Ion Permeability of Free-Suspended Layer-by-Layer (LbL) Films Prepared Using an Alginate Scaffold
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of LbL Films
2.3. Absorption Spectra of LbL Films
2.4. Ion Permeation

3. Results and Discussion
3.1. Preparation of Free-Suspended LbL Film
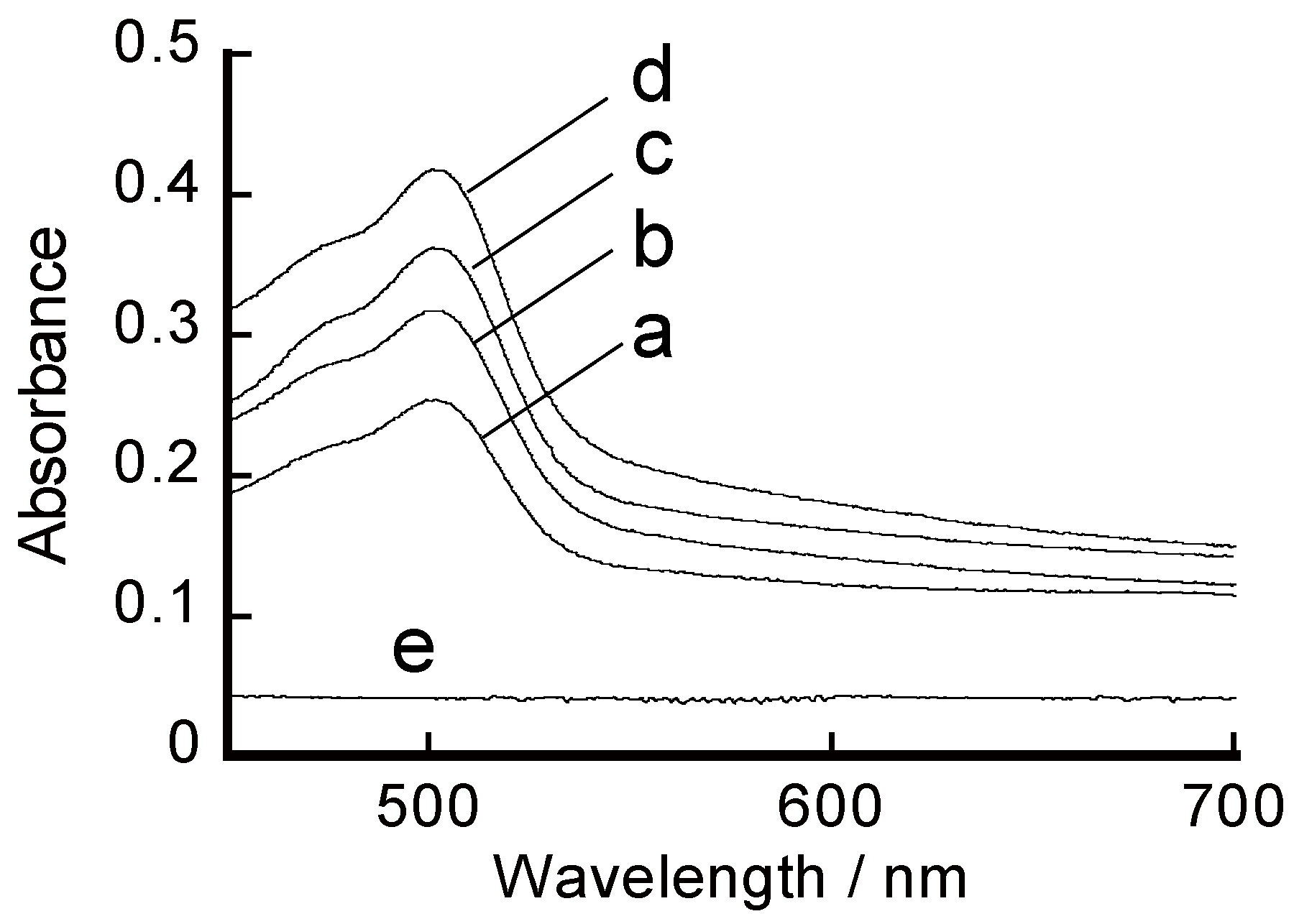
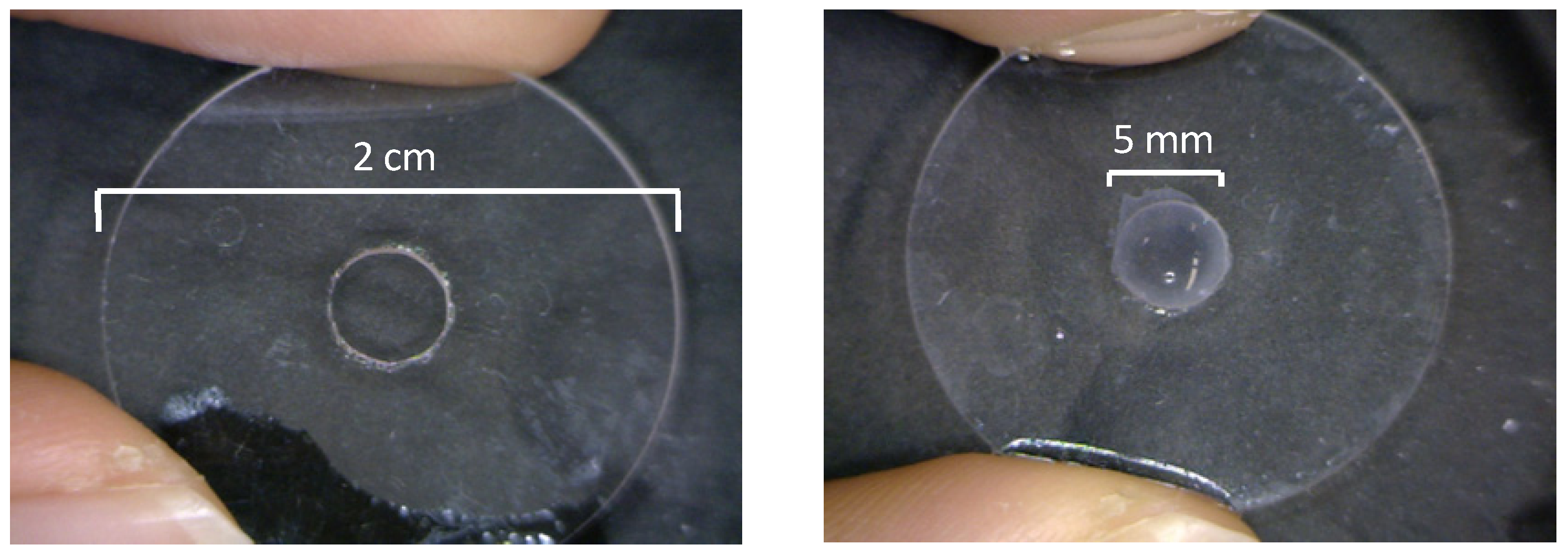
3.2. Ion Permeation


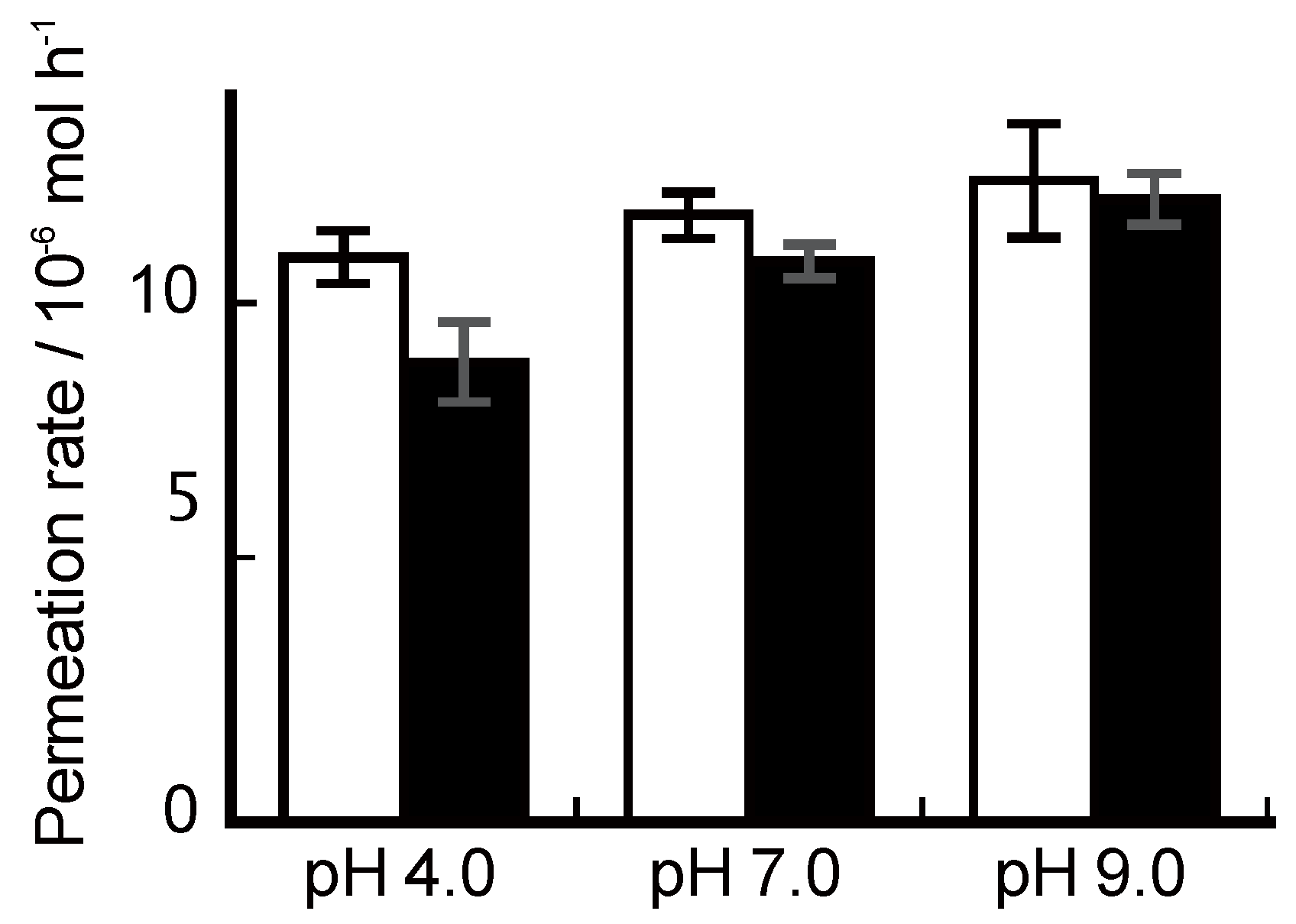

4. Conclusions
Acknowledgments
Conflict of Interest
References
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar]
- Sukhishvili, S.A. Responsive polymer films and capsules via layer-by-layer assembly. Curr. Opin. Colloid Interface Sci. 2005, 10, 37–44. [Google Scholar]
- Tang, Z.; Wang, Y.; Podasiadlo, P.; Kotov, N.A. Biomedical applications of layer-by-layer assemblies: From biomimetics to tissue engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar]
- Marchenko, I.; Yashchenok, A.; German, S.; Inozemtseva, O.; Dorin, D.; Bukreeva, T.; Möhwald, H.; Skirtach, A. Polyelectrolytes: Influence on evaporative self-assembly of particles and assembly of multilayers with polymers, nanoparticles and carbon nanotubes. Polymers 2010, 2, 690–708. [Google Scholar]
- Iost, R.M.; Crespilho, F.N. Layer-by-layer self-assembly and electrochemistry: Applications in biosensing and bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar]
- Tomita, S.; Sato, K.; Anzai, J. Layer-by-layer assembled thin films composed of carboxyl-terminated poly(amidoamine) dendrimers as a pH-sensitive nano-device. J. Colloid Interface Sci. 2008, 326, 35–40. [Google Scholar]
- Dai, Z.; Wilson, J.T.; Chaikof, E.L. Construction of pegylated multilayer architectures via (strept)avidin/biotin interactions. Mater. Sci. Eng. C 2007, 27, 402–408. [Google Scholar]
- Hoshi, T.; Akase, S.; Anzai, J. Preparation of multilayer thin films containing avidin through sugar-lectin interactions and their binding properties. Langmuir 2002, 18, 7024–7028. [Google Scholar]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar]
