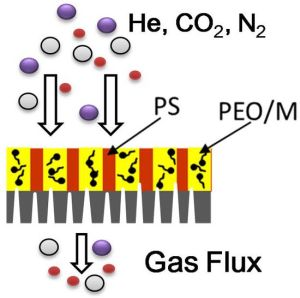
Fabrication of CO2 Facilitated Transport Channels in Block Copolymer through Supramolecular Assembly
Abstract
:1. Introduction
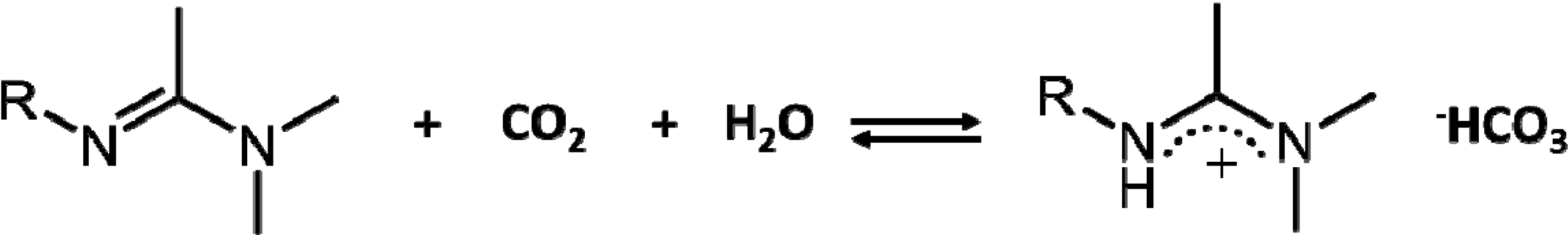
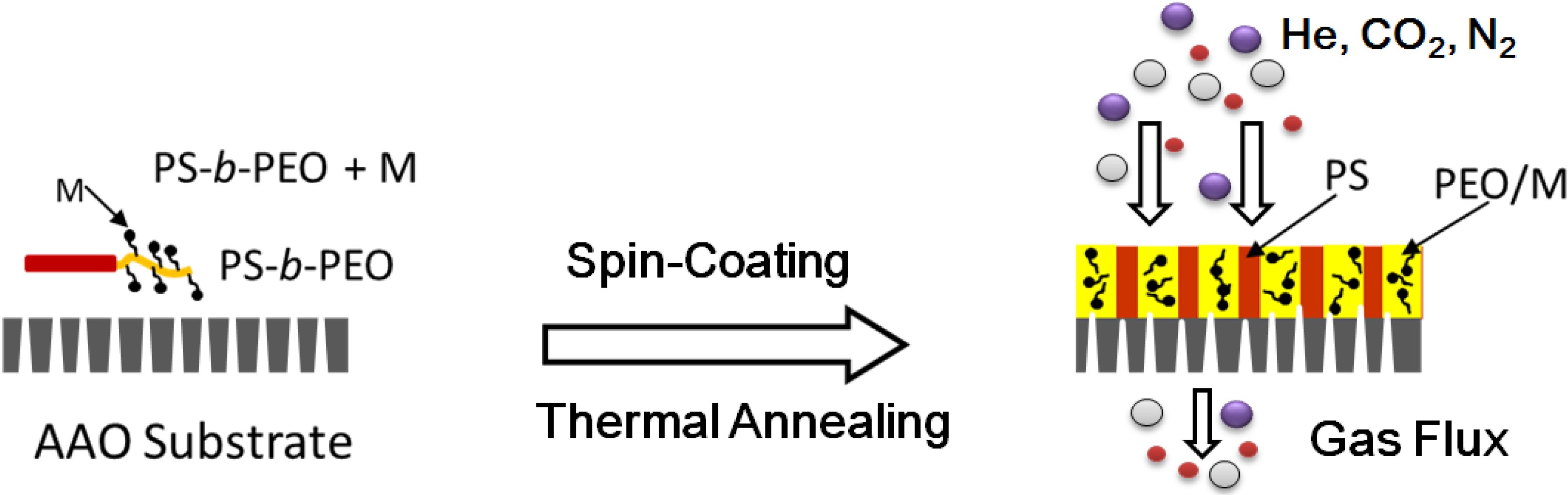
2. Experimental Section
2.1. Materials
2.2. Preparation of Membranes
2.3. Characterization
2.4. Gas Permeation Measurement


3. Results and Discussion
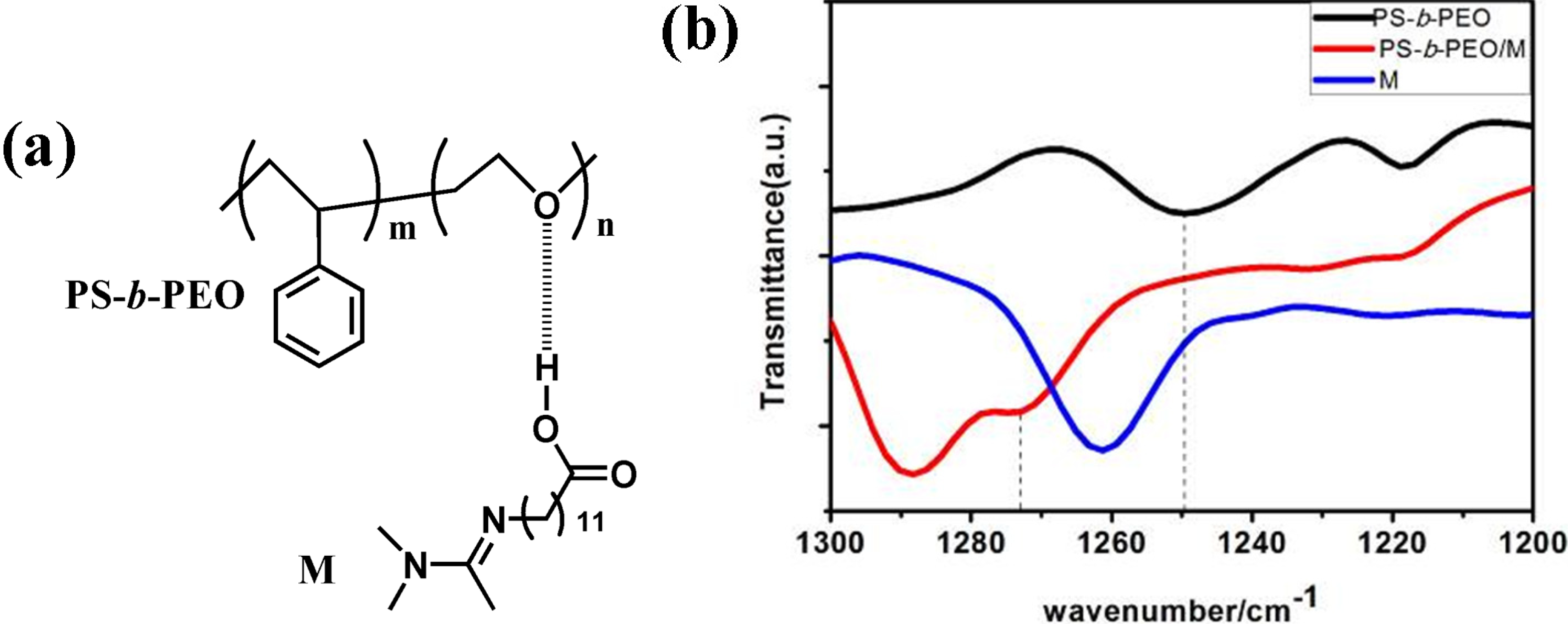
3.1. Structure and Morphology of Membranes
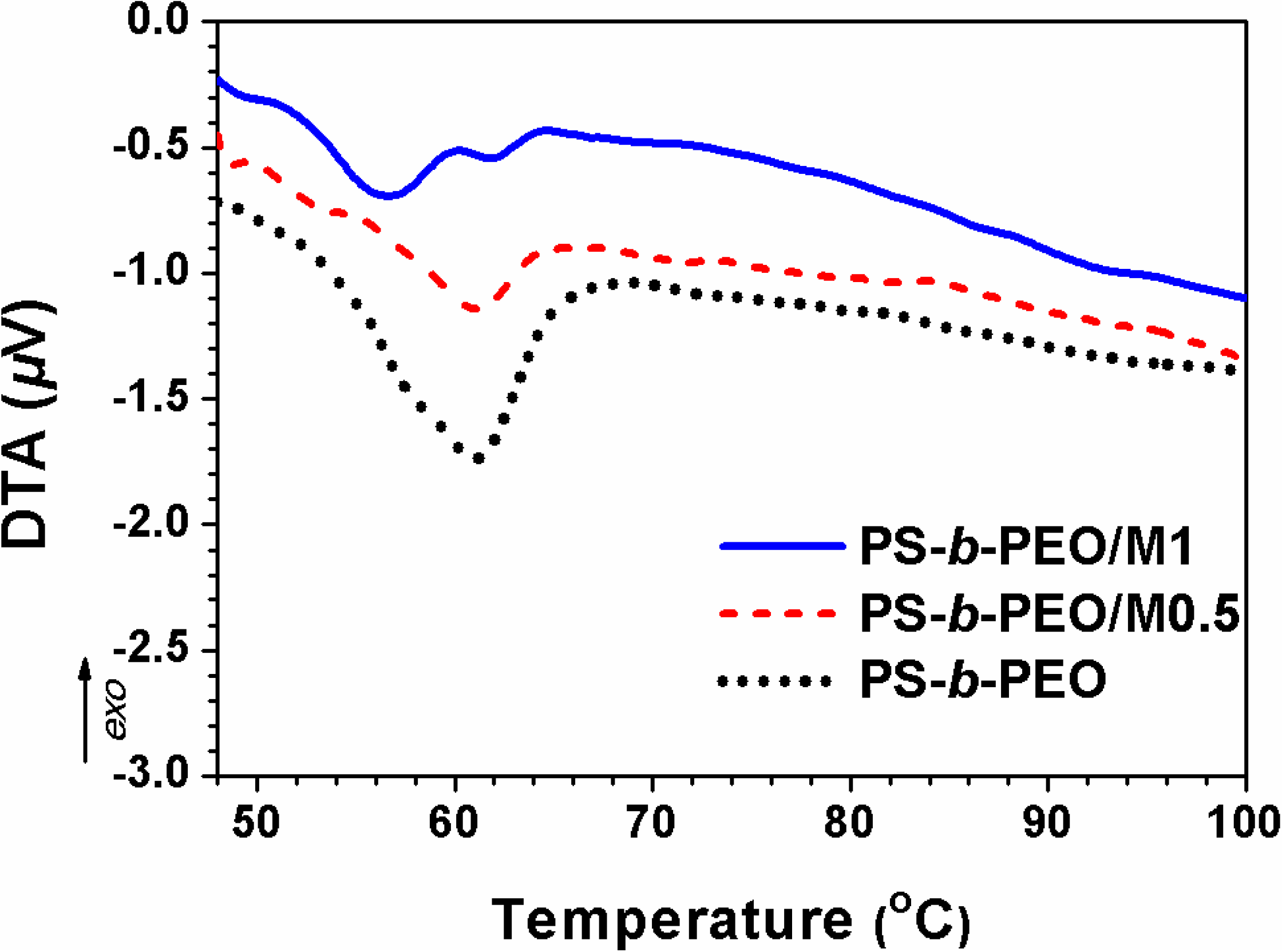
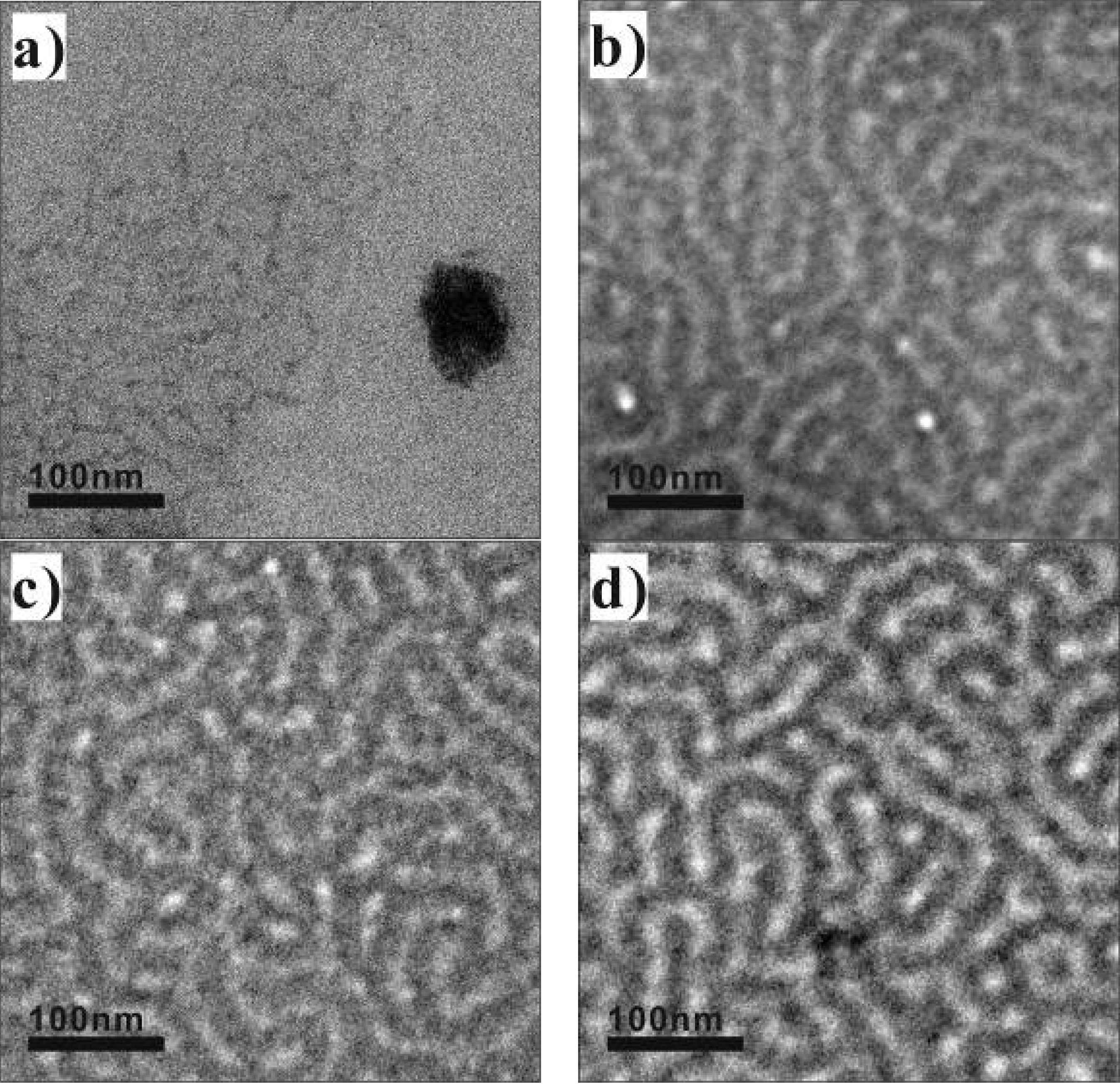

3.2. Gas Permeability of Membranes
| Membrane | Thickness(nm) | Condition | He | N2 | CO2 | αCO2/N2 |
|---|---|---|---|---|---|---|
| PS-b-PEO (dry) | 455 | unannealed | 140.31 | 71.42 | 79.76 | 1.12 |
| PS-b-PEO (humid) | unannealed | 139.62 | 69.05 | 80.00 | 1.15 | |
| PS-b-PEO (dry) | 520 | annealed | 241.76 | 170.97 | 195.46 | 1.14 |
| PS-b-PEO (humid) | annealed | 242.85 | 164.61 | 188.49 | 1.15 | |
| PS-b-PEO/M0.5 (dry) | 387 | unannealed | 169.49 | 80.68 | 87.21 | 1.08 |
| PS-b-PEO/M0.5 (humid) | unannealed | 163.41 | 82.64 | 87.09 | 1.05 | |
| PS-b-PEO/M0.5 (dry) | 350 | annealed | 366.04 | 242.99 | 278.33 | 1.15 |
| PS-b-PEO/M0.5 (humid) | annealed | 372.4 | 240.94 | 290.4 | 1.21 | |
| PS-b-PEO/M1 (dry) | 358 | unannealed | 152.59 | 85.37 | 101.05 | 1.18 |
| PS-b-PEO/M1 (humid) | unannealed | 149.92 | 85.10 | 110.84 | 1.30 | |
| PS-b-PEO/M1 (dry) | 373 | annealed | 231.07 | 149.30 | 201.71 | 1.35 |
