Influence of Glutamic Acid on the Properties of Poly(xylitol glutamate sebacate) Bioelastomer
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PXGS
| Samples | Monomers ratio (xylitol/sebacic acid/Boc-l-glutamic, mol %) | Xylitol (mmol) | Sebacic acid (mmol) | Boc-l-glutamic (mmol) |
|---|---|---|---|---|
| PXS 1:1 | 100/100/0 | 10 | 10 | – |
| PXS 1:2 | 100/200/0 | 10 | 20 | – |
| PXS 2:5 | 100/250/0 | 10 | 20 | – |
| PXS 1:3 | 100/300/0 | 10 | 30 | – |
| PXGS 1:1-5% | 100/95/5 | 10 | 9.5 | 0.5 |
| PXGS 2:3-5% | 100/142.5/7.5 | 10 | 14.25 | 0.75 |
| PXGS 1:2-5% | 100/190/10 | 10 | 19 | 1 |
| PXGS 2:5-5% | 100/237.5/12.5 | 10 | 23.75 | 1.25 |
| PXGS 1:3-5% | 100/285/15 | 10 | 28.5 | 1.5 |
| PXGS 2:5-10% | 100/225/25 | 10 | 22.50 | 2.50 |
| PXGS 2:5-15% | 100/212.5/37.5 | 10 | 21.25 | 3.75 |

2.3. Characterization
2.4. Tensile Tests
2.5. In Vitro Degradation
3. Results and Discussion
3.1. Characterization of Chemical Structure


3.2. Thermal Properties

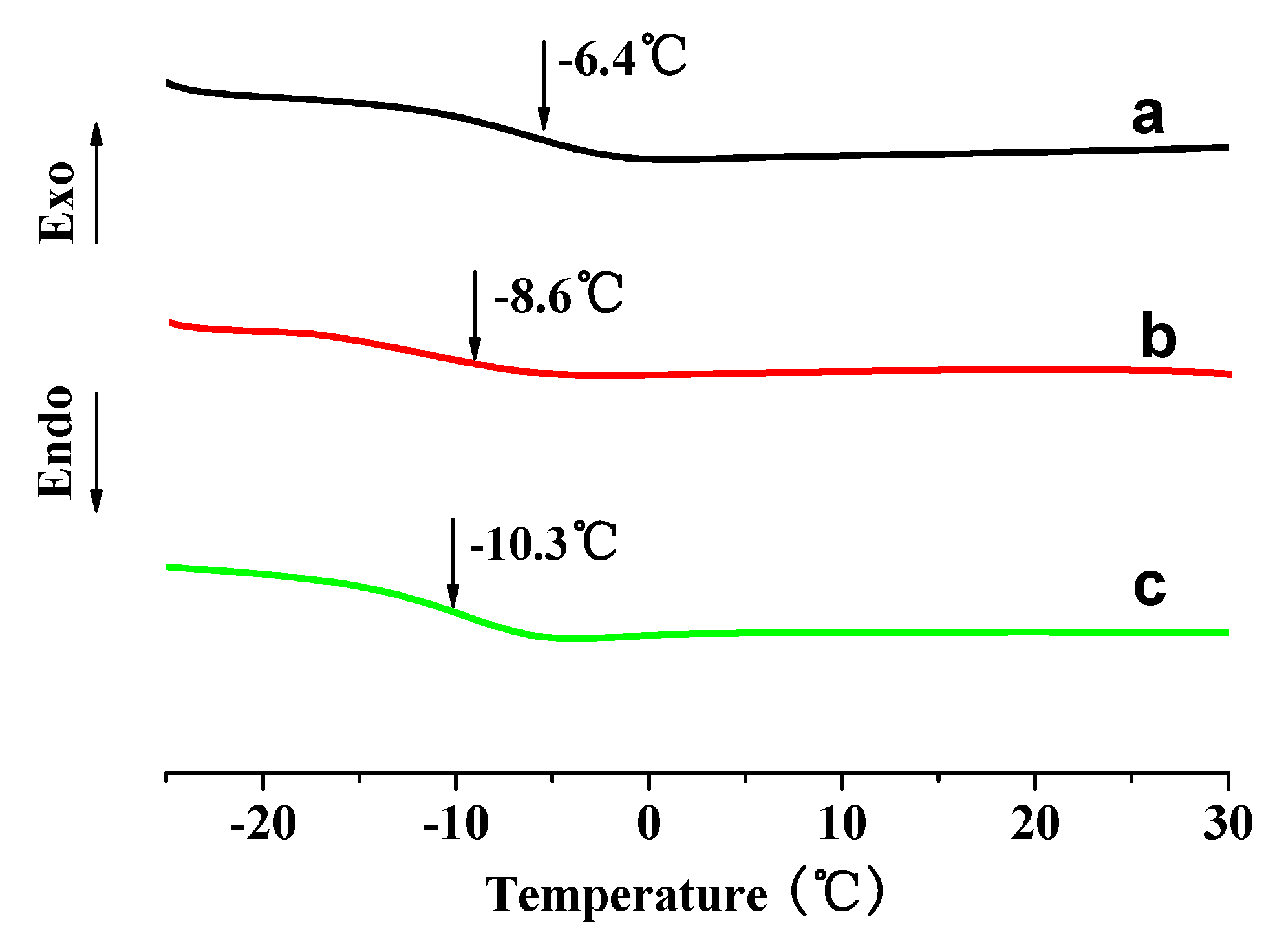
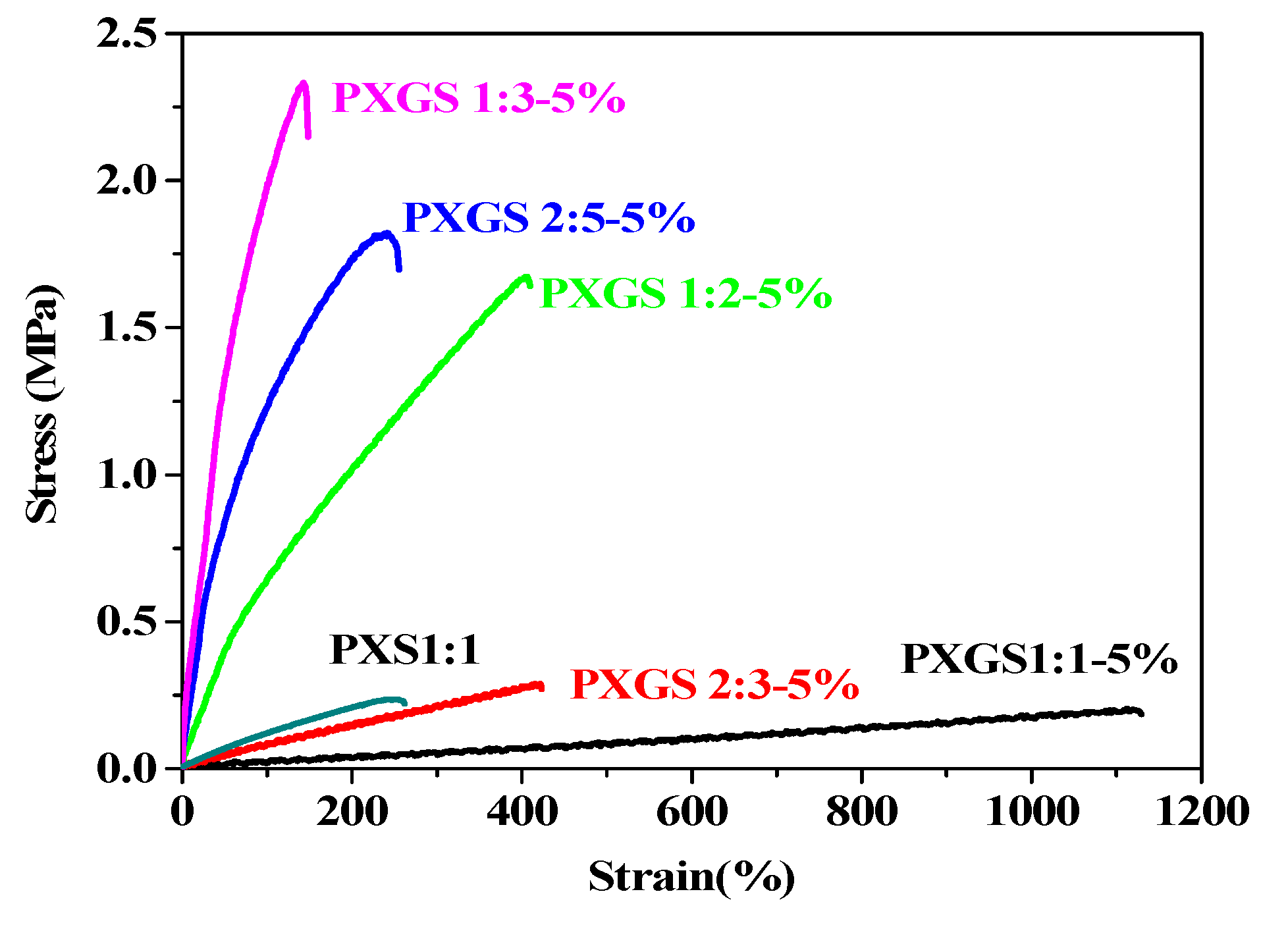
3.3. Mechanical Properties
| Samples | Tensile strength (MPa) | Elongations at break (%) | Tensile permanent deformation (%) | Water contact angle (°) | Gel content (%) |
|---|---|---|---|---|---|
| PXS 1:1 | 0.24 ± 0.18 | 251.7 ± 23.8 | – | 65.5 ± 4.5 | 84.0 ± 1.4 |
| PXS 1:2 | 0.94 ± 0.15 | 114.3 ± 20.0 | – | 81.5 ± 5.1 | 86.1 ± 2.7 |
| PXS 2:5 | 2.20 ± 0.14 | 136.6 ± 23.2 | – | 87.6 ± 7.3 | 89.6 ± 2.2 |
| PXS 1:3 | 2.45 ± 0.20 | 82.0 ± 14.1 | – | 72.7 ± 6.9 | 91.0 ± 5.7 |
| PXGS 1:1-5% | 0.17 ± 0.03 | 971.8 ± 27.8 | 21 | 48.9 ± 5.4 | 77.5 ± 1.1 |
| PXGS 2:3-5% | 0.29 ± 0.04 | 462.3 ± 25.0 | 12 | 53.0 ± 3.5 | 78.8 ± 2.0 |
| PXGS 1:2-5% | 1.64 ± 0.11 | 409.9 ± 38.8 | 4 | 61.5 ± 3.0 | 81.4 ± 6.4 |
| PXGS 2:5-5% | 1.79 ± 0.19 | 226.0 ± 22.2 | 3 | 69.6 ± 7.8 | 86.2 ± 1.7 |
| PXGS 1:3-5% | 2.35 ± 0.18 | 148.6 ± 30.5 | 5 | 63.9 ± 4.6 | 84.5 ± 2.2 |