- Wang, B.; Anzai, J. Redox reactions of ferricyanide ions in layer-by-layer deposited polysaccharide films: A significant effect of the type of polycation in the films. Langmuir 2007, 23, 7378–7384. [Google Scholar]
- Lynn, D.M. Peeling back the layers: Controlled erosion and triggered disassembly of multilayered polyelectrolyte thin films. Adv. Mater. 2007, 19, 4118–4230. [Google Scholar]
- Liu, L.; Chen, Z.; Yang, S.; Jin, X.; Lin, X. A novel inhibition biosensor constructed by layer-by-layer technique based on biospecific affinity for the determination of sulfide. Sens. Actuators B 2008, 129, 218–224. [Google Scholar]
- Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer construction of protein architectures through avidin-biotin and lectin-sugar interactions for biosensor applications. Anal. Bioanal. Chem. 2012, 402, 1749–1758. [Google Scholar] [CrossRef]
- Sato, K.; Kodama, D.; Naka, Y.; Anzai, J. Electrochemically induced disintegration of layer-by-layer-assembled thin films composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules 2006, 7, 3302–3305. [Google Scholar]
- Inoue, H.; Anzai, J. Stimuli-sensitive thin films prepared by a layer-by-layer deposition of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Langmuir 2005, 21, 8354–8359. [Google Scholar] [CrossRef]
- Wood, K.C.; Zacharie, N.S.; Schmidt, D.J.; Wrightman, S.N.; Andaya, B.J.; Hammond, P.T. Electroactive controlled release thin films. Proc. Natl. Acad. Sci. USA 2008, 105, 2280–2285. [Google Scholar]
- Esser-Kahn, A.P.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Triggered release from polymer capsules. Macromolecules 2011, 44, 5539–5553. [Google Scholar]
- Liu, A.; Anzai, J. Ferrocene-containing polyelectrolyte multilayer films: Effects of electrochemically inactive surface layers on the redox properties. Langmuir 2003, 19, 4043–4046. [Google Scholar]