| PS-b-PEO/M1 (humid) | annealed | 236.29 | 146.54 | 214.64 | 1.46 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lackner, K.S. A guide to CO2 sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef]
- Hudson, M.R.; Queen, W.L.; Mason, J.A.; Ficke, D.W.; Lobo, R.F.; Brown, C.M. Unconventional, highly selective CO2 adsorption in Zeolite SSZ-13. J. Am. Chem. Soc. 2012, 134, 1970–1973. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Jiang, L. Research progress of preparation methods of CO2-favored permeation membranes. J. Chin. Univ. 2013, 34, 249–268. [Google Scholar]
- Zhang, Y.; Wang, Z.; Wang, S.C. Selective permeation of CO2 through new facilitated transport membranes. Desalination 2002, 145, 385–388. [Google Scholar] [CrossRef]
- Jessop, P.G.; Phan, L.; Carrier, A.; Robinson, S.; Durr, C.J.; Harjani, J.R. A solvent having switchable hydrophilicity. Green Chem. 2010, 12, 809–814. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, S.L.; Xu, H.P.; Wang, Z.Q.; Zhang, X. Reversible dispersion of single-walled carbon nanotubes based on a CO2-responsive dispersant. Langmuir 2010, 26, 16667–16671. [Google Scholar]
- Li, B.Y.; Jiang, B.B.; Fauth, D.J.; Gray, M.L.; Pennline, H.W.; Richards, G.A. Innovative nano-layered solid sorbents for CO2 capture. Chem. Commun. 2011, 47, 1719–1721. [Google Scholar]
- Frank, S.; Bates, G.H.F. Block copolymers—Designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar]
- Peng, J.; Cui, L.; Luo, C.X.; Xing, R.B.; Han, Y.C. Constructing and tuning polymer surface microstructures. Chin. Sci. Bull. 2009, 54, 679–695. [Google Scholar]
