Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells
Abstract
:1. Introduction
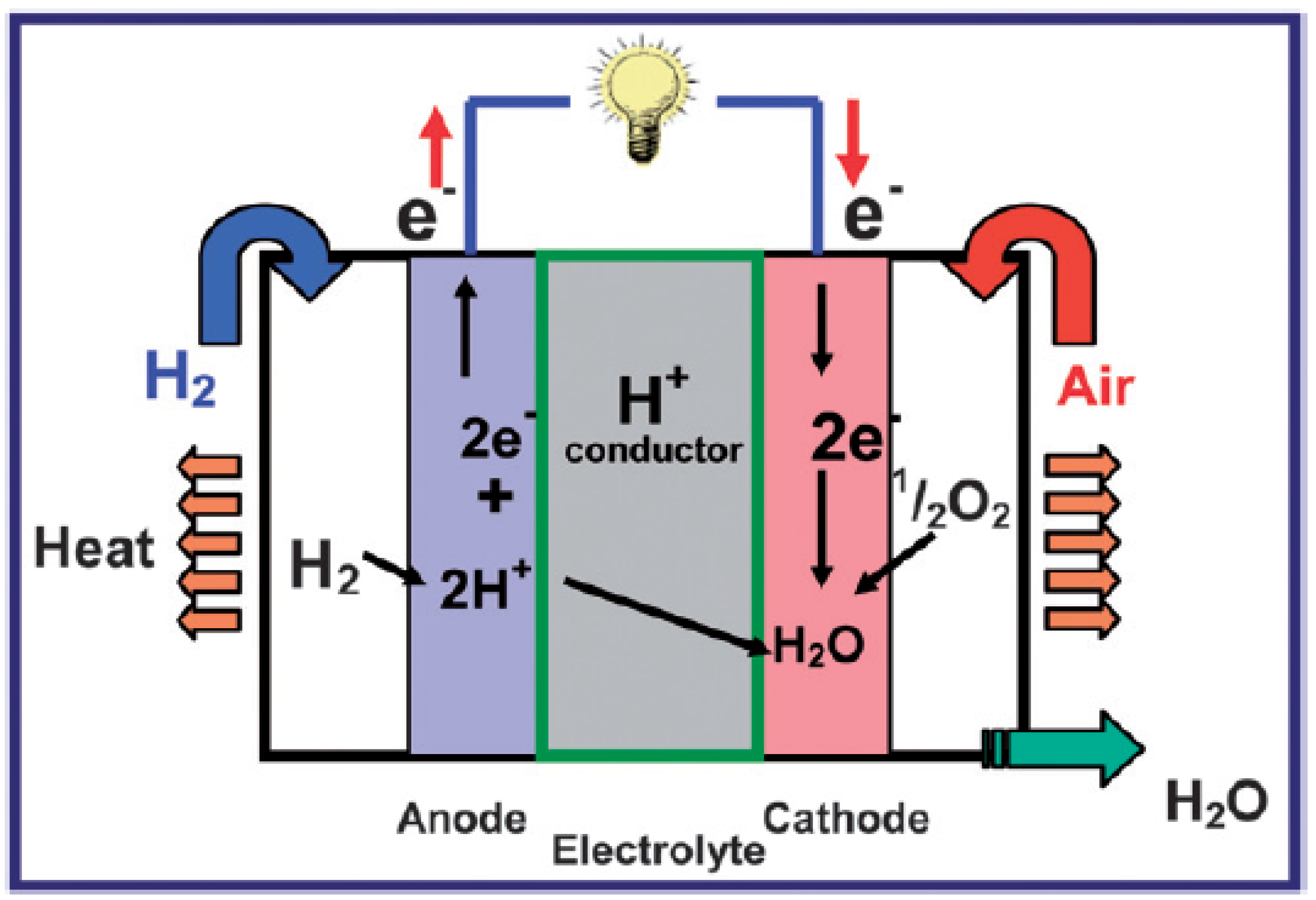
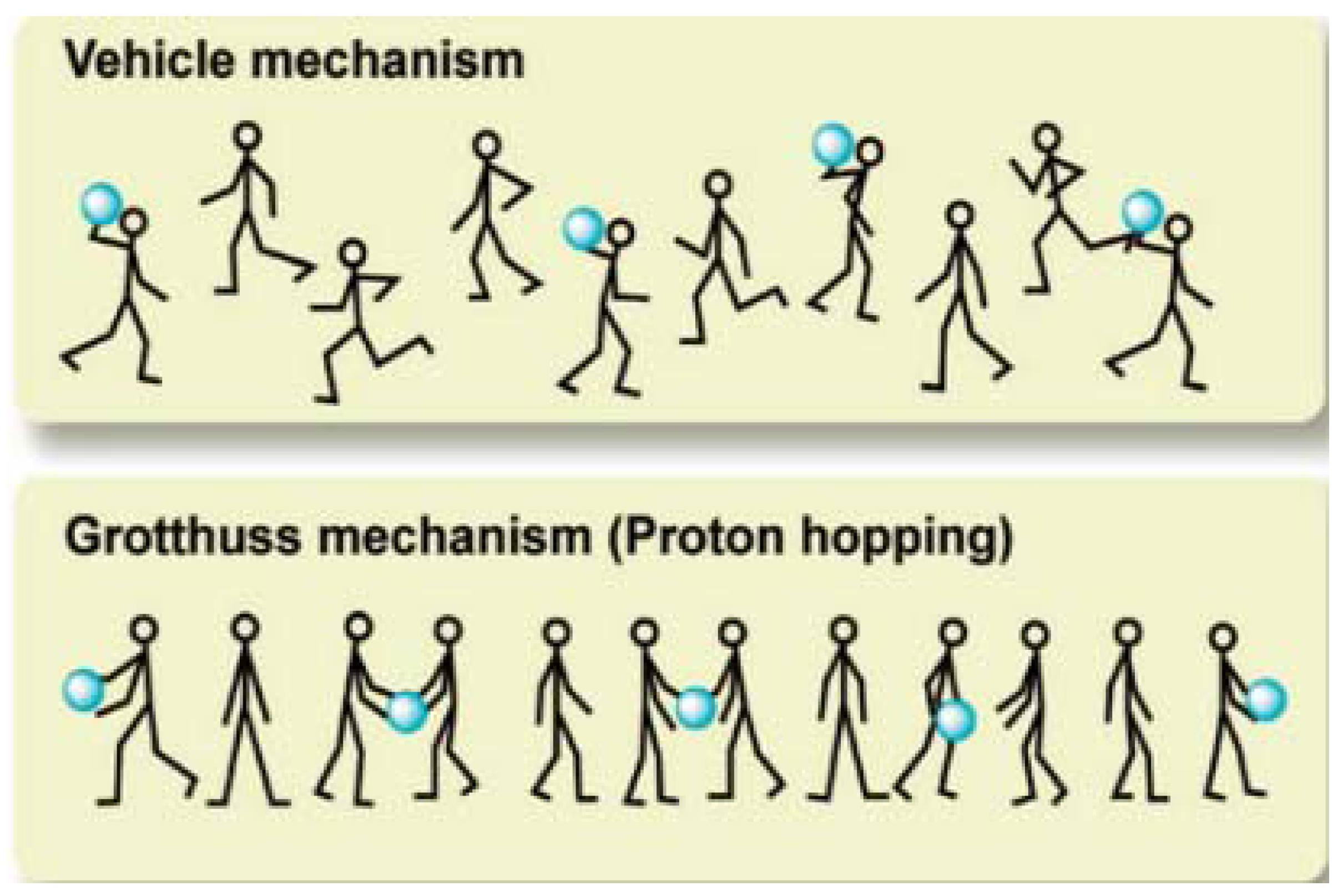
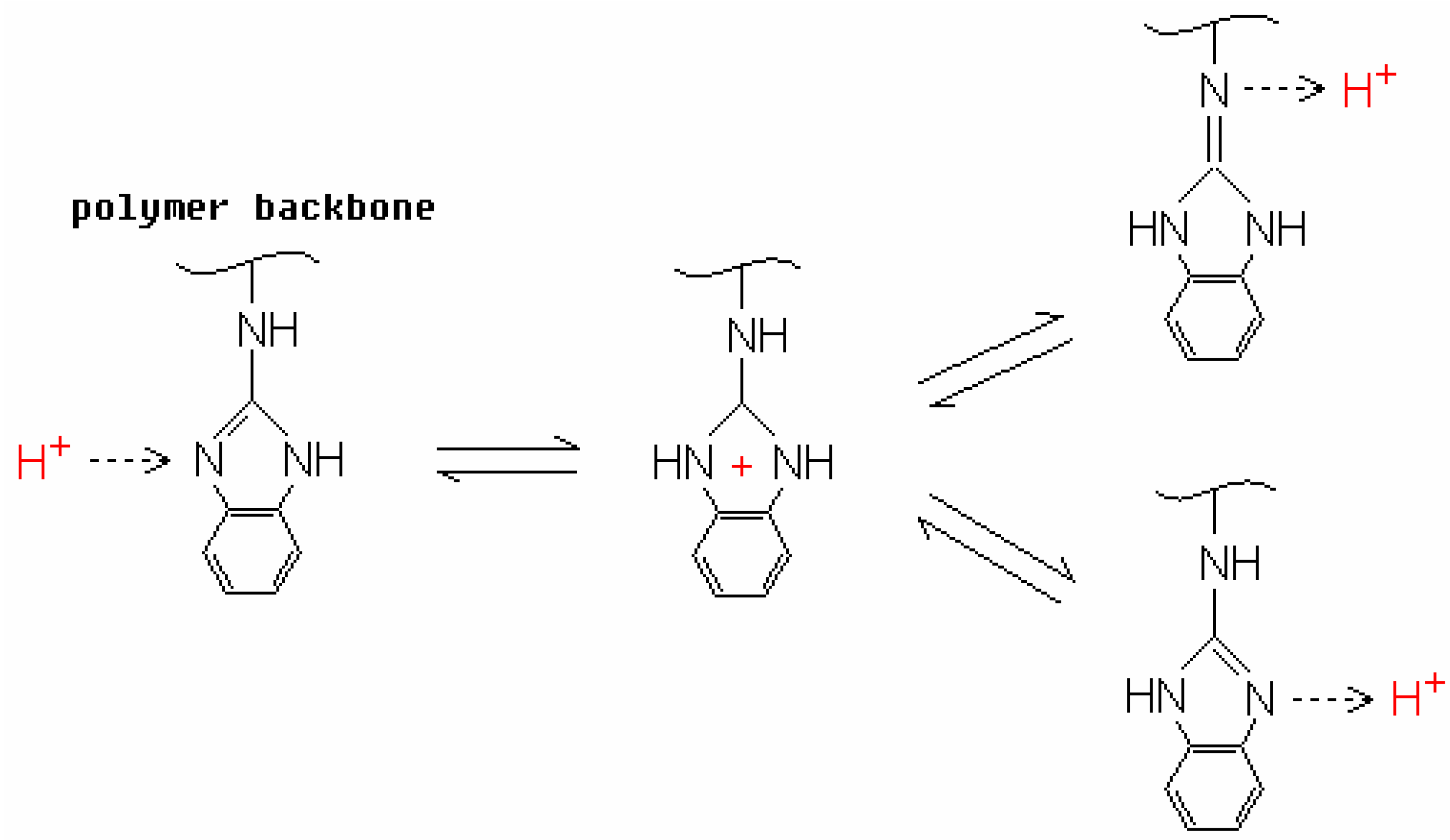
2. Proton-Conducting Mechanisms
3. Acid-Base Membranes for High Temperature PEMFCs
3.1. Nafion-Based Acid-Base Membranes
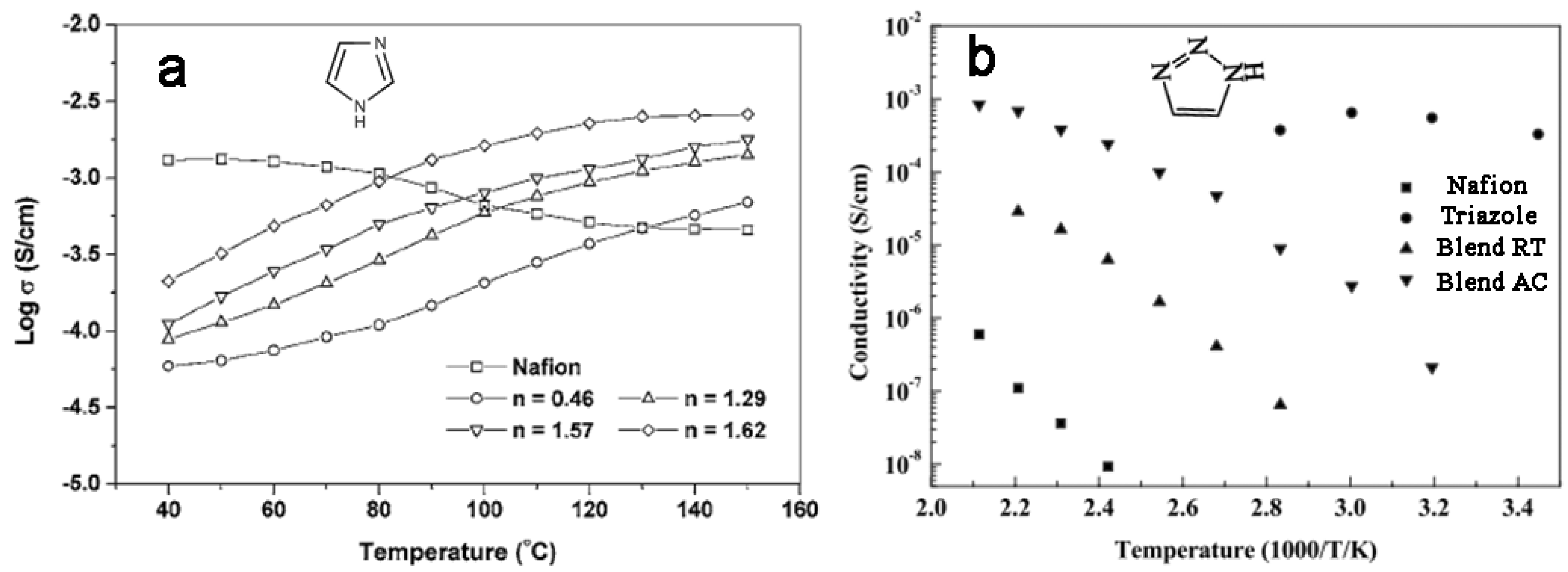
3.2. SPEEK-Based Acid-Base Membranes
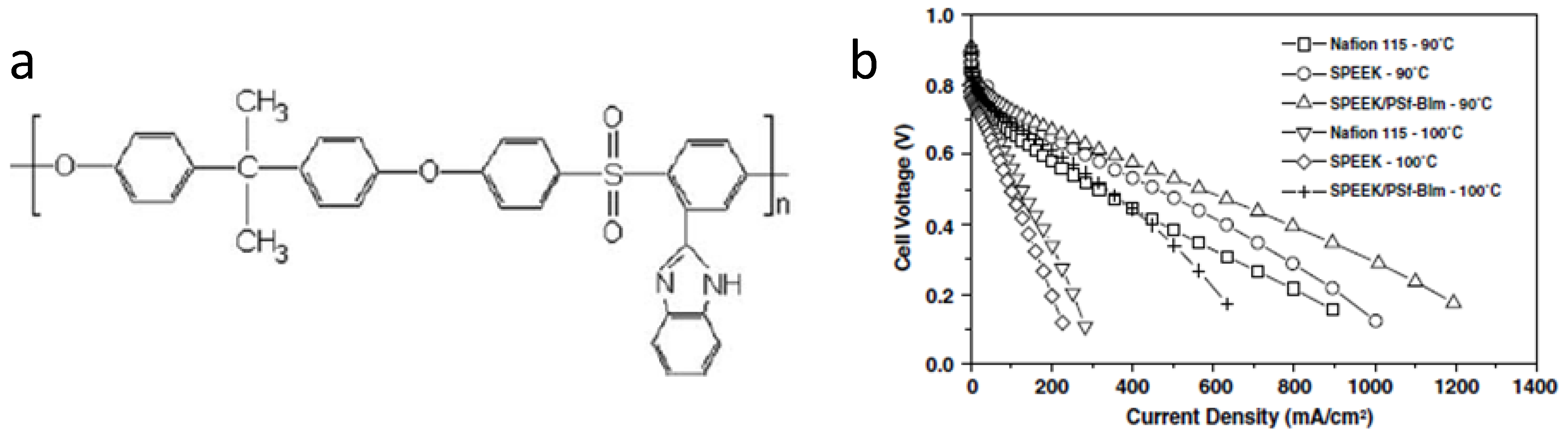
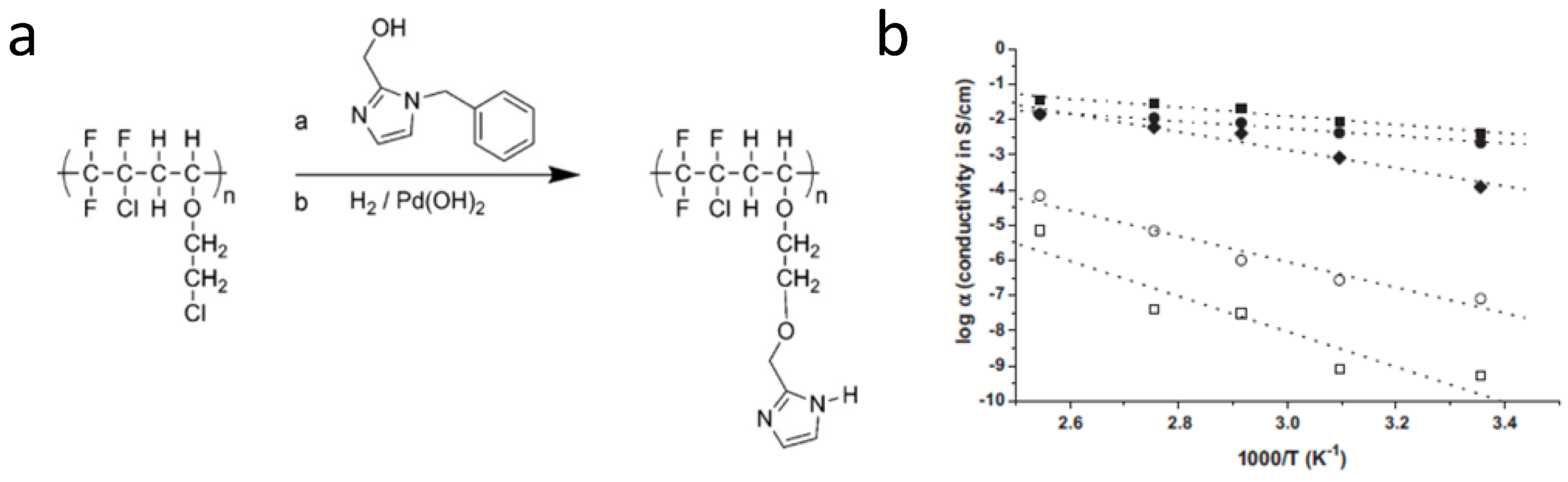
3.3. Polybenzimidazole-Based Acid-Base Membranes

4. Acid-Base Membranes for DMFCs
4.1. Nitrogen-Containing Small Basic Molecules
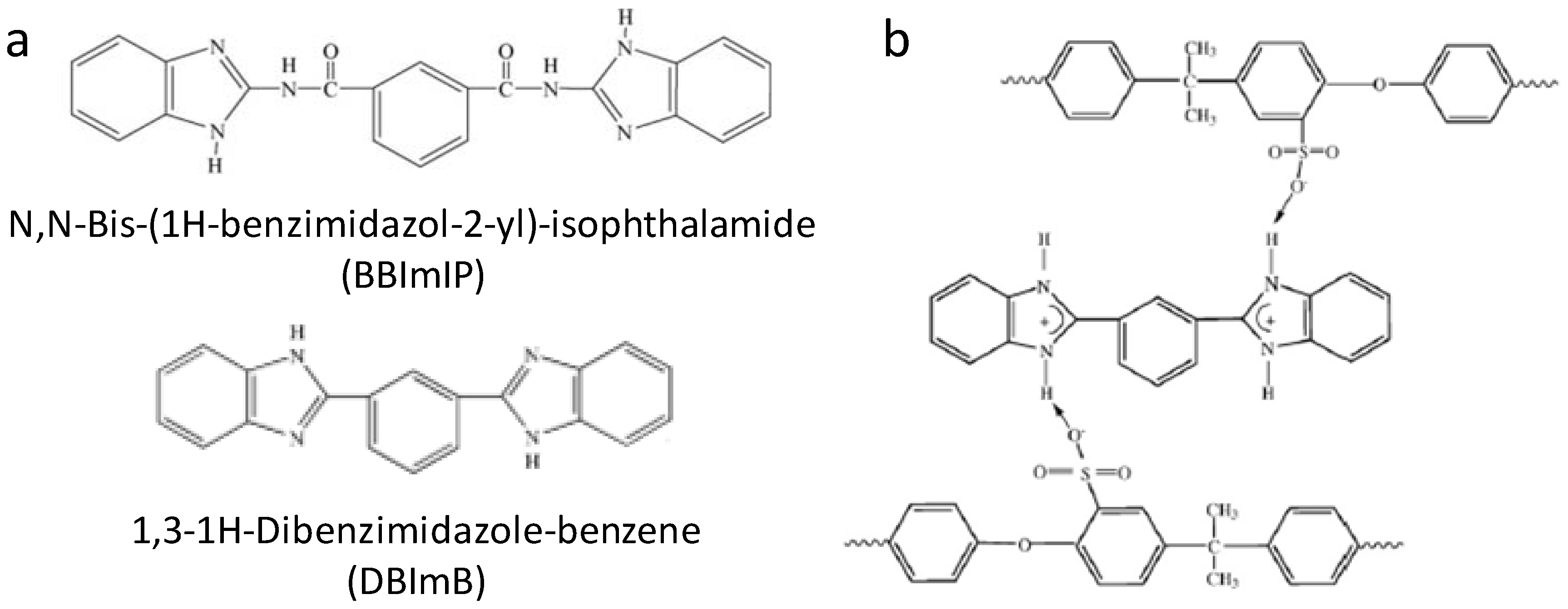
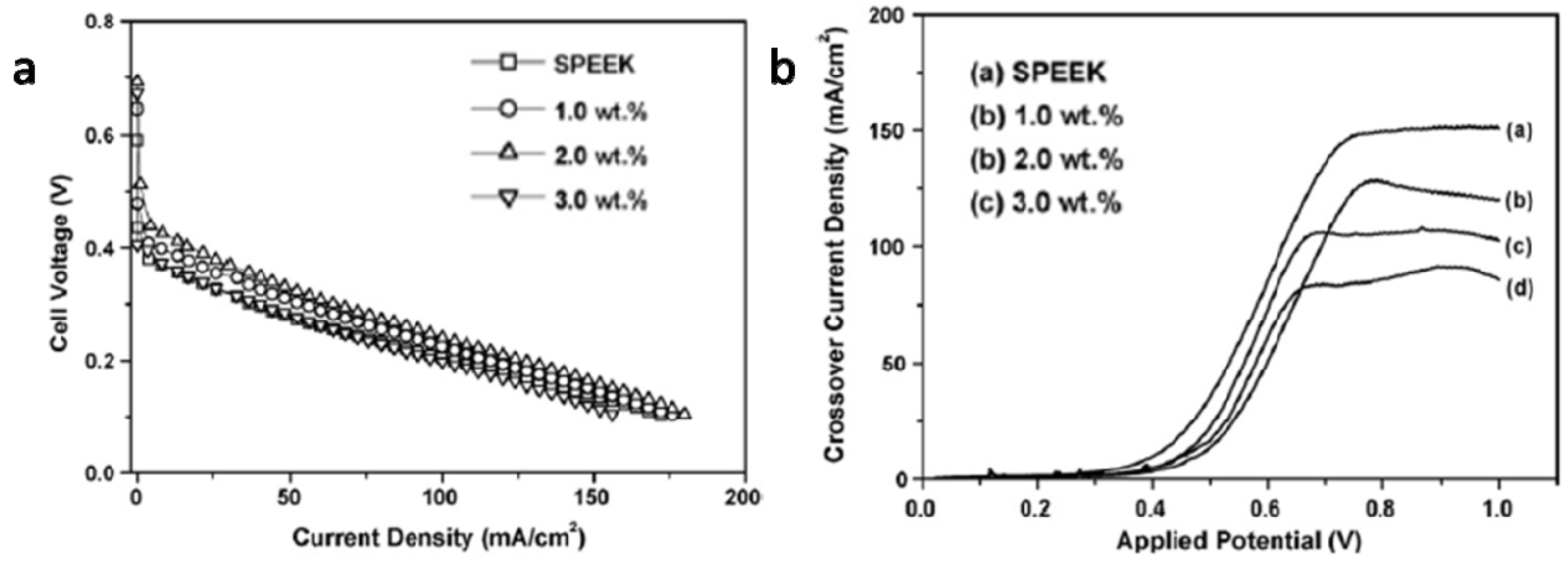
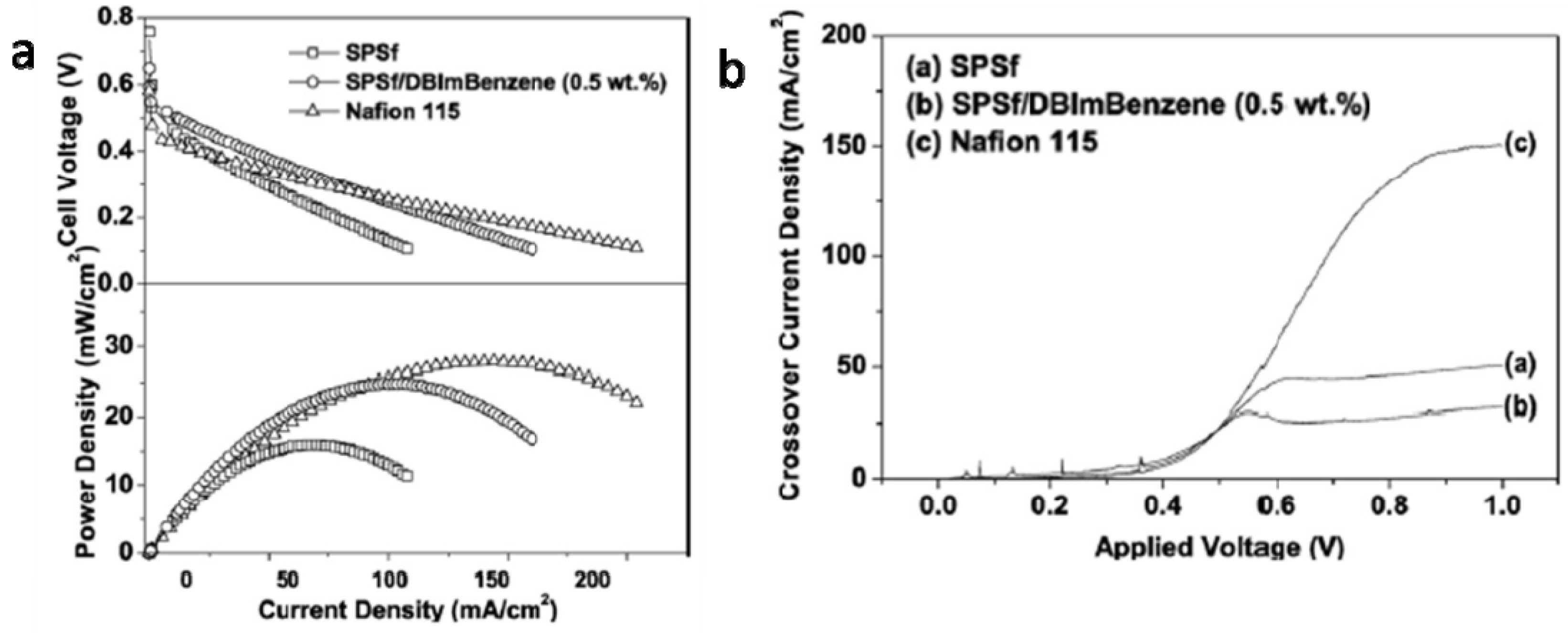
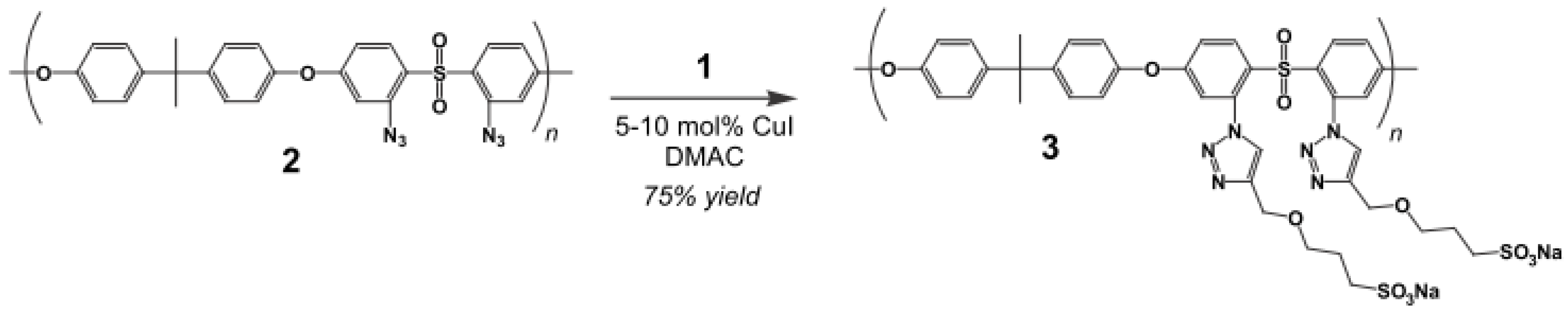
4.2. Nitrogen-Containing Polymers
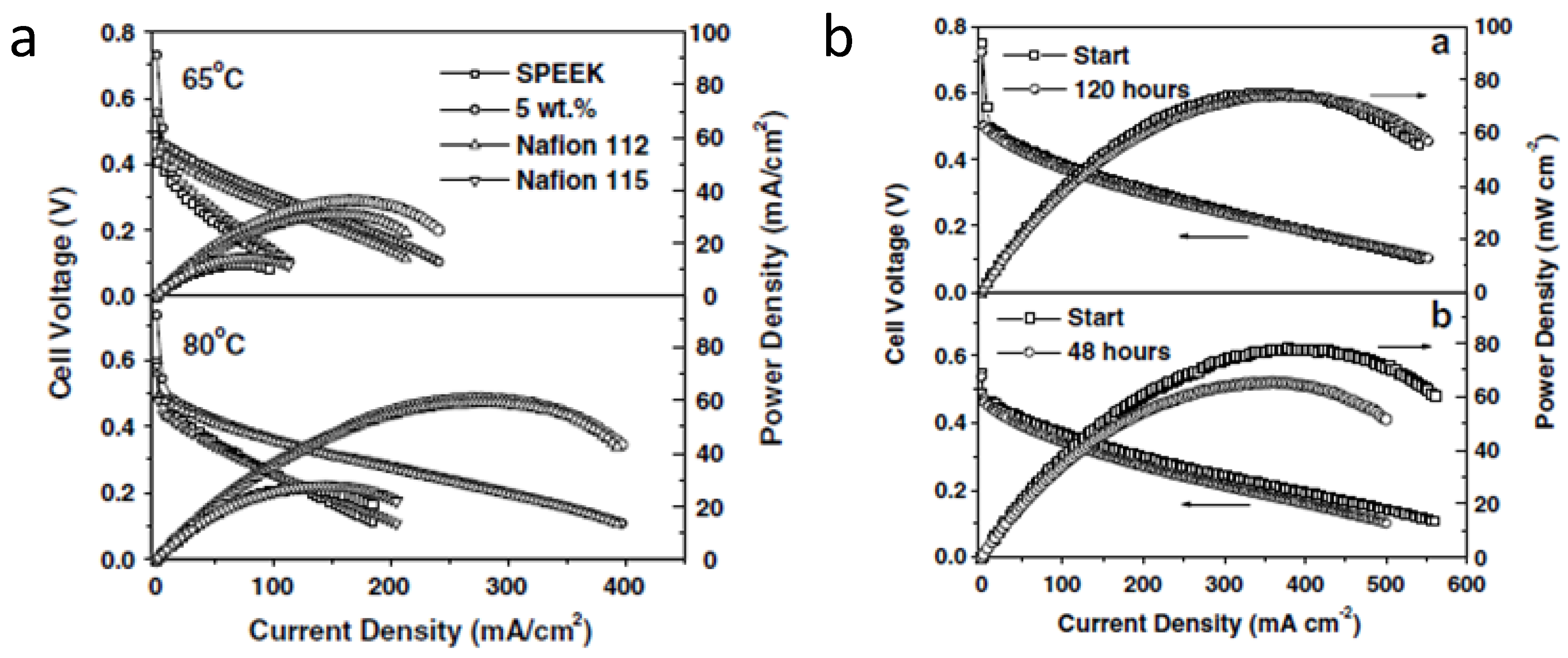
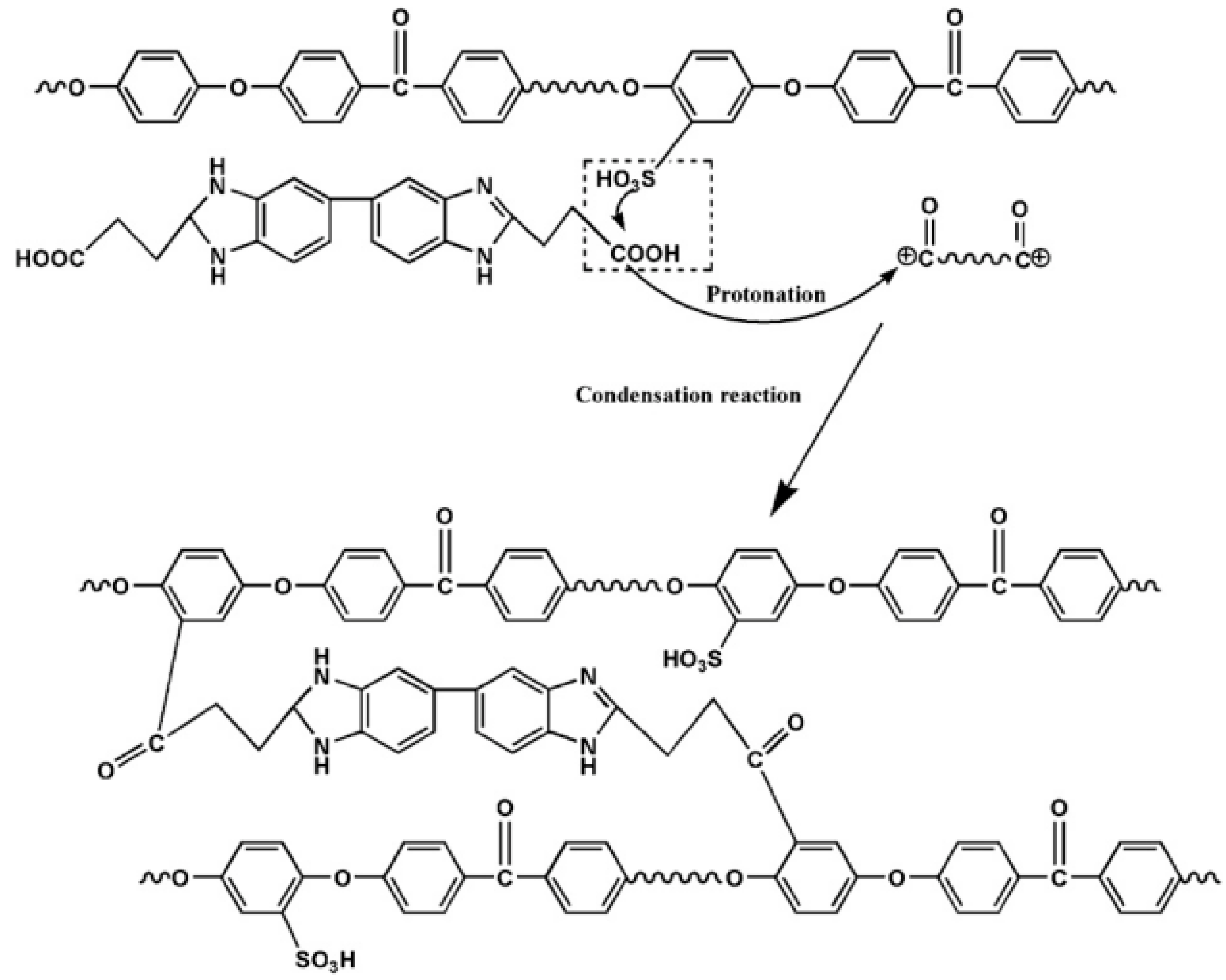
5. Conclusions
Acknowledgments
References
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; Zelenay, P.; More, K.; Stroh, K.; Zawodzinski, T.; Boncella, J.; McGrath, J.E.; Inaba, M.; Miyatake, K.; Hori, M.; Ota, K.; Ogumi, Z.; Miyata, S.; Nishikata, A.; Siroma, Z.; Uchimoto, Y.; Yasuda, K.; Kimijima, K.; Iwashita, N. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Wang, Z.; Gao, Y. Proton exchange membrane fuel cell from low temperature to high temperature: Material challenges. J. Power Sources 2007, 167, 235–242. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, D.S.; Guiver, M.D.; Pivovar, B.S. Interpretation of direct methanol fuel cell electrolyte properties using non-traditional length-scale parameters. J. Membr.Sci. 2011, 374, 49–58. [Google Scholar] [CrossRef]
- Zaidi, S.M.J. Research Trends in Polymer Electrolyte Membranes for PEMFC. In Polymer Membranes for Fuel Cells; Zaidi, S.M.J., Matsuura, T., Eds.; Springer Publishing Co.: New York, NY, USA, 2006; pp. 7–25. [Google Scholar]