- Boulmedais, F.; Tang, C.S.; Keller, B.; Vörös, J. Controlled electrodissolution of polyelectrolyte multilayers: A platform technology toward the surface-initiated delivery of drugs. Adv. Funct. Mater. 2006, 16, 63–70. [Google Scholar] [CrossRef]
- Lutkenhaus, J.L.; Hammond, P.T. Electrochemically enabled polyelectrolyte multilayer devices: From fuel cells to sensors. Soft Matter 2007, 3, 804–816. [Google Scholar]
- Haiyun, L.; Hu, N. Salt-induced swelling and electrochemical property change of hyaluronic acid/myoglobin multilayer films. J. Phys. Chem. 2007, 111, 1984–1993. [Google Scholar]
- Egawa, Y.; Seki, T.; Takahashi, S.; Anzai, J. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater. Sci. Eng. C 2011, 31, 1257–1264. [Google Scholar]
- Nolan, C.M.; Serpe, M.J.; Lyon, L.A. Thermally modulated insulin release from microgel thin films. Biomacromolecules 2004, 5, 1940–1946. [Google Scholar]
- Yoshida, K.; Sato, K.; Anzai, J. Layer-by-layer polyelectrolyte films containing insulin for pH- triggered release. J. Mater. Chem. 2010, 20, 1546–1552. [Google Scholar]
- Becker, A.L.; Johnston, A.P.R.; Caruso, F. Layer-by-layer assembled capsules and films for therapeutic delivery. Small 2010, 6, 1836–1852. [Google Scholar]
- Sato, K.; Yoshida, K.; Takahashi, S.; Anzai, J. pH- and sugar-sensitive layer-by-layer films and microcapsules for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 809–821. [Google Scholar]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar]
- Skirtach, A.G.; Karageorgiev, P.; Bédard, M.F.; Sukhorukov, G.B.; Möhwald, H. Reversibly permeable nanomembranes of polymeric microcapsules. J. Am. Chem. Soc. 2008, 130, 11572–11573. [Google Scholar]
- Farhat, T.R.; Schlenoff, J.B. Ion transport and equilibria in polyelectrolyte multilayers. Langmuir 2001, 17, 1184–1192. [Google Scholar]
- Stoeve, P.; Vasquez, V.; Coelho, M.A.N.; Rabolt, J.F. Gas transfer in supported films made by molecular self-assembly of ionic polymers. Thin Solid Films 1996, 284, 708–712. [Google Scholar]