- Ji, S.; Liu, C.C.; Liao, W.; Fenske, A.L.; Craig, G.S.W.; Nealey, P.F. Domain orientation and grain coarsening in cylinder-forming poly(styrene-b-methyl methacrylate) films. Macromolecules 2011, 44, 4291–4300. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, E.; Lee, M. Nanofibers with tunable stiffness from self-assembly of an amphiphilic wedge-coil molecule. Angew. Chem. Int. Ed. 2006, 45, 7195–7198. [Google Scholar] [CrossRef]
- Bracco, S.; Comotti, A.; Ferretti, L.; Sozzani, P. Supramolecular aggregation of block copolymers in the solid state as assisted by the selective formation of inclusion crystals. J. Am. Chem. Soc. 2011, 133, 8982–8994. [Google Scholar] [CrossRef]
- Taniguchi, I.; Duan, S.; Kazama, S.; Fujioka, Y. Facile fabrication of a novel high performance CO2 separation membrane: Immobilization of poly(amidoamine) dendrimers in poly(ethylene glycol) networks. J. Membr. Sci. 2008, 322, 277–280. [Google Scholar] [CrossRef]
- Xia, G.; Jeong, S.J.; Kim, J.E.; Kim, B.H.; Koo, C.M.; Kim, S.O. Spin coating nanopatterned multielemental materials via self-assembled nanotemplates. Nanotechnology 2009, 20, 225301. [Google Scholar] [CrossRef]
- Farrell, R.A.; Petkov, N.; Morris, M.A.; Holmes, J.D. Self-assembled templates for the generation of arrays of 1-dimensional nanostructures: From molecules to devices. J. Colloid Interface Sci. 2010, 349, 449–472. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, C.; Choi, S.Y.; Kim, S.H. Block copolymer-surfactant complexes in thin films for multiple usages from hierarchical structure to nano-objects. Macromolecules 2010, 43, 442–447. [Google Scholar] [CrossRef]