- Ahmed, M.; Dincer, I. A review on methanol crossover in direct methanol fuel cells: Challenges and achievements. Int. J. Energy Res. 2011, 35, 1213–1228. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.-T.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Zhu, Y.; Zieren, S.; Manthiram, A. Novel crosslinked membranes based on sulfonated poly(ether ether ketone) for direct methanol fuel cells. Chem. Commun. 2011, 47, 7410–7412. [Google Scholar]
- Hande, V.R.; Rao, S.; Rath, S.K.; Thakur, A.; Patri, M. Crosslinking of sulphonated poly (ether ether ketone) using aromatic bis(hydroxymethyl) compound. J. Membr.Sci. 2008, 322, 67–73. [Google Scholar] [CrossRef]
- Ishikawa, J.-I.; Fujiyama, S.; Inoue, K.; Omi, T.; Tamai, S. Highly sulfonated poly(aryl ether ketone) block coplymers having a cross-linking structure. J. Membr.Sci. 2007, 298, 48–55. [Google Scholar] [CrossRef]
- Lee, K.-S.; Jeong, M.-H.; Lee, J.-P.; Lee, J.-S. End-group cross-linked poly(arylene ether) for proton exchange membranes. Macromolecules 2009, 42, 584–590. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Chen, W.-Y.; Huang, Y.-J.; Cheng, M.-Y.; Yen, Y.-C.; Cheng, C.-C.; Chang, F.-C. Preparation and characterization of high-durability zwitterionic crosslinked proton exchange membranes. J. Membr.Sci. 2010, 362, 29–37. [Google Scholar] [CrossRef]
- Kayser, M.J.; Reinholdt, M.X.; Kaliaguine, S. Amine grafted silica/SPEEK nanocomposites as proton exchange membranes. J. Phys. Chem. B 2010, 114, 8387–8395. [Google Scholar]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized graphene oxide nanocomposite membrane for low humidity and high temperature proton exchange membrane fuel cells. J. Phy. Chem. C 2011, 115, 20774–20781. [Google Scholar]
