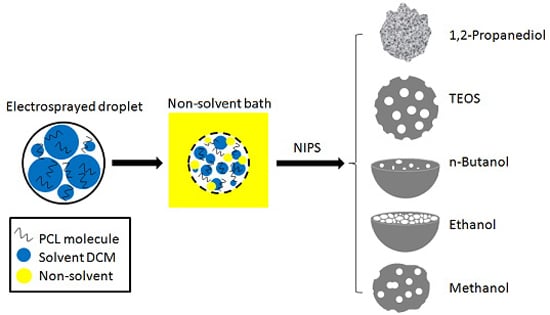
Tuning Microparticle Porosity during Single Needle Electrospraying Synthesis via a Non-Solvent-Based Physicochemical Approach
Abstract
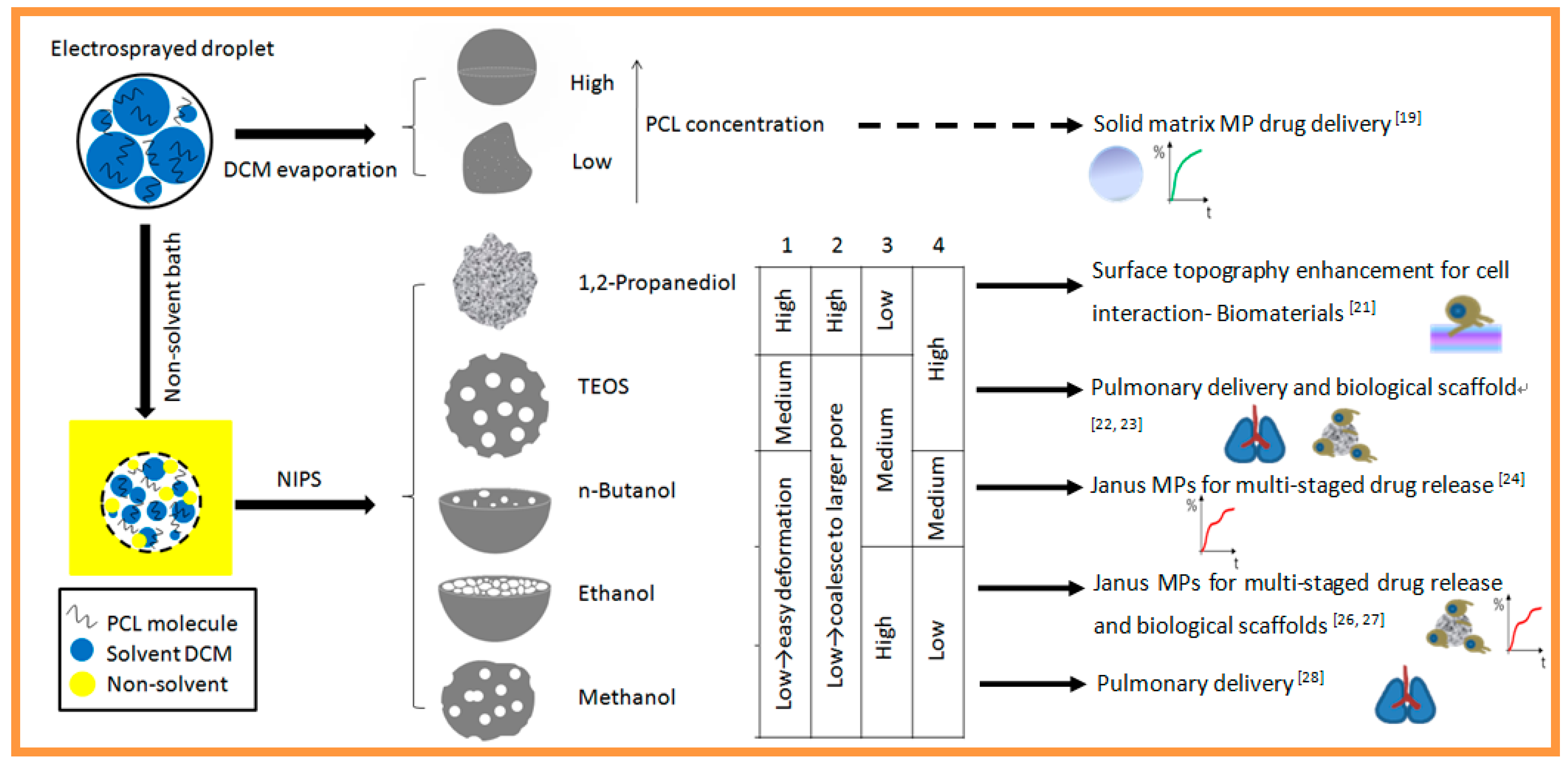
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. PCL Solution Preparation
2.2.2. Preparation of Microparticles
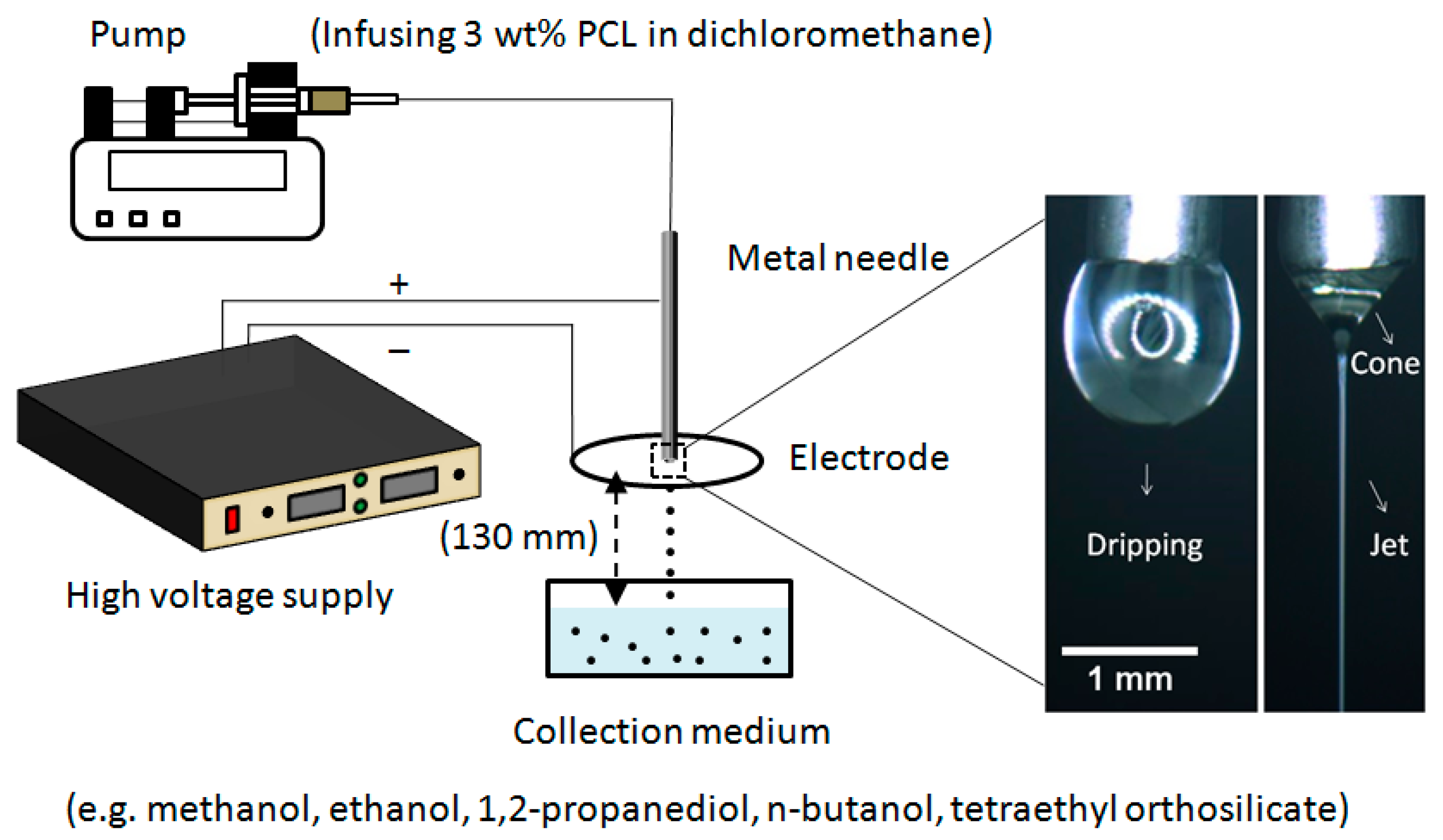
2.2.3. Microparticle Characterization
2.2.4. Surface Measurements and Solvent Characterizations
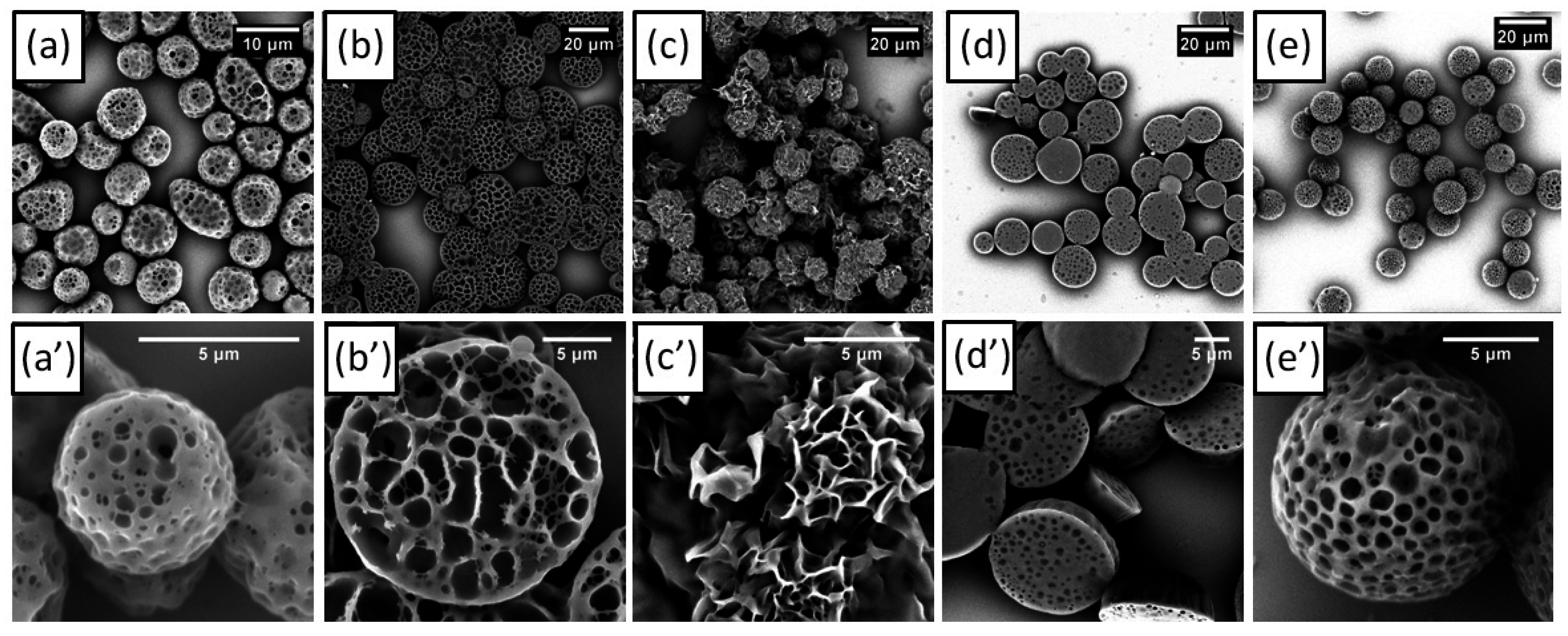
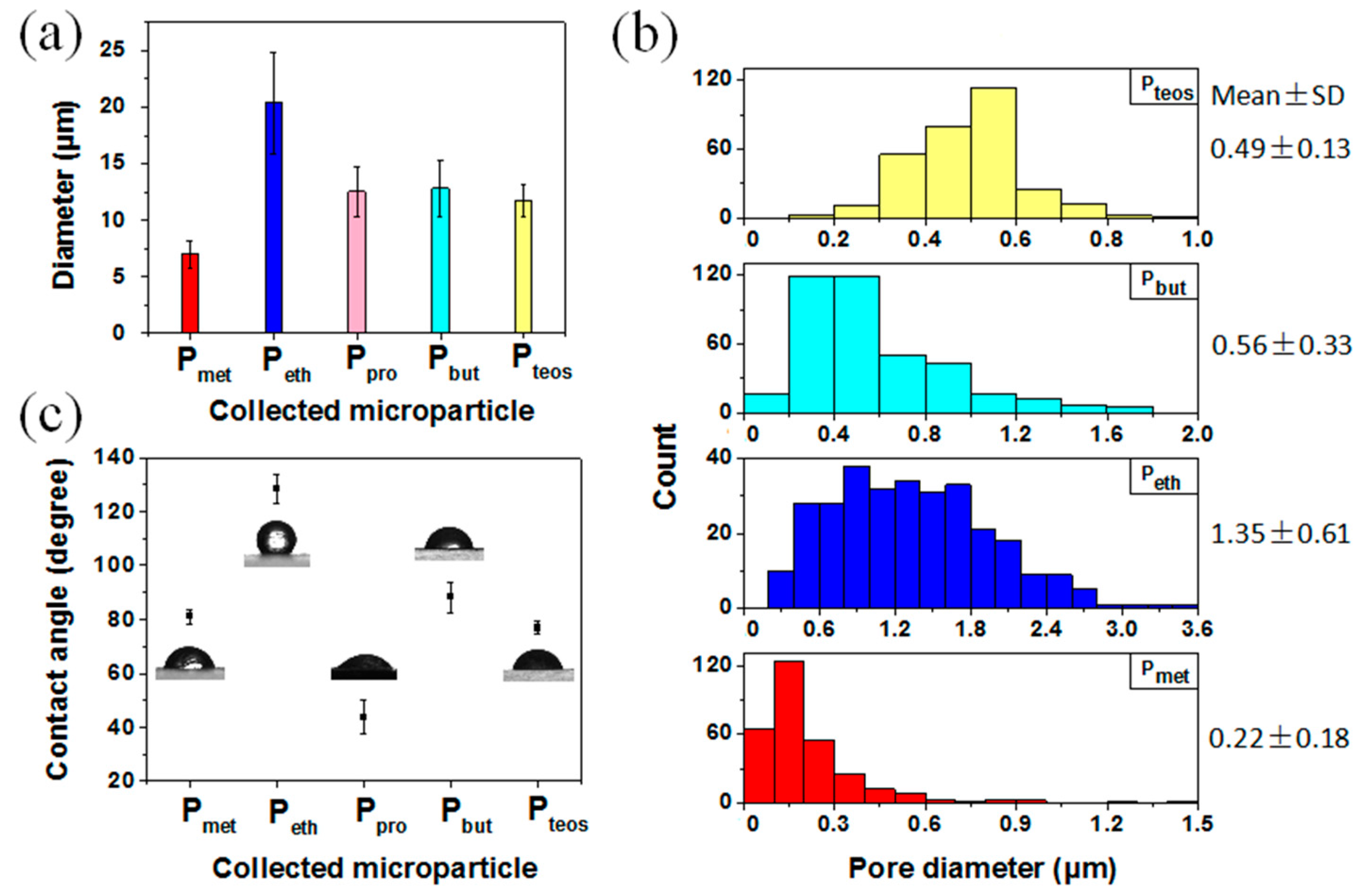
3. Results and Discussion
| Items | Molecular Weight (g·mol−1) | Vapour Pres a (kPa) | Surface Tension b (mN·m−1) | Viscosity c (mPa·s) |
|---|---|---|---|---|
| Methanol | 32 | 12.97 | 22.6 | 0.6 |
| Ethanol | 46 | 5.87 | 22.3 | 1.2 |
| 1,2-Propanediol | 76 | ~0 | 72.0 | 60.5 |
| n-Butanol | 74 | 0.58 | 24.6 | 2.1 |
| TEOS | 208 | 0.13 | 23.4 | 17.9 |
| DCM | 85 | 47.40 | 23.1 | 0.4 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oh, Y.J.; Lee, J.; Seo, J.Y.; Rhim, T.; Kim, S.H.; Yoon, H.J.; Lee, K.Y. Preparation of budesonide-loaded porous PLGA microparticles and their therapeutic efficacy in a murine asthma model. J. Control. Release 2011, 150, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Kuhn, P.; Weber, J.; Titirici, M.M.; Antonietti, M. Porous polymers: Enabling solutions for energy applications. Macromol. Rapid Commun. 2009, 30, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Li, Y.B.; Yang, M.; Chang, Y.Q.; Zhang, J.P.; Sun, Z.W.; Wang, Y.P. Enhanced light absorption in porous particles for ultra-NIR-sensitive biomaterials. ACS Macro Lett. 2015, 4, 392–397. