Interfacial Properties of Methylcelluloses: The Influence of Molar Mass
Abstract
:1. Introduction
2. Experimental Section
| Polymer Reference | [η] (mL·g−1) a,b | MV (g·mol−1) c | DS d |
|---|---|---|---|
| A15LV | 193 | 42,100 | 1.8 |
| A4C | 573 | 212,000 | 1.7 |
| A15C | 740 | 304,600 | 1.8 |
| A4M | 933 | 423,400 | 1.7 |
3. Results and Discussion
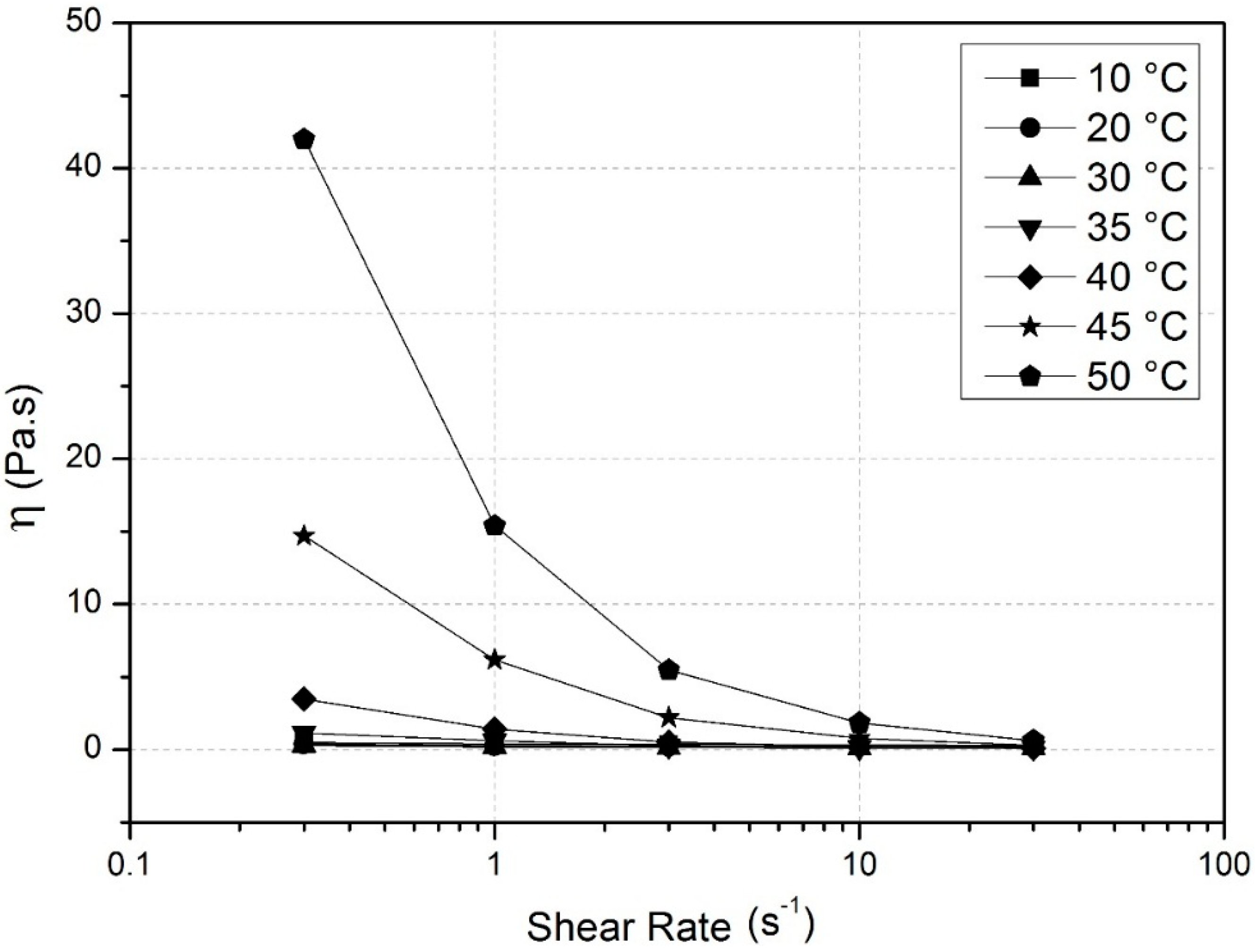
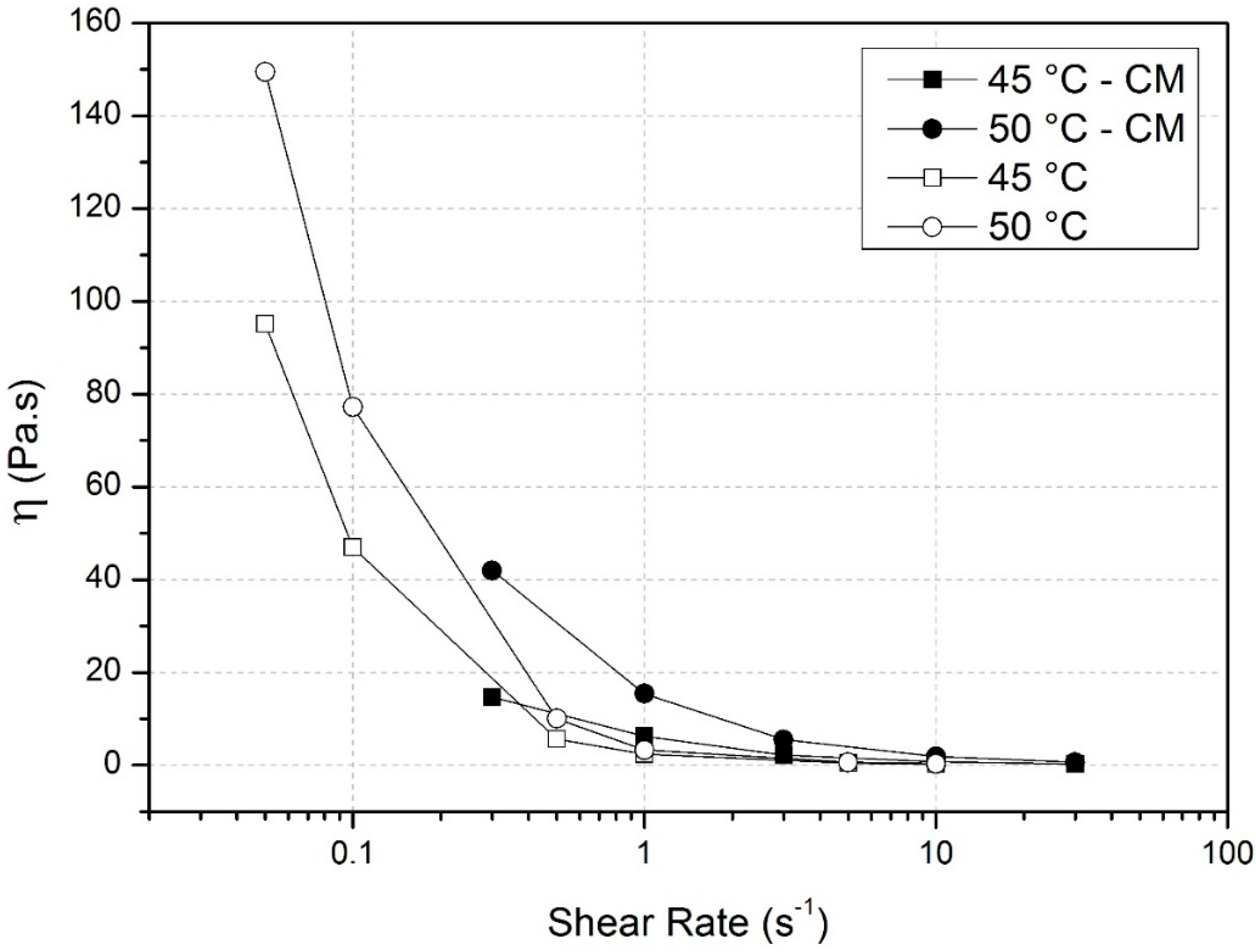
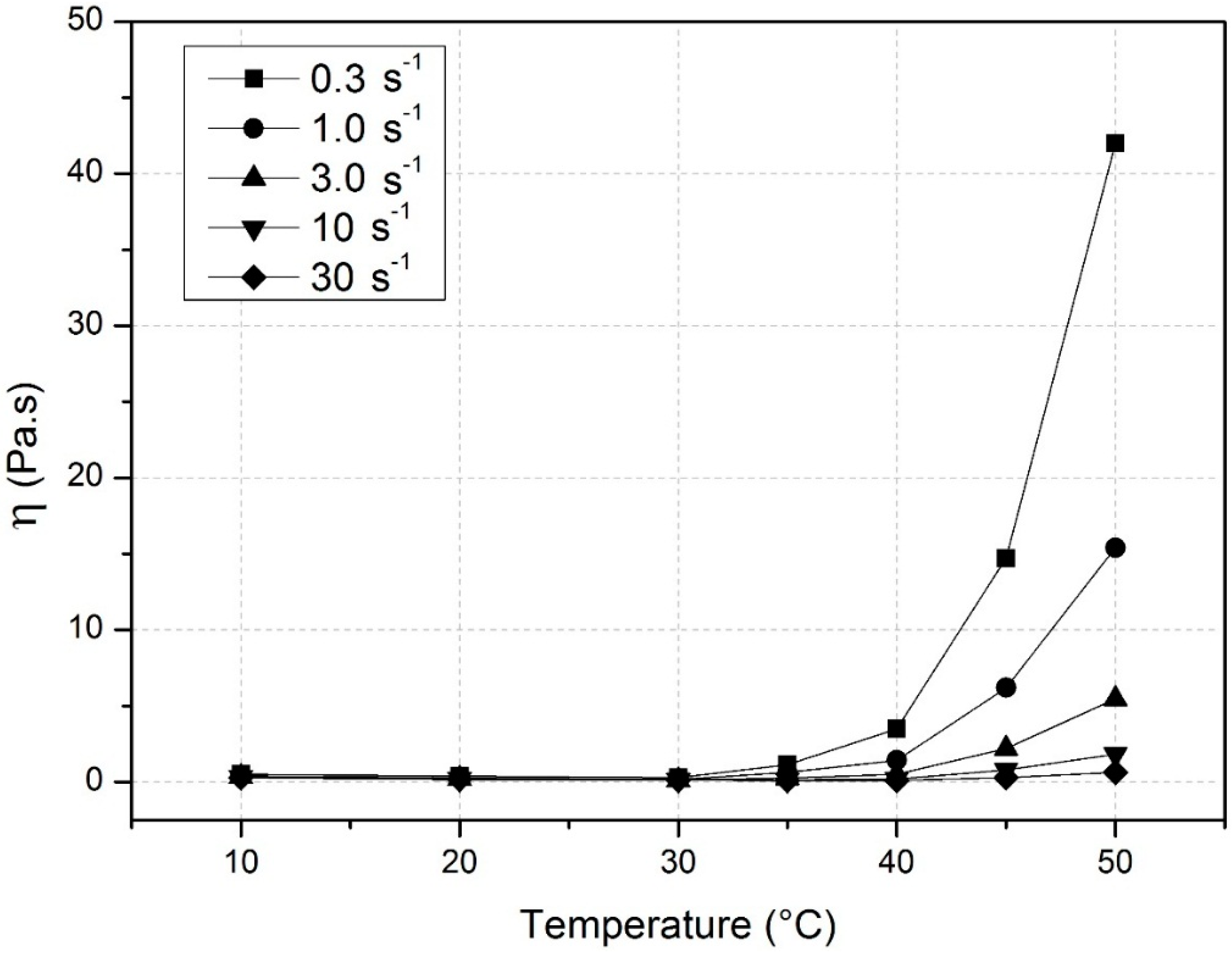
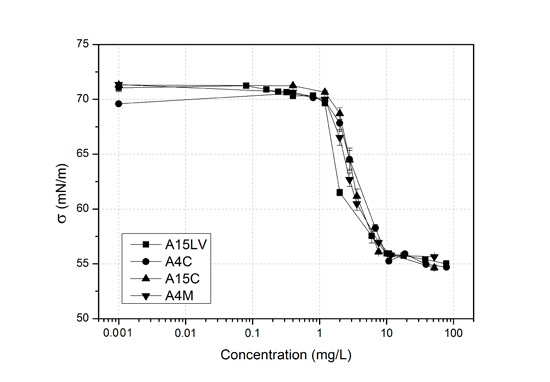
3.1. Surface Activity of Methylcelluloses
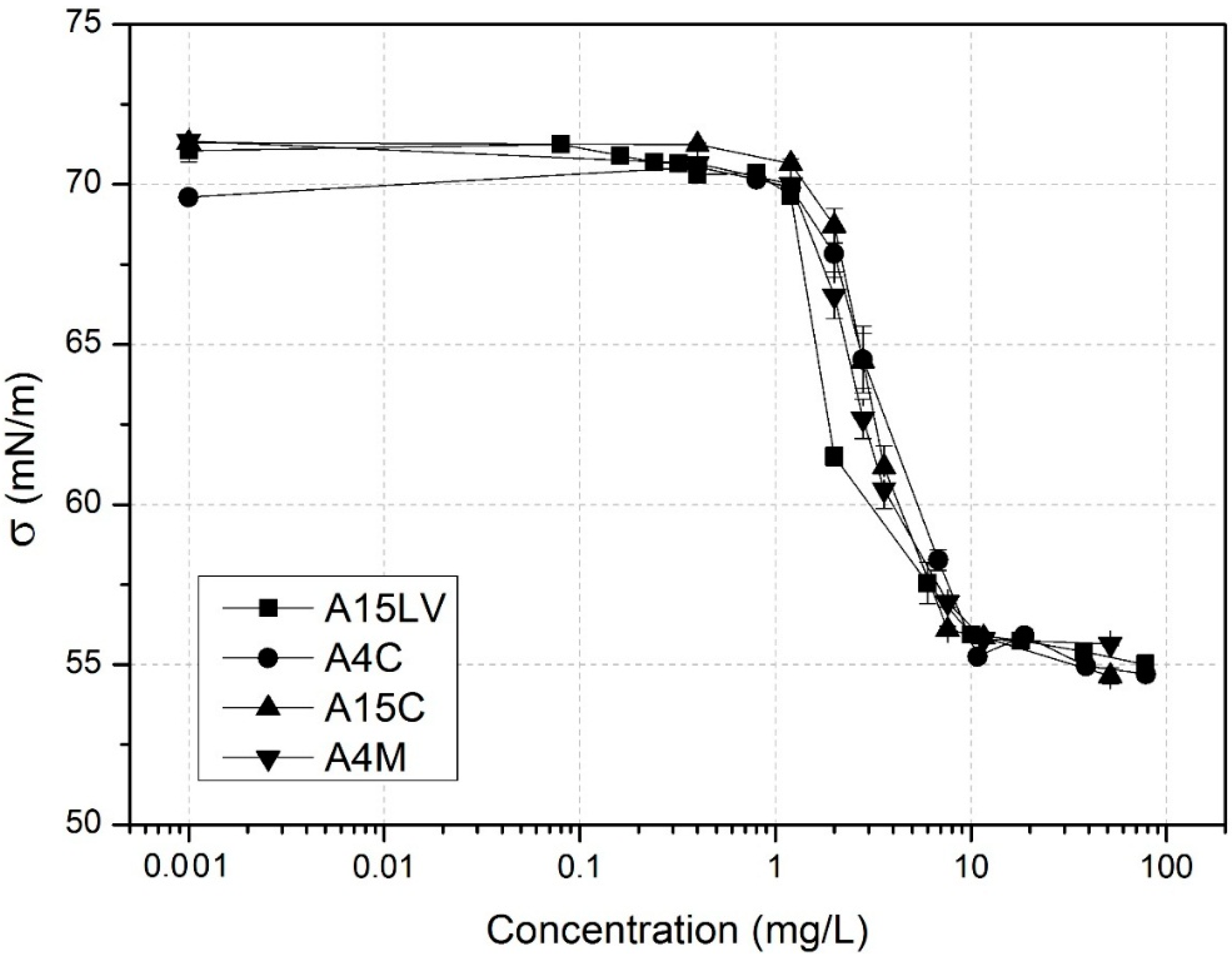

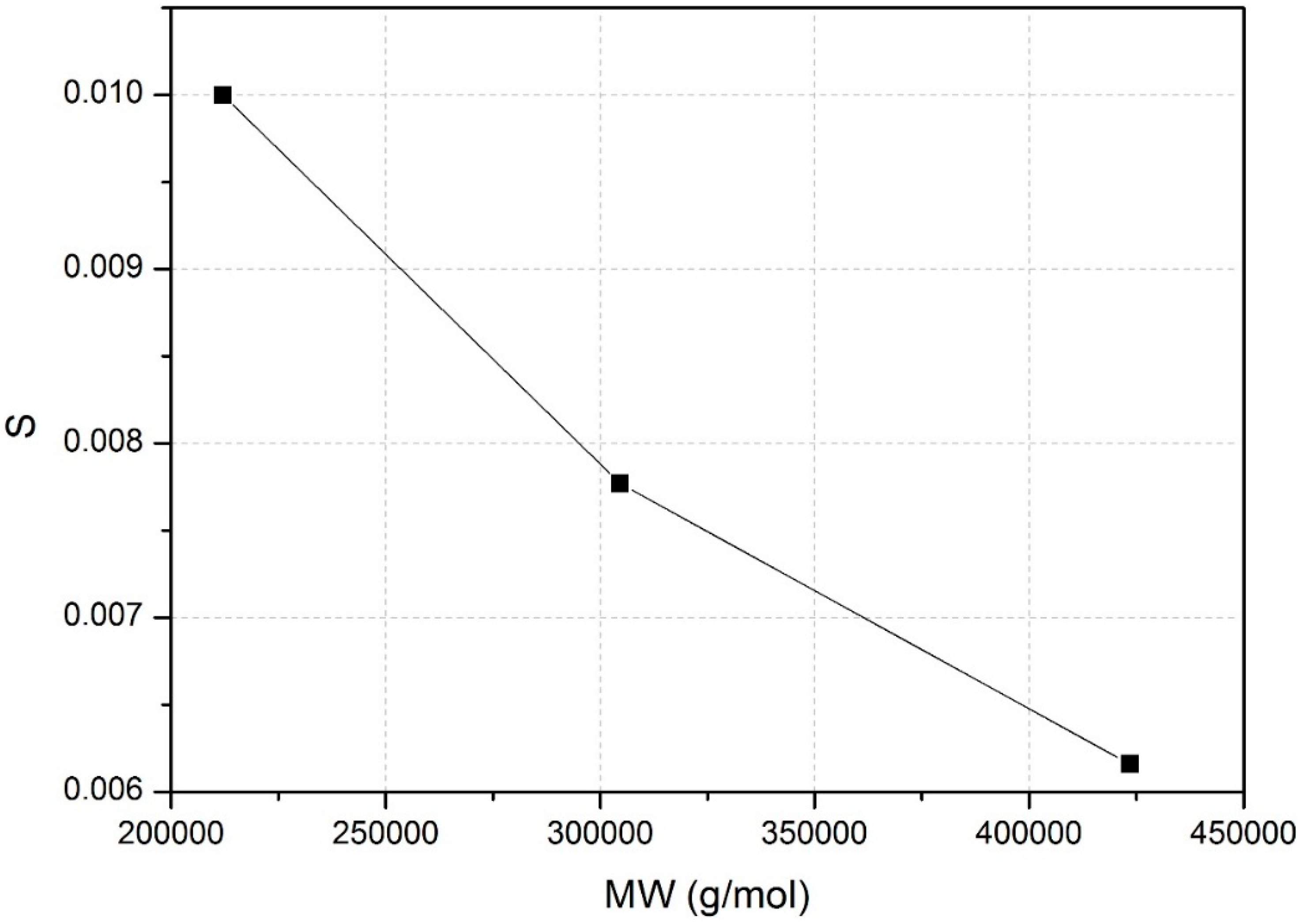
3.2. Adsorption Isotherm
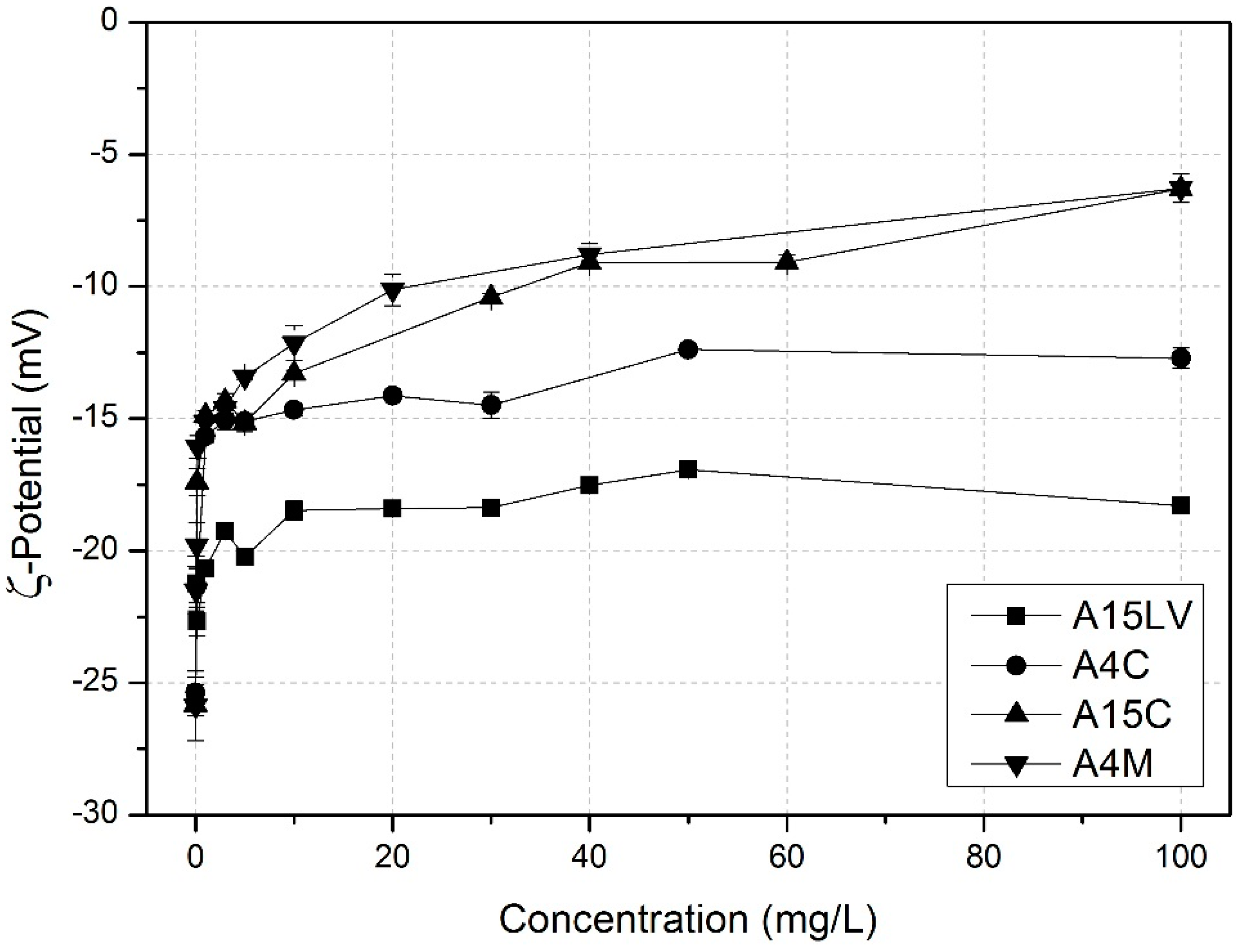
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dickinson, E. Milk protein interfacial layers and the relationship to emulsion stability and rheology. Colloids Surf. B Biointerfaces 2001, 20, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Rinaudo, M.; Quemeneur, F.; Pepin-Donat, B. Stabilization of liposomes by polyelectrolytes: Mechanism of interaction and role of experimental conditions. Macromol. Symp. 2009, 278, 67–79. [Google Scholar] [CrossRef]
- Quemeneur, F.; Rinaudo, M.; Maret, G.; Pepin-Donat, B. Decoration of lipid vesicles by polyelectrolytes: Mechanism and structure. Soft Matter 2010, 6, 4471–4481. [Google Scholar] [CrossRef]
- Lopez-Franco, Y.L.; Valdez, M.A.; Hernandez, J.; Calderon de la Barca, A.M.; Rinaudo, M.; Goycoolea, F.M. Macromolecular dimensions and mechanical properties of monolayer films of sonorean mesquite gum. Macromol. Biosci. 2004, 4, 865–874. [Google Scholar] [PubMed]
- Babak, V.G.; Auzely, R.; Rinaudo, M. Effect of electrolyte concentration on the dynamic surface tension and dilational viscoelasticity of adsorption layers of chitosan and dodecyl chitosan. J. Phys. Chem. B 2007, 111, 9519–9529. [Google Scholar] [CrossRef] [PubMed]
- Desbrieres, J.; Rinaudo, M.; Babak, V.; Vikhoreva, G. Surface activity of water soluble amphiphilic chitin derivatives. Polym. Bull. 1997, 39, 209–215. [Google Scholar] [CrossRef]
- Babak, V.; Lukina, I.; Vikhoreva, G.; Desbrieres, J.; Rinaudo, M. Interfacial properties of dynamic association between chitin derivatives and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 1999, 147, 139–148. [Google Scholar] [CrossRef]
- Hesselink, F.T.; Vrij, A.; Overbeek, J.T.G. Theory of stabilization of dispersions by absorbed macromolecules. II. Interactions between two flat particles. J. Phys. Chem. 1971, 75, 2094–2103. [Google Scholar]
- Park, Y.; Huang, R.; Corti, D.S.; Franses, E.I. Colloidal dispersion stability of unilamellar DPPC vesicle in aqueous electrolyte solutions and comparison to predictions of the DLVO theory. J. Colloid Interface Sci. 2010, 342, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.; Barta, A. Pharmacopeial cellulose ethers as oil-in-water emulsifiers. 