Poly(alkyl methacrylate) Tooth Coatings for Dental Care: Evaluation of the Demineralisation-Protection Benefit Using a Time-Resolved In Vitro Method
Abstract
: An in vitro method for the time-resolved quantification of acid-mediated tooth demineralisation has been developed and evaluated against putative non-permanent protective formulations based on a series of poly(alkyl methacrylate)s. Using a thermostatted carousel, dentally relevant substrates consisting of hydroxyapatite discs or sections of bovine teeth have been exposed to aqueous citric acid under controlled conditions, before and after being treated with the polymeric coatings. The dissolution of phosphate was monitored by the determination of 31P by Inductively Coupled Plasma— Mass Spectrometry and by the spectrophotometric phosphovanadomolybdate method. Dose-response plots constructed for both groups of treated substrates have revealed that the coatings significantly reduce erosion rates but are less effective at inhibiting tooth demineralisation than the standard fluoride treatment. The approach has enabled an evaluation of the erosion-protection efficiency of each coating.1. Introduction
Early stage dental erosion involves the reversible demineralisation of enamel through the dissolution of calcium hydroxyapatite (HA) and related substituted apatitic mineral phases by acids of non-bacterial origin [1-3]. In healthy individuals, the potentially deleterious effects of dietary acids on dental hard tissues are mitigated by the continuous production of saliva, whose inherent buffer capacity serves to raise pH in the oral cavity whilst at the same time diluting the acid challenge and promoting clearance [4]. Salivary proteins also play an important role in protecting teeth from acid insult, by being adsorbed selectively onto the tooth surface in the form of a tightly bound thin film. The salivary pellicle (thickness 0.1–1.0 μm depending on location and maturity) provides a significant barrier to acid attack on the underlying mineral surface [5,6]. The widespread use of fluoride in dentifrice formulations has been largely responsible for the marked decline in the incidence of dental caries in many populations over the last 40 years, but there are indications that this decline has slowed, while over the last 10 years dental erosion has become a clinical concern [7,8]. In particular, high levels of erosive wear have been observed in children and adolescents [9,10]. Risk factors include diet and social deprivation; but erosion and associated wear appear to be associated primarily with developed nations. Although consumption levels of acidic soft drinks has been positively correlated with increased levels of erosion, subjects exhibiting inappropriate consumption patterns are likely to be at highest risk [11,12]. Awareness by individuals and provision of timely advice by health care professionals are considered to be at least as important as therapeutic intervention in preventing progression of bulk tissue loss which in extremis can lead to complete loss of function [13].
The aetiology of dental caries requires the presence of bacterial plaque as one of several prerequisites, while dental erosion does not involve bacteria [9,14]. Early caries lesions form in the sub-surface of the tooth due to the presence of an overlying plaque layer; erosive lesions, by contrast, are surface defects formed by “top-down” demineralization [15]. Progression of early erosive lesions results in the formation of concavities, the appearance of which is accelerated by abrasive damage or by attrition-induced wear. Caries and erosion, however, do both involve acid-mediated demineralisation: in the case of caries the source is plaque acid, whilst in erosion the acid challenge derives from the stomach and/or from diet; acids associated with dental erosion are markedly more aggressive than plaque acids [12,15].
Fluoride is known to be highly efficacious in inhibiting the development of caries, and does so by substituting for −OH groups in HA, Ca10(PO4)6 (OH)2. This results in the formation of partially or fully fluoridated apatitic phases, Ca10(PO4)6Fx(OH)2-x, whose resistance to acid-mediated dissolution is much greater than that of the parent non-fluoridated apatite [16-19]. Fluoride also enhances remineralisation of early caries lesions by promoting mineral uptake as the less soluble fluoridated apatitic phase. The same fluoride chemistries apply in the case of dental erosion, but unlike dental caries, where there is a wealth of longitudinal clinical data supporting the efficacy of sodium monofluorophosphate and ionic fluorides, such as sodium fluoride (NaF) [20], evidence supporting the use of fluoride as an anti-erosion agent is currently limited to in vitro and in situ clinical studies [21]. The current view is that higher doses of fluoride may be required to achieve clinically significant levels of erosion control compared with that required in the prevention and management of dental caries [21-23]. The most convenient access to fluoridated medicaments is through the over-the-counter availability of dentifrices and rinses. The levels of fluoride contained in these are dictated by the anti-caries monographs of regulatory agencies. In the absence of a specific anti-erosion monograph, and given the concerns for fluorosis associated with the use of higher levels of fluoride in children, technologies that augment the anti-erosion efficacy of medicaments containing fluoride at current concentrations (900–1,500 mg/kg for dentifrices and 90–450 mg/kg for mouth rinses), are of particular interest for future development.