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, T.G. Comparative study on sustained release of human growth hormone from semi-crystalline poly(l-lactic acid) and amorphous poly(d,l-lactic-co-glycolic acid) microspheres: Morphological effect on protein release. J. Control. Release 2004, 98, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Yu, H.S.; Kim, H.W. Tissue engineering polymeric microcarriers with macroporous morphology and bone-bioactive surface. Macromol. Biosci. 2009, 9, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.M.; Wu, R.R.; Dai, X.H.; Yin, Y.J.; Pan, G.Q.; Meng, M.J.; Shi, W.D.; Yan, Y.S. A hierarchical porous bowl-like PLA@MSNs-COOH composite for pH-dominated long-term controlled release of doxorubicin and integrated nanoparticle for potential second treatment. Biomacromolecules 2015, 16, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Reignier, J.; Huneault, M.A. Preparation of interconnected poly(epsilon-caprolactone) porous scaffolds by a combination of polymer and salt particulate leaching. Polymer 2006, 47, 4703–4717. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lim, K.S.; Oh, J.E.; Yoon, A.R.; Joo, W.S.; Kim, H.S.; Yun, C.O.; Kim, S. Development of porous PLGA/PEI1.8k biodegradable microspheres for the delivery of mesenchymal stem cells (MSCs). J. Control. Release 2015, 205, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Takai, C.; Hotta, T.; Shiozaki, S.; Boonsongrit, Y.; Abe, H. Unique porous microspheres with dense core and a porous layer prepared by a novel S/O/W emulsion technique. Chem. Commun. 