- Yang, C.-C.; Lue, S.J.; Shih, J.-Y. A novel organic/inorganic polymer membrane based on poly(vinyl alcohol)/poly(2-acrylamido-2-methyl-1-propanesulfonic acid/3-glycidyloxypropyl trimethoxysilane polymer electrolyte membrane for direct methanol fuel cells. J. Power Sources 2011, 196, 4458–4467. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Kim, H.K.; Lee, J.S. New sulfonic acid moiety grafted on montmorillonite as filler of organic-inorganic composite membrane for non-humidified proton-exchange membrane fuel cells. J. Power Sources 2010, 195, 4653–4659. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoshida, T.; Kawamura, G.; Muto, H.; Sakai, M.; Matsuda, A. Inorganic-organic composite electrolytes consisting of polybenzimidazole and Cs-substituted heteropoly acids and their application for medium temperature fuel cells. J. Mater. Chem. 2010, 20, 6359–6366. [Google Scholar] [CrossRef]
- Lin, H.L.; Hu, C.R.; Lai, S.W.; Yu, T.L. Polybenzimidazole and butylsulfonate grafted polybenzimidazole blends for proton exchange membrane fuel cells. J. Membr.Sci. 2012, 389, 399–406. [Google Scholar] [CrossRef]
- Acar, O.; Sen, U.; Bozkurt, A.; Ata, A. Proton conducting membranes based on Poly(2,5-benzimidazole) (ABPBI)-Poly(vinylphosphonic acid) blends for fuel cells. Int. J. Hydrog.Energy 2009, 34, 2724–2730. [Google Scholar] [CrossRef]
- Li, W.; Fu, Y.Z.; Manthiram, A.; Guiver, M.D. Blend membranes consisting of sulfonated poly(ether ether ketone) and polysulfone bearing 4-nitrobenzimidazole for direct methanol fuel cells. J. Electrochem. Soc. 2009, 156, B258–B263. [Google Scholar] [CrossRef]
- Li, W.; Manthiram, A.; Guiver, M.D. Blend membranes consisting of sulfonated poly(ether ether ketone) and 1h-perimidine tethered polysulfone for direct methanol fuel cells. Electrochem. Solid State Lett. 2009, 12, B180–B184. [Google Scholar] [CrossRef]
- Goodenough, J.B. Proton Conductors: Solids, Membranes and Gels-Materials and Devices; Colomban, P., Ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Kreuer, K.D. On the complexity of proton conduction phenomena. Solid State Ionics 2000, 136-137, 149–160. [Google Scholar] [CrossRef]