- Kumar, S.K.; Hong, J.D. Photoresponsive ion gating function of an azobenzene polyelectrolyte multilayer spin-self-assembled on a nanoporous support. Langmuir 2008, 24, 4190–4193. [Google Scholar]
- Kösters, J.; Schönhoff, M.; Stolwijk, N.A. Ion transport effects in a solid electrolyte due to salt substitution and addition using an ion liquid. J. Phys. Chem. B 2013, 117, 2527–2534. [Google Scholar]
- Harris, J.J.; Stair, J.L.; Bruening, M.L. Layered polyelectrolyte films as selective, ultrathin barriers for anion transport. Chem. Mater. 2000, 12, 1941–1946. [Google Scholar]
- Krasemann, L.; Tieke, B. Selective ion transport across self-assembled alternating multilayers of cationic and anionic polyelectrolytes. Langmuir 2000, 16, 287–290. [Google Scholar] [CrossRef]
- Toutianoush, A.; Tieke, B. Selective transport and incorporation of highly charged metal and metal complex ions in self-assembled polyelectrolyte multilayer membranes. Mater. Sci. Eng. C 2002, 22, 135–139. [Google Scholar]
- Ono, S.S.; Decher, G. Preparation of ultrathin self-standing polyelectrolyte multilayer membranes at physiological conditions using pH-responsive film segments as sacrificial layers. Nano Lett. 2006, 6, 592–598. [Google Scholar]
- Park, J.; Kim, J.; Lee, S.; Bang, J.; Kim, B.J.; Kim, Y.S.; Cho, J. Free-standing film electronics using photo-crosslinking layer-by-layer assembly. J. Mater. Chem. 2009, 19, 4488–4490. [Google Scholar]
- Sato, K.; Shiba, T.; Anzai, J. Preparation of free-suspended polyelectrolyte multilayer films using an alginate scaffold and their ion permeability. Mater. Sci. Eng. C 2012, 32, 2649–2653. [Google Scholar]
- Zhu, H.; Srivastava, R.; McShane, M.J. Spontaneous loading of positively charged macromolecules into alginate-templated polyelectrolyte microcapsules. Biomacromolecules 2005, 6, 2221–2228. [Google Scholar]
