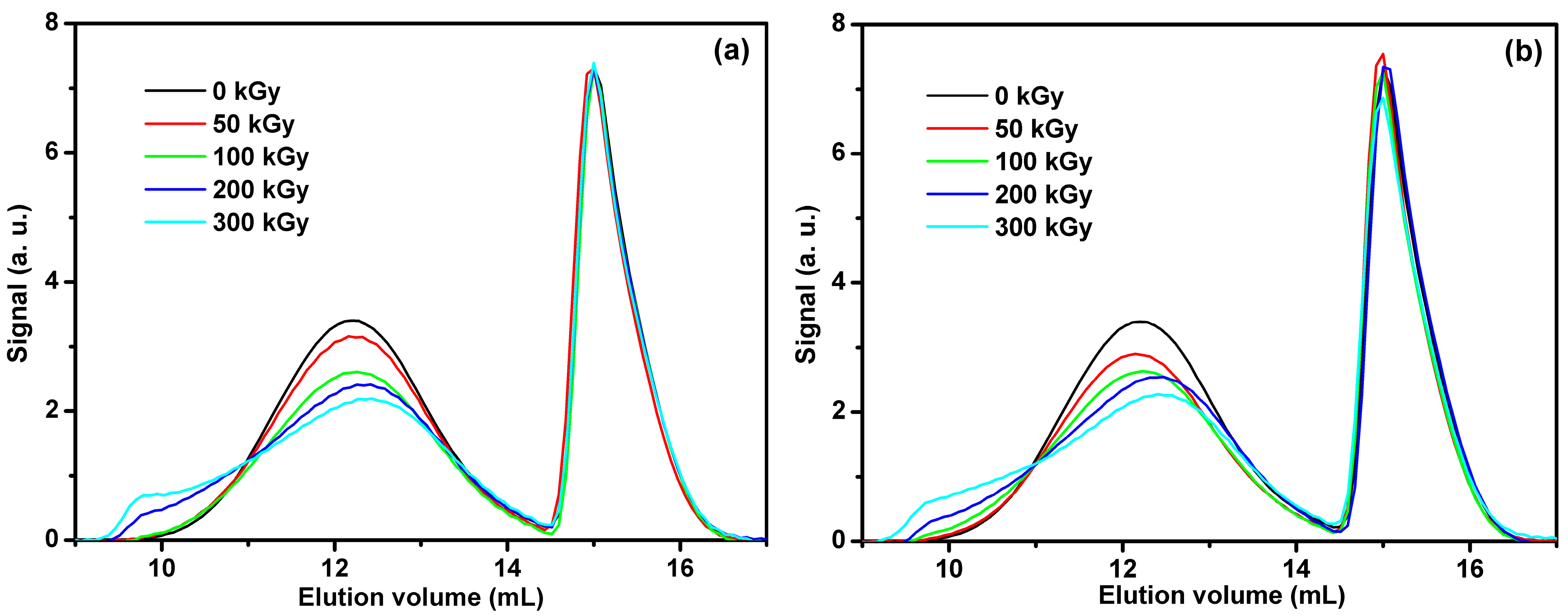
3.2. Gel Permeation Chromatography (GPC)
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. X-Ray Diffraction (XRD)
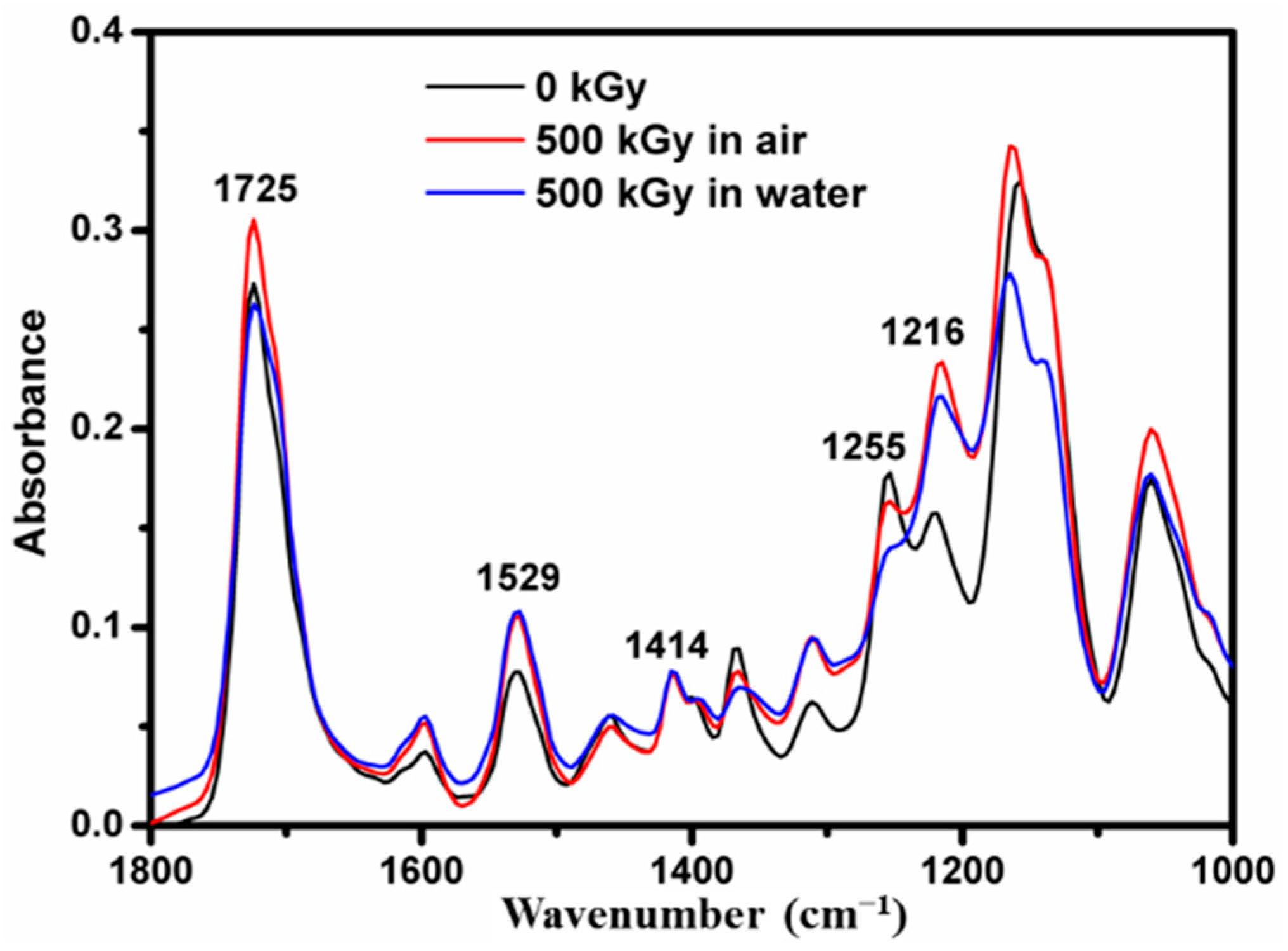
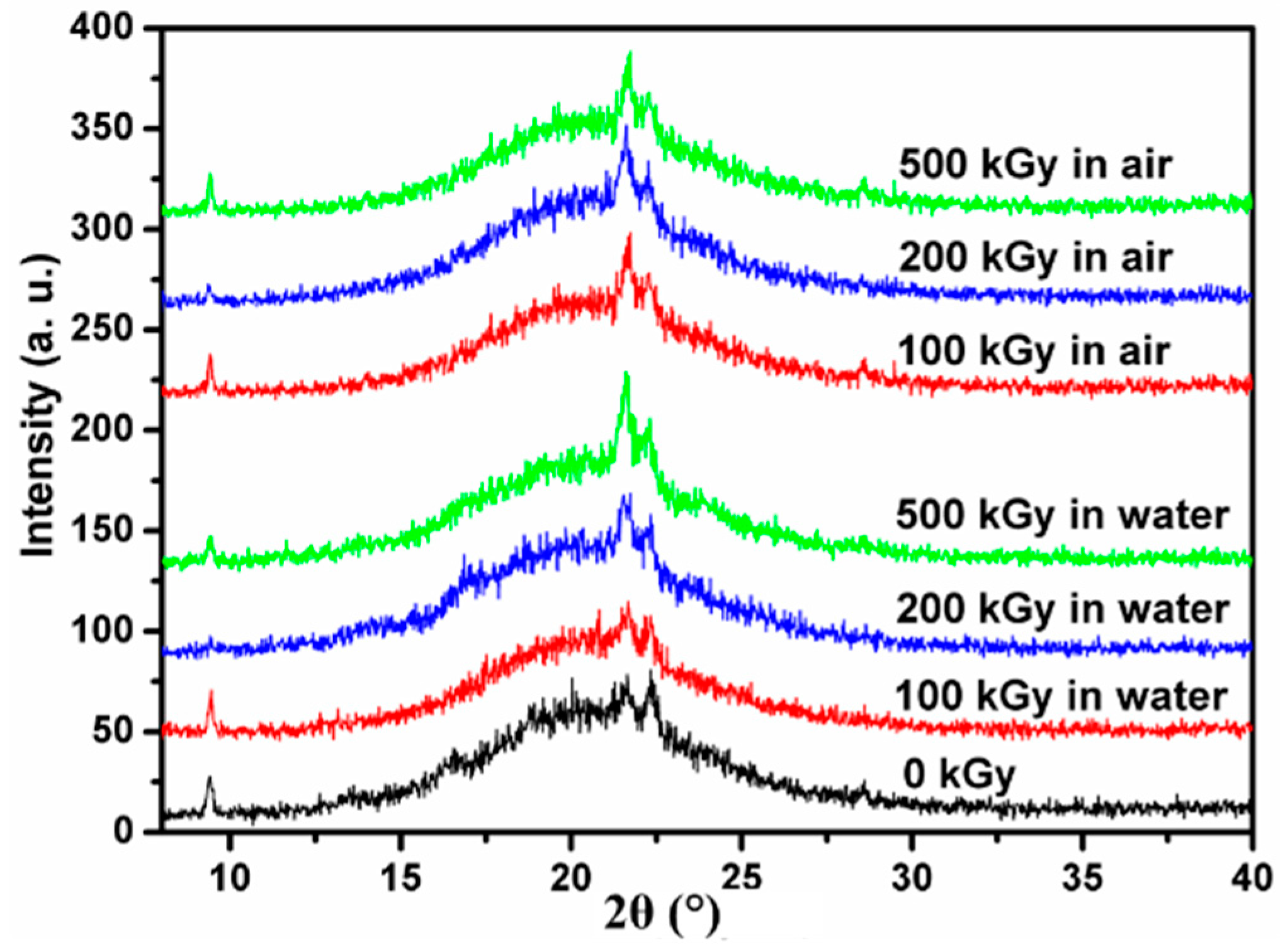
4. Discussion
4.1. Phase Mixing Induced by γ Irradiation

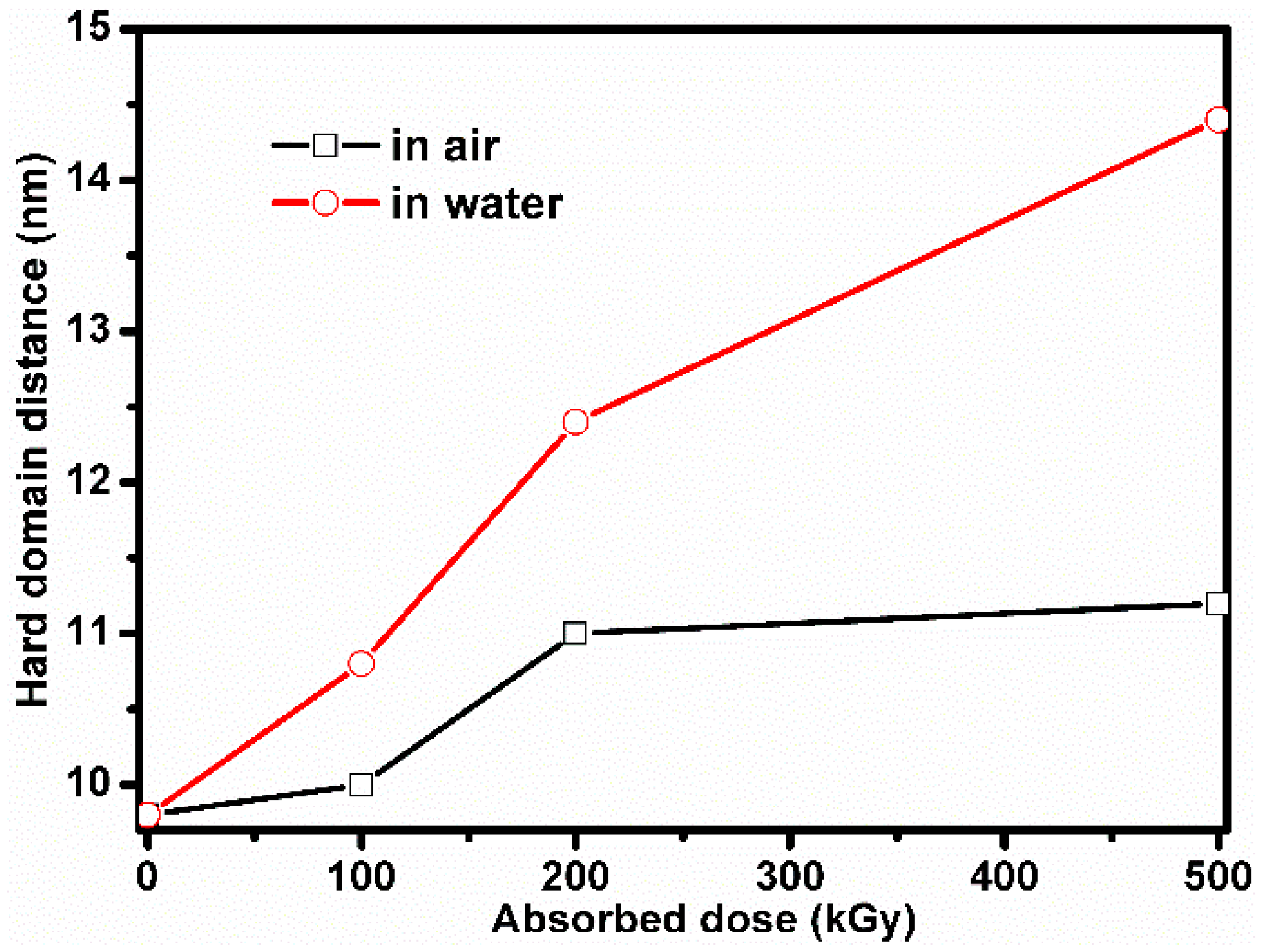
4.2. The Effects of the Water and Air Environments


5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prisacariu, C. Polyurethane Elastomers: From Morphology to Mechanical Aspects; Springer Verlag: Wien, Austria, 2011; pp. 3–16. [Google Scholar]
- Kusano, J.; Uno, Y. Radiation resistivity of polymeric materials with data tables. Available online: http://jolissrch-inter.tokai-sc.jaea.go.jp/pdfdata/JAERI-Data-Code-2003-015.pdf (accessed on 5 September 2015).