- Peckham, T.J.; Holdcroft, S. Structure-morphology-property relationships of non-perfluorinated proton-conducting membranes. Adv. Mater. 2010, 22, 4667–4690. [Google Scholar] [CrossRef]
- Ueki, T.; Watanabe, M. Macromolecules in ionic liquids: Progress, challenges, and opportunities. Macromolecules 2008, 41, 3739–3749. [Google Scholar] [CrossRef]
- Yamada, M.; Honma, I. Anhydrous proton conducting polymer electrolytes based on poly(vinylphosphonic acid)-heterocycle composite material. Polymer 2005, 46, 2986–2992. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.L.; Lu, X.F.; Zhang, C.C.; Wang, Z.J.; Kong, L.R.; Wang, C.; Liu, X.C. Composite membranes based on sulfonated poly(aryl ether ketone)s containing the hexafluoroisopropylidene diphenyl moiety and poly(amic acid) for proton exchange membrane fuel cell application. Int. J. Hydrog. Energy 2011, 36, 14622–14631. [Google Scholar] [CrossRef]
- Verma, A.; Scott, K. Development of high-temperature PEMFC based on heteropolyacids and polybenzimidazole. J. Solid State Electrochem. 2010, 14, 213–219. [Google Scholar] [CrossRef]
- Fontanella, J.J.; Wintersgill, M.C.; Wainright, J.S.; Savinell, R.F.; Litt, M. High pressure electrical conductivity studies of acid doped polybenzimidazole. Electrochim. Acta 1998, 43, 1289–1294. [Google Scholar] [CrossRef]
- Yang, C.; Costamagna, P.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Approaches and technical challenges to high temperature operation of proton exchange membrane fuel cells. J. Power Sources 2001, 103, 1–9. [Google Scholar] [CrossRef]
- Fu, Y.-Z.; Manthiram, A. Nafion-imidazole-H3PO4 composite membranes for proton exchange membrane fuel cells. J. Electrochem. Soc. 2007, 154, B8–B12. [Google Scholar] [CrossRef]