| PXGS 2:5-10% | 2.67 ± 0.14 | 349.6 ± 25.7 | 2 | 56.5 ± 6.0 | 75.5 ± 1.1 |
| PXGS 2:5-15% | 2.02 ± 0.15 | 471.6 ± 30.1 | 3 | 57.0 ± 4.7 | 61.0 ± 2.1 |

3.4. Hydrophilicity

3.5. In Vitro Degradation

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Allen, J.; Khan, S. Characterization of porcine circulating progenitor cells: Toward a functional endothelium. Tissue Eng. A 2008, 14, 183–191. [Google Scholar]
- Alperin, C.; Zandstra, P.W. Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials 2005, 26, 7377–7386. [Google Scholar] [CrossRef]
- Nagata, M.; Kanechika, M. Biodegradable network elastomeric polyesters from multifunctional aromatic carboxylic acids and poly(ε-caprolactone) diols. J. Polym. Sci. A Polym. Chem. 2002, 40, 4523–4529. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, J. A new biodegradable polyester elastomer for cartilage tissue engineering. J. Biomed. Mater. Res. 2006, 77, 331–339. [Google Scholar] [CrossRef]
- Puskas, J.E.; Chen, Y.H. Biomedical application of commercial polymers and novel polyisobutylene-based thermoplastic elastomers for soft tissue replacement. Biomacromolecules 2004, 5, 1141–1154. [Google Scholar] [CrossRef]
- Mihye, K.; Bohee, H. Composite system of PLCL scaffold and heparin-based hydrogel for regeneration of partial-thickness cartilage defects. Biomacromolecules 2012, 13, 2287–2298. [Google Scholar] [CrossRef]
- Sundback, C.A.; Shyu, J.Y. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5465. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Impallomeni, G. Evidence for selective hydrolysis of aliphatic copolyesters induced by lipase catalysis. Biomacromolecules 2004, 5, 433–444. [Google Scholar] [CrossRef]