2009, 5533–5535. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Stride, E.; Edirisinghe, M. A new method for the preparation of monoporous hollow microspheres. Langmuir 2010, 26, 5115–5121. [Google Scholar] [CrossRef] [PubMed]
- Fantini, D.; Zanetti, M.; Costa, L. Polystyrene microspheres and nanospheres produced by electrospray. Macromol. Rapid Commun. 2006, 27, 2038–2042. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Clark, R.L. Controllable porous polymer particles generated by electrospraying. J. Colloid Interface Sci. 2007, 310, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F.; Li, W.; Wong, J.S.P.; Hu, M.J.; Li, R.K.Y. Controllable morphology and wettability of polymer microspheres prepared by nonsolvent assisted electrospraying. Polymer 2014, 55, 2913–2920. [Google Scholar] [CrossRef]
- Labbaf, S.; Deb, S.; Cama, G.; Stride, E.; Edirisinghe, M. Preparation of multicompartment sub-micron particles using a triple-needle electrohydrodynamic device. J. Colloid Interface Sci. 2013, 409, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.H.; Martin, D.C.; Lahann, J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 2005, 4, 759–763. [Google Scholar] [CrossRef] [PubMed]
- De la Ossa, D.H.P.; Ligresti, A.; Gil-Alegre, M.E.; Aberturas, M.R.; Molpeceres, J.; Di Marzo, V.; Suarez, A.I.T. Poly-epsilon-caprolactone microspheres as a drug delivery system for cannabinoid administration: Development, characterization and