- Subbaraman, R.; Ghassemi, H.; Zawodzinski, T. Triazole and triazole derivatives as proton transport facilitators in polymer electrolyte membrane fuel cells. Solid State Ionics 2009, 180, 1143–1150. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Fuchs, A.; Ise, M.; Spaeth, M.; Maier, J. Imidazole and pyrazole-based proton conducting polymers and liquids. Electrochim. Acta 1998, 43, 1281–1288. [Google Scholar] [CrossRef]
- Herz, H.G.; Kreuer, K.D.; Maier, J.; Scharfenberger, G.; Schuster, M.F.H.; Meyer, W.H. New fully polymeric proton solvents with high proton mobility. Electrochim. Acta 2003, 48, 2165–2171. [Google Scholar] [CrossRef]
- Schuster, M.F.H.; Meyer, W.H.; Schuster, M.; Kreuer, K.D. Toward a new type of anhydrous organic proton conductor based on immobilized imidazole. Chem. Mater. 2004, 16, 329–337. [Google Scholar] [CrossRef]
- Subbaraman, R.; Ghassemi, H.; Zawodzinski, T.A. 4,5-dicyano-1H-[1,2,3]-triazole as a proton transport facilitator for polymer electrolyte membrane fuel cells. J. Am. Chem. Soc. 2007, 129, 2238–2239. [Google Scholar] [CrossRef]
- Kim, J.D.; Mori, T.; Hayashi, S.; Honma, I. Anhydrous proton-conducting properties of Nafion-1,2,4-triazole and Nafion-benzimidazole membranes for polymer electrolyte fuel cells. J. Electrochem. Soc. 2007, 154, A290–A294. [Google Scholar] [CrossRef]
- Sen, U.; Bozkurt, A.; Ata, A. Nafion/poly(1-vinyl-1,2,4-triazole) blends as proton conducting membranes for polymer electrolyte membrane fuel cells. J. Power Sources 2010, 195, 7720–7726. [Google Scholar] [CrossRef]
- Kim, J.-D.; Oba, Y.; Ohnuma, M.; Jun, M.-S.; Tanaka, Y.; Mori, T.; Choi, Y.-W.; Yoon, Y.-G. Physico-chemical properties of highly flexible temperature tolerant anhydrous Nafion-1,2,3-triazole blend membranes. J. Electrochem. Soc. 2010, 157, B1872–B1877. [Google Scholar] [CrossRef]