- Adem, E.; Angulo-Cervera, E.; González-Jiménez, A.; Valentín, J.L.; Marcos-Fernández, A. Effect of dose and temperature on the physical properties of an aliphatic thermoplastic polyurethane irradiated with an electron beam. Radiat. Phys. Chem. 2015, 112, 61–70. [Google Scholar]
- Shintani, H.; Kikuchi, H.; Nakamura, A. Effects of gamma ray irradiation on the change of characteristics of polyurethane. J. Appl. Polym. Sci. 1990, 41, 661–675. [Google Scholar] [CrossRef]
- Pierpoint, S.; Silverman, J.; Al-Sheikhly, M. Effects of ionizing radiation on the aging of polyester based polyurethane binder. Radiat. Phys. Chem. 2001, 62, 163–169. [Google Scholar] [CrossRef]
- Murray, K.A.; Kennedy, J.E.; McEvoy, B.; Vrain, O.; Ryan, D.; Cowman, R.; Higginbotham, C.L. The influence of electron beam irradiation conducted in air on the thermal, chemical, structural and surface properties of medical grade polyurethane. Eur. Polym. J. 2013, 49, 1782–1795. [Google Scholar] [CrossRef]
- Burillo, G.; Beristain, M.F.; Sanchez, E.; Ogawa, T. Effects of aromatic diacetylenes on polyurethane degradation by gamma irradiation. Polym. Degrad. Stab. 2013, 98, 1988–1992. [Google Scholar] [CrossRef]
- Sui, H.; Liu, X.; Zhong, F.; Li, X.; Wang, B.; Ju, X. Relationship between free volume and mechanical properties of polyurethane irradiated by gamma rays. J. Radioanal. Nucl. Chem. 2014, 300, 701–706. [Google Scholar] [CrossRef]
- Wang, C.B.; Cooper, S.L. Morphology and properties of segmented polyether polyurethaneureas. Macromolecules 1983, 16, 775–786. [Google Scholar] [CrossRef]
- Li, Y.; Gao, T.; Liu, J.; Linliu, K.; Desper, C.R.; Chu, B. Multiphase structure of a segmented polyurethane: Effects of temperature and annealing. Macromolecules 1992, 25, 7365–7372. [Google Scholar] [CrossRef]
- Sui, T.; Baimpas, N.; Dolbnya, I.P.; Prisacariu, C.; Korsunsky, A.M. Multiple-length-scale deformation analysis in a thermoplastic polyurethane. Nat. Commun. 2015, 6, 6583. [Google Scholar] [CrossRef] [PubMed]