in vitro evaluation of their antitumoral efficacy. J. Control. Release 2012, 161, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Ma, L.X.; Liu, J. Chemistry and Chemical Engineering Physical Property Data Handbook; Chemical Industry Press: Beijing, China, 2002. [Google Scholar]
- Feng, J.T.; Lin, L.; Chen, P.P.; Hua, W.D.; Sun, Q.M.; Ao, Z.; Liu, D.S.; Jiang, L.; Wang, S.T.; Han, D. Topographical binding to mucosa-exposed cancer cells: Pollen-mimetic porous microspheres with tunable pore sizes. ACS Appl. Mater. Interfaces 2015, 7, 8961–8967. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Yang, M.S.; Baldursdottir, S.; Kristensen, J.; Dyas, M.; Stride, E.; Edirisinghe, M. Particle formation and characteristics of celecoxib-loaded poly(lactic-co-glycolic acid) microparticles prepared in different solvents using electrospraying. Polymer 2012, 53, 3220–3229. [Google Scholar] [CrossRef]
- Xie, J.W.; Lim, L.K.; Phua, Y.Y.; Hua, J.S.; Wang, C.H. Electrohydrodynamic atomization for biodegradable polymeric particle production. J. Colloid Interface Sci. 2006, 302, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Shi, X.T.; Ren, L.; Wang, C.M.; Wang, D.A. Porous poly(lactic-co-glycolide) microsphere sintered scaffolds for tissue repair applications. Mater. Sci. Eng. 2009, 29, 2502–2507. [Google Scholar] [CrossRef]
- Courrier, H.M.; Butz, N.; Vandamme, T.F. Pulmonary drug delivery systems: Recent developments and prospects. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 425–498. [Google Scholar] [CrossRef]