- Fu, Y.; Manthiram, A.; Guiver, M.D. Blend membranes based on sulfonated poly(ether ether ketone) and polysulfone bearing benzimidazole side groups for proton exchange membrane fuel cells. Electrochem. Commun. 2006, 8, 1386–1390. [Google Scholar] [CrossRef]
- Ameduri, B. From vinylidene fluoride (VDF) to the applications of VDF-containing polymers and copolymers: Recent developments and future trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef]
- Frutsaert, G.; David, G.; Ameduri, B.; Jones, D.J.; Rozière, J.; Glipa, X. Synthesis and characterisation of novel fluorinated polymers bearing pendant imidazole groups and blend membranes: New materials for PEMFC operating at low relative humidity. J. Membr.Sci. 2011, 367, 127–133. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.-T.; Savinell, R.F.; Litt, M.; Moaddel, H.; Rogers, C. Acid-doped polybenziimidazole, a new polymer electrolyte. Proc. Electrochem. Soc. 1994, 94, 255–264. [Google Scholar]
- Yang, J.; Li, Q.; Cleemann, L.N.; Xu, C.; Jensen, J.O.; Pan, C.; Bjerrum, N.J.; He, R. Synthesis and properties of poly(aryl sulfone benzimidazole) and its copolymers for high temperature membrane electrolytes of fuel cells. J. Mater. Chem. 2012, 22, 11185–11195. [Google Scholar]
- Glipa, X.; El Haddad, M.; Jones, D.J.; Rozière, J. Synthesis and characterisation of sulfonated polybenzimidazole: A highly conducting proton exchange polymer. Solid State Ionics 1997, 97, 323–331. [Google Scholar] [CrossRef]
- Kawahara, M.; Rikukawa, M.; Sanui, K.; Ogata, N. Synthesis and proton conductivity of sulfopropylated poly(benzimidazole) films. Solid State Ionics 2000, 136-137, 1193–1196. [Google Scholar] [CrossRef]
- Beattie, P.D.; Orfino, F.P.; Basura, V.I.; Zychowska, K.; Ding, J.; Chuy, C.; Schmeisser, J.; Holdcroft, S. Ionic conductivity of proton exchange membranes. J. Electroanal. Chem. 2001, 503, 45–56. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Manthiram, A. Synthesis and characterization of sulfonated polysulfone membranes for direct methanol fuel cells. J. Power Sources 2006, 157, 222–225. [Google Scholar] [CrossRef]