- Kuila, B.K.; Rama, M.S.; Stamm, M. Supramolecular assembly of poly(styrene)-b-poly(4-vinylpyridine) and ferroceneacetic acid: An easy way to large-scale controllable periodic arrays of iron oxide nanomaterials. Adv. Mater. 2011, 23, 1797–1800. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.P.; Peinemann, K.-V. CO2-philic polymer membrane with extremely high separation performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.; Eckert, C.A.; Liotta, C.L. Green chemistry: Reversible nonpolar-to-polar solvent. Nature 2005, 436, 1102–1102. [Google Scholar]
- Pérez, E.R.; da Silva, M.O.; Costa, V.C.; Rodrigues-Filho, U.P.; Franco, D.W. Efficient and clean synthesis of N-alkyl carbamates by transcarboxylation and O-alkylation coupled reactions using a DBU-CO2 zwitterionic carbamic complex in aprotic polar media. Tetrahedron Lett. 2002, 43, 4091–4093. [Google Scholar] [CrossRef]
- Ochiai, B.; Yokota, K.; Fujii, A.; Nagai, D.; Endo, T. Reversible trap-release of CO2 by polymers bearing DBU and DBN moieties. Macromolecules 2008, 41, 1229–1236. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Jessop, P.G.; Thomas, C.A.; Eckert, C.A.; Liotta, C.L. The reaction of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) with carbon dioxide. J. Org. Chem. 2005, 70, 5335–5338. [Google Scholar] [CrossRef]