- Dressick, W.J.; Wahl, K.J.; Bassim, N.D.; Stroud, R.M. Divalent-anion salt effects in polyelectrolyte multilayer depositions. Langmuir 2012, 28, 15831–15843. [Google Scholar]
- Pau, P.C.F.; Berg, J.O.; McMillan, W.G. Application of Stokes’ law to ions in aqueous solution. J. Phys. Chem. 1990, 94, 2671–2679. [Google Scholar]
- Jin, W.; Toutianoush, A.; Pyrasch, M.; Schnepf, J.; Gottschalk, H.; Rammensee, W.; Tieke, B. Self-assembled films of Prussian Blue and analogues: Structure and Morphology, elemental composition, film growth, and nanosieving of ions. J. Phys. Chem. 2003, 107, 12062–12070. [Google Scholar]
- Tieke, B.; El-Hashani, A.; Toutianoush, A.; Fendt, A. Multilayered films based on macrocyclic polyamines, calixarenes and cyclodextrins and transport properties of the corresponding membranes. Thin Solid Films 2008, 516, 8814–8820. [Google Scholar]
- El-Hashani, A.; Toutianoush, A.; Tieke, B. Layer-by-layer assembled membranes of protonated 18-azacrown-6 and polyvinylsulfonate and their application for highly efficient anion separation. J. Phys. Chem. B 2007, 111, 8582–8588. [Google Scholar] [CrossRef]
- Mauser, T.; Déjugnat, C.; Sukhorukov, G.B. Reversible pH-dependent properties of multilayer microcapsules made of weak polyelectrolytes. Macromol. Rapid Commun. 2004, 25, 1781–1785. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sato, K.; Shiba, T.; Anzai, J.-i. Ion Permeability of Free-Suspended Layer-by-Layer (LbL) Films Prepared Using an Alginate Scaffold. Polymers 2013, 5, 696-705. https://doi.org/10.3390/polym5020696
Sato K, Shiba T, Anzai J-i. Ion Permeability of Free-Suspended Layer-by-Layer (LbL) Films Prepared Using an Alginate Scaffold. Polymers. 2013; 5(2):696-705. https://doi.org/10.3390/polym5020696
Chicago/Turabian StyleSato, Katsuhiko, Takuto Shiba, and Jun-ichi Anzai. 2013. "Ion Permeability of Free-Suspended Layer-by-Layer (LbL) Films Prepared Using an Alginate Scaffold" Polymers 5, no. 2: 696-705. https://doi.org/10.3390/polym5020696