- Yang, B.; Manthiram, A. Sulfonated poly(ether ether ketone) membranes for direct methanol fuel cells. Electrochem. Solid State Lett. 2003, 6, A229–A231. [Google Scholar] [CrossRef]
- Li, W.; Bellay, A.; Fu, Y.Z.; Manthiram, A. N,N'-Bis-(1H-benzimidazol-2-yl)-isophthalamide as an additive in sulfonated polymer membranes for direct methanol fuel cells. J. Power Sources 2008, 180, 719–723. [Google Scholar] [CrossRef]
- Fu, Y.; Li, W.; Manthiram, A. Sulfonated polysulfone with 1,3-1H-dibenzimidazole-benzene additive as a membrane for direct methanol fuel cells. J. Membr.Sci. 2008, 310, 262–267. [Google Scholar] [CrossRef]
- Ren, X.; Springer, T.E.; Gottesfeld, S. Water and methanol uptakes in nafion membranes and membrane effects on direct methanol cell performance. J. Electrochem. Soc. 2000, 147, 92–98. [Google Scholar] [CrossRef]
- Fu, Y.; Manthiram, A.; Guiver, M.D. Acid-base blend membranes based on 2-amino-benzimidazole and sulfonated poly(ether ether ketone) for direct methanol fuel cells. Electrochem. Commun. 2007, 9, 905–910. [Google Scholar] [CrossRef]
- Lee, J.K.; Li, W.; Manthiram, A.; Guiver, M.D. Blend membranes based on acid-base interactions for operation at high methanol concentrations. J. Electrochem. Soc. 2009, 156, B46–B50. [Google Scholar] [CrossRef]
- Ye, Y.S.; Huang, Y.J.; Cheng, C.C.; Chang, F.C. A new supramolecular sulfonated polyimide for use in proton exchange membranes for fuel cells. Chem. Commun. 2010, 46, 7554–7556. [Google Scholar] [CrossRef]
- Han, M.; Zhang, G.; Li, M.; Wang, S.; Zhang, Y.; Li, H.; Lew, C.M.; Na, H. Considerations of the morphology in the design of proton exchange membranes: Cross-linked sulfonated poly(ether ether ketone)s using a new carboxyl-terminated benzimidazole as the cross-linker for PEMFCs. Int. J. Hydrog.Energy 2011, 36, 2197–2206. [Google Scholar] [CrossRef]