- Lindstrom, A.; Albertsson, A.C. Quantitative determination of degradation products, an effective means to study early stages of egradation in linear and branched poly(butylene adipate) and poly(butylene succinate). Polym. Degrad. Stab. 2004, 83, 487–493. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, Y.M.; Langer, R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. A 2003, 66, 192–197. [Google Scholar] [CrossRef]
- Zhao, W.; Xing, Z. Synthesis and characterization of novel soybean-oil-based elastomers with favorable processability and tunable properties. Macromolecules 2012, 45, 9010–9019. [Google Scholar] [CrossRef]
- Lei, L.J.; Ding, T. Synthesis, characterization and in vitro degradation of a novel degradable poly[(1,2-propanediol-sebacate)-citrate] bioelastomer. Polym. Degrad. Stab. 2007, 92, 389–396. [Google Scholar] [CrossRef]
- Guo, B.C.; Chen, Y.W. Biobased poly(propylene sebacate) as shape memory polymer with tunable switching temperature for potential biomedical applications. Biomacromolecules 2011, 12, 1312–1321. [Google Scholar] [CrossRef]
- Bruggeman, J.P.; Bettinger, C.J. Biodegradable xylitol-based polymers. Adv. Mater. 2008, 20, 1922–1927. [Google Scholar] [CrossRef]
- Joost, P.; Bruggeman, C.J. Biodegradable xylitol-based elastomers: In vivo behavior and biocompatibility. J. Biomed. Mater. Res. A 2010, 1, 92–104. [Google Scholar]
- Ellingsworth, L.R.; DeLustro, F. The human immune response to reconstituted bovine collagen. J. Immunol. 1986, 136, 877–882. [Google Scholar]
- Lupton, J.R.; Alster, T.S. Cutaneous hypersensitivity reaction to injectable hyaluronic acid gel. Dermatol. Surg. 2000, 26, 135–137. [Google Scholar] [CrossRef]
- Borschel, G.H.; Kia, K.F. Mechanical properties of acellular peripheral nerve. J. Surg. Res. 2003, 114, 133–139. [Google Scholar] [CrossRef]
- Clerin, V.; Nichol, J.W. Tissue engineering of arteries by directed remodeling of intact arterial segments. Tissue Eng. 2003, 9, 461–472. [Google Scholar] [CrossRef]