- Yang, Y.; Bajaj, N.; Xu, P.; Ohn, K.; Tsifansky, M.D.; Yeo, Y. Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 2009, 30, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, Y.J.; Deng, T.Z.; Jin, F.; Liu, S.X.; Zhang, Y.J.; Feng, F.; Jin, Y. Facile modification of gelatin-based microcarriers with multiporous surface and proliferative growth factors delivery to enhance cell growth. J. Alloy. Compd. 2008, 460, 639–645. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, C.S.; Cho, K.Y. Enhanced cell adhesion to the dimpled surfaces of golf-ball-shaped microparticles. ACS Appl. Mater. Interfaces 2014, 6, 16493–16497. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, J.M.; Ji, W.H.; Wang, W.; He, S.H.; Jiang, X.Z.; Wiedmann, T.; Wang, C.; Wang, J.P. Biocompatible Fe–Si nanoparticles with adjustable self-regulation of temperature for medical applications. ACS Appl. Mater. Interfaces 2015, 7, 12649–12654. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Chung, H.J.; Park, T.G. Biodegradable polymeric microspheres with "open/closed" pores for sustained release of human growth hormone. J. Control. Release 2006, 112, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, Y.J.; Lee, S.K.; Lee, K.Y. Facile control of porous structures of polymer microspheres using an osmotic agent for pulmonary delivery. J. Control. Release 2010, 146, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; He, Y.N.; Fan, Y.; Wang, X.G. Fabricating super-hydrophobic lotus-leaf-like surfaces through soft-lithographic imprinting. Macromol. Rapid Commun. 2006, 27, 1859–1864. [Google Scholar] [CrossRef]