1. Interfacial properties. Eur. J. Pharm. Biopharm. 1994, 40, 128–133. [Google Scholar]
- Sarkar, N. Structural interpretation of the interfacial properties of aqueous solutions of methylcellulose and hydroxypropylcellulose. Polymer 1984, 25, 481–486. [Google Scholar] [CrossRef]
- Gaonkar, A.G. Surface and interfacial activities and emulsion characteristics of some food hydrocolloids. Food Hydrocoll. 1991, 5, 329–337. [Google Scholar] [CrossRef]
- Arboleya, J.C.; Wilde, P.J. Competitive adsorption of proteins with methylcellulose and hydroxypropylmethylcellulose. Food Hydrocoll. 2005, 19, 485–491. [Google Scholar] [CrossRef]
- Stanley, D.W.; Goff, H.D.; Smith, A.K. Texture-structure relationships in foamed dairy emulsions. Food Res. Int. 1996, 29, 1–13. [Google Scholar] [CrossRef]
- Kato, T.; Yokoyama, M.; Takahashi, A. Melting temperatures of thermally reversible gels. IV. Methyl cellulose-water gels. Colloid Polym. Sci. 1978, 256, 15–21. [Google Scholar] [CrossRef]
- Hirrien, M.; Chevillard, C.; Descrières, J.; Axelos, M.A.V.; Rinaudo, M. Thermogelation of methylcelluloses: New evidence for understanding the gelation mechanism. Polym. J. 1998, 39, 6251–6259. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Influence of molar mass and concentration on the thermogelation of methylcelluloses. Int. J. Polym. Anal. Charact. 2014, in press. [Google Scholar]
- Funami, T.; Kataoka, Y.; Hiroe, M.; Asai, I.; Takahashi, R.; Nishinari, K. Thermal aggregation of methylcellulose with different molecular weights. Food Hydrocoll. 2007, 21, 46–58. [Google Scholar] [CrossRef]
- Ibbett, R.N.; Philp, K.; Price, D.M. 13C N.M.R. Studies of the thermal behavior of aqueous solutions of cellulose ethers. Polymer 1992, 33, 4087–4094. [Google Scholar] [CrossRef]