- Tian, T.; Chen, X.; Li, H.; Wang, Y.; Guo, L.; Jiang, L. Amidine-based fluorescent chemosensor with high applicability for detection of CO2: A facile way to “See” CO2. Analyst 2013, 138, 991–994. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Koech, P.K.; Ang, M.T.C.; Liang, C.; Rainbolt, J.E.; Yonker, C.R.; Jessop, P.G. Reversible zwitterionic liquids, the reaction of alkanol guanidines, alkanol amidines, and diamines with CO2. Green Chem. 2010, 12, 713–721. [Google Scholar] [CrossRef]
- Mihara, M.; Jessop, P.; Cunningham, M. Redispersible polymer colloids using carbon dioxide as an external trigger. Macromolecules 2011, 44, 3688–3693. [Google Scholar] [CrossRef]
- Li, X.W.; Tian, T.; Leolukman, M.; Wang, Y.; Jiang, L. A supramolecular approach to probing the influence of micro-phase structure on gas permeability of block copolymer membranes. Sci. Adv. Mater. 2013, 5, 719–726. [Google Scholar] [CrossRef]
- Xue, B.; Li, X.; Gao, L.; Gao, M.; Wang, Y.; Jiang, L. CO2-selective free-standing membrane by self-assembly of a UV-crosslinkable diblock copolymer. J. Mater. Chem. 2012, 22, 10918–10923. [Google Scholar] [CrossRef]
- Wang, Y.; Janout, V.; Regen, S.L. Creating poly(ethylene oxide)-based polyelectrolytes for thin film construction using an ionic linker strategy. Chem. Mater. 2010, 22, 1285–1287. [Google Scholar] [CrossRef]
- Wang, Y.; Stedronsky, E.; Regen, S.L. Defects in a polyelectrolyte multilayer: The inside story. J. Am. Chem. Soc. 2008, 130, 16510–16511. [Google Scholar] [CrossRef]
- Lee, J.Y.; Painter, P.C.; Coleman, M.M. Hydrogen bonding in polymer blends. 4. Blends involving polymers containing methacrylic acid and vinylpyridine groups. Macromolecules 1988, 21, 954–960. [Google Scholar] [CrossRef]