- Laity, P.R.; Taylor, J.E.; Wong, S.S.; Khunkamchoo, P.; Norris, K.; Cable, M.; Andrewsc, G.T.; Johnsonb, A.F.; Cameron, R.E. A review of small-angle scattering models for random segmented poly(ether-urethane) copolymers. Polymer 2004, 45, 7273–7291. [Google Scholar] [CrossRef]
- Mang, J.T.; Peterson, P.D.; Orler, E.B.; Wrobleski, D.A.; Langlois, D.A.; Espada, L.I.; Hjelm, R.P. Small-angle neutron scattering study of a thermally aged, segmented poly(ester urethane) binder. Neutron News 2003, 14, 26–28. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Russell, T.P. Simultaneous SAXS-DSC study of multiple endothermic behavior in polyether-based polyurethane block copolymers. Macromolecules 1986, 19, 714–720. [Google Scholar] [CrossRef]
- Taylor, J.E.; Laity, P.R.; Freeburn, S.; Wong, S.S.; Norris, K.; Khunkamchoo, P.; Andrews, G.; Johnson, A.F.; Cameron, R.E. Effect of processing route and acetone pre-treatment on the biostability of pellethane materials used in medical device applications. Biomaterials 2005, 26, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Almásy, L.; Yan, G.; Sun, G.; Zhou, X.; Liu, J.; Rosta, L.; Chen, B. Small-angle neutron scattering investigation of polyurethane aged in dry and wet air. Express Polym. Lett. 2014, 8, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Keiderling, U. The new “BerSANS-PC” software for reduction and treatment of small angle neutron scattering data. Appl. Phys. A 2002, 74, s1455–s1457. [Google Scholar] [CrossRef]
- Breßler, I.; Kohlbrecher, J.; Thünemann, A.F. SASfit: A comprehensive tool for small-angle scattering data analysis. Available online: http://arxiv.org/ftp/arxiv/papers/1506/1506.02958.pdf (accessed on 5 September 2015).