- Hjortdal, J.O. Regional elastic performance of the human cornea. J. Biomech. 1996, 29, 931–934. [Google Scholar] [CrossRef]
- Kyle, S.; Aggeli, A. Production of self-assembling biomaterials for tissue engineering. Trends Biotechnol. 2009, 27, 423–433. [Google Scholar] [CrossRef]
- Chun, L. Poly(l-glutamic acid)-anticancer drug conjugates. Adv. Drug Deliv. Rev. 2002, 54, 695–713. [Google Scholar] [CrossRef]
- Markland, P.; Amidon, G.L. Modified polypeptides containing γ-benzyl glutamic acid as drug delivery platforms. Int. J. Pharm. 1999, 178, 183–192. [Google Scholar] [CrossRef]
- Cao, B.; Yin, J.B. Porous scaffolds based on cross-linking of poly(l-glutamic acid). Macromol. Biosci. 2011, 11, 427–434. [Google Scholar] [CrossRef]
- John, A.W.; Lovina, J.F. Solid-phase syntheses of peptoids using fmoc-protected N-substituted glycines: The synthesis of (retro)peptoids of leu-enkephalin and substance P. Chem. Eur. J. 1998, 4, 1570–1580. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dong, W.; Li, T.; Xiang, S.; Ma, P.; Chen, M. Influence of Glutamic Acid on the Properties of Poly(xylitol glutamate sebacate) Bioelastomer. Polymers 2013, 5, 1339-1351. https://doi.org/10.3390/polym5041339
Dong W, Li T, Xiang S, Ma P, Chen M. Influence of Glutamic Acid on the Properties of Poly(xylitol glutamate sebacate) Bioelastomer. Polymers. 2013; 5(4):1339-1351. https://doi.org/10.3390/polym5041339
Chicago/Turabian StyleDong, Weifu, Ting Li, Shuangfei Xiang, Piming Ma, and Mingqing Chen. 2013. "Influence of Glutamic Acid on the Properties of Poly(xylitol glutamate sebacate) Bioelastomer" Polymers 5, no. 4: 1339-1351. https://doi.org/10.3390/polym5041339
APA StyleDong, W., Li, T., Xiang, S., Ma, P., & Chen, M. (2013). Influence of Glutamic Acid on the Properties of Poly(xylitol glutamate sebacate) Bioelastomer. Polymers, 5(4), 1339-1351. https://doi.org/10.3390/polym5041339