- Kim, M.R.; Lee, S.; Park, J.K.; Cho, K.Y. Golf ball-shaped PLGA microparticles with internal pores fabricated by simple O/W emulsion. Chem. Commun. 2010, 46, 7433–7435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Liu, J.; Wang, X.J.; Li, M.X.; Yang, J. Controlling internal nanostructures of porous microspheres prepared via electrospraying. Colloid Polym. Sci. 2010, 288, 1385–1391. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Bai, Y.; Zhao, D.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Tuning Microparticle Porosity during Single Needle Electrospraying Synthesis via a Non-Solvent-Based Physicochemical Approach. Polymers 2015, 7, 2701-2710. https://doi.org/10.3390/polym7121531
Gao Y, Bai Y, Zhao D, Chang M-W, Ahmad Z, Li J-S. Tuning Microparticle Porosity during Single Needle Electrospraying Synthesis via a Non-Solvent-Based Physicochemical Approach. Polymers. 2015; 7(12):2701-2710. https://doi.org/10.3390/polym7121531
Chicago/Turabian StyleGao, Yuan, Yuntong Bai, Ding Zhao, Ming-Wei Chang, Zeeshan Ahmad, and Jing-Song Li. 2015. "Tuning Microparticle Porosity during Single Needle Electrospraying Synthesis via a Non-Solvent-Based Physicochemical Approach" Polymers 7, no. 12: 2701-2710. https://doi.org/10.3390/polym7121531
APA StyleGao, Y., Bai, Y., Zhao, D., Chang, M.-W., Ahmad, Z., & Li, J.-S. (2015). Tuning Microparticle Porosity during Single Needle Electrospraying Synthesis via a Non-Solvent-Based Physicochemical Approach. Polymers, 7(12), 2701-2710. https://doi.org/10.3390/polym7121531