- Ruokolainen, J.; Brinke, G.T.; Ikkala, O.; Torkkeli, M.; Serimaa, R. Mesomorphic structures in flexible polymer-surfactant systems due to hydrogen bonding: Poly(4-vinylpyridine)-pentadecylphenol. Macromolecules 1996, 29, 3409–3415. [Google Scholar] [CrossRef]
- Yeh, S.W.; Wei, K.H.; Sun, Y.S.; Jeng, U.S.; Liang, K.S. Morphological transformation of PS-b-PEO diblock copolymer by selectively dispersed colloidal CdS quantum dots. Macromolecules 2003, 36, 7903–7907. [Google Scholar] [CrossRef]
- Dirany, M.; Lacroix-Desmazes, P.; Vayer, M.; Erre, R.; Boutevin, B.; Sinturel, C. Polystyrene-block-polylactide obtained by the combination of atom transfer radical polymerization and ring-opening polymerization with a commercial dual initiator. J. Appl. Polym. Sci. 2011, 122, 2944–2951. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Shang, Y.; Li, X.; Tian, T.; Gao, L.; Jiang, L. Fabrication of CO2 Facilitated Transport Channels in Block Copolymer through Supramolecular Assembly. Polymers 2014, 6, 1403-1413. https://doi.org/10.3390/polym6051403
Wang Y, Shang Y, Li X, Tian T, Gao L, Jiang L. Fabrication of CO2 Facilitated Transport Channels in Block Copolymer through Supramolecular Assembly. Polymers. 2014; 6(5):1403-1413. https://doi.org/10.3390/polym6051403
Chicago/Turabian StyleWang, Yao, Ying Shang, Xianwu Li, Tong Tian, Longcheng Gao, and Lei Jiang. 2014. "Fabrication of CO2 Facilitated Transport Channels in Block Copolymer through Supramolecular Assembly" Polymers 6, no. 5: 1403-1413. https://doi.org/10.3390/polym6051403