- Hirrien, M.; Desbrières, J.; Rinaudo, M. Physical properties of methylcelluloses in relation with the conditions for cellulose modification. Carbohydr. Polym. 1996, 31, 243–253. [Google Scholar]
- Hirrien, M. Comportement des Méthylcelluloses en Relation avec leur Structure. Ph.D. thesis, University Joseph Fourier, Grenoble, France, 19 December 1996. [Google Scholar]
- Cox, W.P.; Merz, E.H. Correlaion of dynamic and steady flow viscosities. J. Polym. Sci. 1958, 28, 619–622. [Google Scholar] [CrossRef]
- Nahringbauer, I. Dynamic surface tension of aqueous polymer solutions. I: Ethyl (hydroxyethyl) cellulose (BERMOCOLL cst-103). J. Colloid Interface Sci. 1995, 176, 318–328. [Google Scholar] [CrossRef]
- Varoqui, R. Effect of polymer adsorption on the electrophoretic mobility of colloids. New J. Chem. 1998, 6, 187–189. [Google Scholar]
- Ruta, B.; Czakkel, O.; Chusshkin, Y.; Pignon, F.; Zontone, F.; Rinaudo, M. Silica nanoparticles as tracers of the gelation dynamics of a natural biopolymer physical gel. Soft Matter 2014, 10, 4547–4554. [Google Scholar] [CrossRef] [PubMed]
- Hoeve, C.A.J.; DiMarzio, E.A.; Peyser, P. Adsorption of polymer molecules at low surface coverage. J. Chem. Phys. 1965, 42, 2558–2563. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Aqueous phase/nanoparticles interface: Hydroxypropyl cellulose adsorption and desorption triggered by temperature and inorganic salts. Soft Matter 2012, 8, 3627–3633. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, M.; Noik, C. Asorption of polysaccharides on a calcite using spin labeled polymers. Polym. Bull. 1983, 9, 543–547. [Google Scholar] [CrossRef]
- Salemis, P. Caractérisation des amidons. Application à l'étude du mécanisme de flottation des minerais. Thèse de 3ème Cycle, University Joseph Fourier, Grenoble, France, 1984. [Google Scholar]
- Alloul, H.; Roques-Carmes, T.; Hamieh, T.; Razafitianamaharavo, A.; Barres, O.; Toufaily, J.; Villiéras, F. Effect of chemical modification on surface free energy components of aerosil silica powders determined with capillary rise technique. Powder Technol. 2013, 246, 575–582. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Interfacial Properties of Methylcelluloses: The Influence of Molar Mass. Polymers 2014, 6, 2961-2973. https://doi.org/10.3390/polym6122961
Nasatto PL, Pignon F, Silveira JLM, Duarte MER, Noseda MD, Rinaudo M. Interfacial Properties of Methylcelluloses: The Influence of Molar Mass. Polymers. 2014; 6(12):2961-2973. https://doi.org/10.3390/polym6122961
Chicago/Turabian StyleNasatto, Pauline L., Frédéric Pignon, Joana L. M. Silveira, Maria Eugênia R. Duarte, Miguel D. Noseda, and Marguerite Rinaudo. 2014. "Interfacial Properties of Methylcelluloses: The Influence of Molar Mass" Polymers 6, no. 12: 2961-2973. https://doi.org/10.3390/polym6122961