- Debye, P.; Anderson, R.; Brumberger, H. Scattering by an inhomogeneous solid. II. The correlation function and its application. J. Appl. Phys. 1957, 28, 679–683. [Google Scholar] [CrossRef]
- Krakovský, I.; Bubenikova, Z.; Urakawa, H.; Kajiwara, K. Inhomogeneous structure of polyurethane networks based on poly(butadiene)diol: 1. The effect of the poly(butadiene)diol content. Polymer 1997, 38, 3637–3643. [Google Scholar] [CrossRef]
- Linliu, K.; Chen, S.A.; Yu, T.L.; Lin, T.L.; Lee, C.H.; Kai, J.J.; Chang, S.L.; Lin, J.S. A small-angle X-ray scattering study of microphase separation transition of polyurethanes: Effect of hard segments. J. Polym. Res. 1995, 2, 63–70. [Google Scholar] [CrossRef]
- Deng, Y.W.; Yu, T.L.; Ho, C.H. Effect of aging under strain on the physical properties of polyester-urethane elastomer. Polym. J. 1994, 26, 1368–1376. [Google Scholar] [CrossRef]
- Schoonover, J.R.; Thompson, D.G.; Osborn, J.C.; Orler, E.B.; Wrobleski, D.A.; Marsh, A.L.; Wang, H.; Ralmer, R.A. Infrared linear dichroism study of a hydrolytically degraded poly(ester urethane). Polym. Degrad. Stab. 2001, 74, 87–96. [Google Scholar] [CrossRef]
- Wang, H.J.; Feng, H.P.; Guo, P.Y.; Zhao, T.S.; Ren, L.F.; Qiang, X.H.; Xiang, Y.H. Effects of crystallization temperature and blend ratio on the crystal structure of poly(butylene adipate) in the poly(butylene adipate)/poly(butylene succinate) blends. Chin. J. Polym. Sci. 2014, 32, 488–496. [Google Scholar] [CrossRef]
- Gan, Z.; Kuwabara, K.; Abe, H.; Iwata, T.; Doi, Y. Metastability and transformation of polymorphic crystals in biodegradable poly(butylene adipate). Biomacromolecules 2004, 5, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, V.; Šmit, I.; Hace, D.; Sućeska, M.; Mudri, I.; Bravar, M. Role of the polyurethane component in the adhesive composition on the hydrolytic stability of the adhesive. Int. J. Adhes. Adhes. 1993, 13, 126–136. [Google Scholar] [CrossRef]
- Blackwell, J.; Nagarajan, M.R.; Hoitink, T.B. Structure of polyurethane elastomers. X-ray diffraction and conformational analysis of MDI-propandiol and MDI-ethylene glycol hard segments. Polymer 1981, 22, 1534–1539. [Google Scholar] [CrossRef]
- Shintani, H.; Nakamura, A. Formation of 4,4′-methylenedianiline in polyurethane potting materials by either γ-ray or autoclave sterilization. J. Biomed. Mater. Res. 1991, 25, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xiong, J.; Chen, X.; Gao, X.; Xu, Y.; Fu, Y. Study on the radiation degradation of polyether-polyurethane induced by electron beam. J. Radioanal. Nucl. Chem. 2007, 274, 525–530. [Google Scholar]
- Walo, M.; Przybytniak, G.; Łyczko, K.; Piątek-Hnat, M. The effect of hard/soft segment composition on radiation stability of poly(ester-urethane)s. Radiat. Phys. Chem. 2014, 94, 18–21. [Google Scholar] [CrossRef]
- Yanagihara, Y.; Osaka, N.; Iimori, S.; Murayama, S.; Saito, H. Relationship between modulus and structure of annealed thermoplastic polyurethane. Mater. Today Commun. 2015, 2, e9–e15. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Bothe, E.; Ulanski, P.; Adhikary, A. Radical transfer reactions in polymers. Radiat. Phys. Chem. 1999, 55, 599–603. [Google Scholar] [CrossRef]
- Chapiro, A. Radiation chemistry of polymers. Radiat. Res. Suppl. 1964, 4, 179–191. [Google Scholar] [CrossRef]
- Pârvu, R.; Podina, C.; Zaharescu, T.; Jipa, S. Stability evaluation of polyurethane coatings by gamma irradiation. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 1815–1818. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Takács, E.; Krakovský, I.; Horváth, Z.E.; Rosta, L.; Almásy, L. Study on the Microstructure of Polyester Polyurethane Irradiated in Air and Water. Polymers 2015, 7, 1755-1766. https://doi.org/10.3390/polym7091481
Tian Q, Takács E, Krakovský I, Horváth ZE, Rosta L, Almásy L. Study on the Microstructure of Polyester Polyurethane Irradiated in Air and Water. Polymers. 2015; 7(9):1755-1766. https://doi.org/10.3390/polym7091481
Chicago/Turabian StyleTian, Qiang, Erzsébet Takács, Ivan Krakovský, Zsolt Endre Horváth, László Rosta, and László Almásy. 2015. "Study on the Microstructure of Polyester Polyurethane Irradiated in Air and Water" Polymers 7, no. 9: 1755-1766. https://doi.org/10.3390/polym7091481