- Zhu, Y.; Manthiram, A. Synthesis and characterization of polysulfone-containing sulfonated side chains for direct methanol fuel cells. J. Power Sources 2011, 196, 7481–7487. [Google Scholar] [CrossRef]
- Li, W.; Manthiram, A. Sulfonated poly(arylene ether sulfone) as a methanol-barrier layer in multilayer membranes for direct methanol fuel cells. J. Power Sources 2010, 195, 962–968. [Google Scholar] [CrossRef]
- Norris, B.C.; Li, W.; Lee, E.; Manthiram, A.; Bielawski, C.W. Click”-functionalization of poly(sulfone)s and a study of their utilities as proton conductive membranes in direct methanol fuel cells. Polymer 2010, 51, 5352–5358. [Google Scholar]
- Huang, Y.J.; Ye, Y.S.; Yen, Y.C.; Tsai, L.D.; Hwang, B.J.; Chang, F.C. Synthesis and characterization of new sulfonated polytriazole proton exchange membrane by click reaction for direct methanol fuel cells (DMFCs). Int. J. Hydrog.Energy 2011, 36, 15333–15343. [Google Scholar] [CrossRef]
- Boaventura, M.; Ponce, M.L.; Brandão, L.; Mendes, A.; Nunes, S.P. Proton conductive membranes based on doped sulfonated polytriazole. Int. J. Hydrogen Energy 2010, 35, 12054–12064. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zuo, Z.; Fu, Y.; Manthiram, A. Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells. Polymers 2012, 4, 1627-1644. https://doi.org/10.3390/polym4041627
Zuo Z, Fu Y, Manthiram A. Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells. Polymers. 2012; 4(4):1627-1644. https://doi.org/10.3390/polym4041627
Chicago/Turabian StyleZuo, Zicheng, Yongzhu Fu, and Arumugam Manthiram. 2012. "Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells" Polymers 4, no. 4: 1627-1644. https://doi.org/10.3390/polym4041627
APA StyleZuo, Z., Fu, Y., & Manthiram, A. (2012). Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells. Polymers, 4(4), 1627-1644. https://doi.org/10.3390/polym4041627