In vitro erosion models have been effective in establishing the relative erosion potentials of foods and drinks, factors affecting these potentials, and the effects of specific interventions on the physicochemical properties of dentally-relevant substrates subject to acid insult. Data from such models indicate broad effects and trends, and the models are valuable tools for the screening of potential inhibitors of acid demineralisation. Due to the strict regulatory environment associated with the acquisition, processing, tracking, storage and disposal of human teeth, alternative substrates such as HA powder, beads or discs have been employed as non-biological enamel substitutes. Enamel and dentine specimens prepared from bovine incisors have also been widely used, because they can be acquired from the same stock at similar age (typically 30 months). Despite the differences between the histology of human and bovine teeth (the latter being more porous), the literature indicates a good correlation between the behaviour of human and bovine specimens in both caries and erosion models [24,25]. A simple means of determining the extent of erosive demineralisation is through the analysis of released phosphate using a single-point phosphovanadomolybdate assay. However, this spectrophotometric method does not (by definition) monitor the progression of erosion; furthermore, the assay is not specific to orthophosphate since linear condensed phosphates also react with the vanadomolybdate reagent. A pH-stat model has been used to monitor erosive demineralisation in real time, and to investigate the effect of specific interventions such as the pre-treatment of HA discs with fluoride, or modification of the erosive challenge itself by addition of film-forming polymers [26]. This model, however, may be difficult to employ for multiple specimens.
Driven by the need to develop a rapid and time-resolved experimental method, a thermostatted carousel has been used in the present study to provide a controlled environment in which citric acid-mediated erosion of HA discs and of pre-prepared sections of bovine teeth could be monitored by discontinuous sampling and subsequent determination of released phosphate. The phosphovanadomolybdate assay and the determination of 31P by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) have been used for this analysis; the sensitivity and precision of these techniques are compared for this application. The protocol for the experimental method aims to facilitate the longitudinal monitoring of multiple erosion experiments (n = 12) that are conducted in parallel under identical conditions of temperature and stirring rate. The methodology is used to evaluate the anti-erosion efficacy of a series of biomedically relevant poly(alkyl methacrylate) coating materials [27].
2. Material and Methods
2.1. Substrates
A single batch of HA discs (diameter 12.5 mm, thickness 1.0–1.3 mm; ca. 0.46 g) was supplied by Himed HiMed, (Old Bethpage, NY, USA). Prior to use, discs (n = 6) were etched × 2 in 500 mL of 10 g/L citric acid (adjusted to pH 3.5) for 30 min at room temperature. Excess acid was removed by rinsing with deionised (DI) water before storage in a humidified atmosphere.
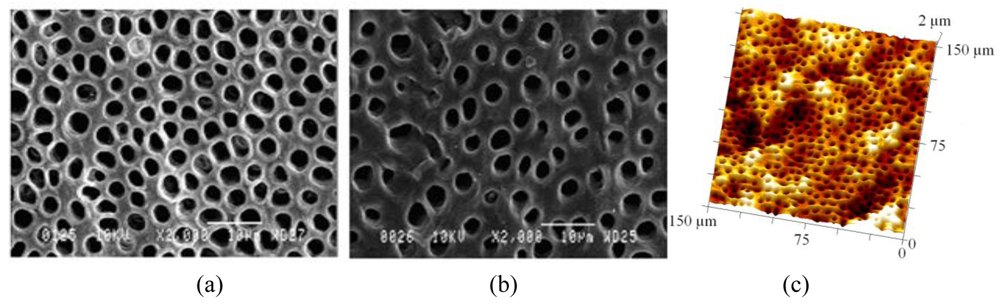
Decay-free bovine incisors were sterilised by immersion in 50 g/L NaOCl for 2 min, followed by 300 mL/L H2O2 for a further 2 min. Thereafter, specimens were stored in 9 g/kg NaCl for a maximum of six weeks. Tooth sections (ca. 15 mm × 10 mm × 5 mm; 1.52 g) were cut from the buccal aspect using an Isomet™ 1000 precision saw (Buehler®, Düsseldorf, Germany). Since one larger face of each specimen consisted of exposed dentine whilst the other was unpolished enamel, some variability was to be expected both within and between the specimens [28,29]. The amorphous smear layer, formed by cutting, was removed by placing the tooth sections in 100 g/L citric acid (pH 2.2) for 2 min. After rinsing in DI water, the specimens were stored in sterile saline. Extracted human molars and pre-molars were stored in an aqueous solution of saturated thymol for up to two weeks after removal of the roots and pulp. Prior to use, teeth were thoroughly rinsed with DI water and visually examined for evidence of damage, decay and white spot lesions. Transverse sections of coronal dentine, 100 μm in thickness, were cut with an Isomet 1000 precision saw and etched (citric acid, 100 g/L; 2 min) to remove the smear layer. Specimens (bovine and human) were sputter-coated with a palladium-gold alloy before imaging using scanning electron microscopy (SEM) at an operating potential of 10 kV (Jeol JSM 6100, Welwyn Garden City, Hertfordshire, UK). Imaging of uncoated bovine specimens was performed by atomic force microscopy (AFM) in contact mode, using a MultiMode Nanoscope IV Scanning Probe Microscope (Digital Instruments, Santa Barbara, CA, USA).
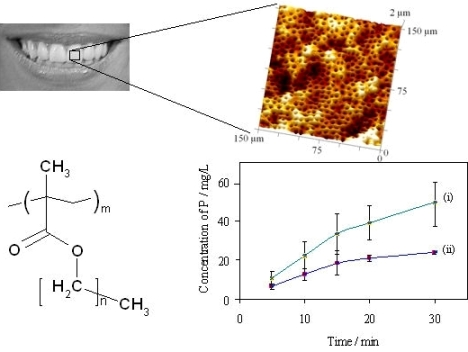
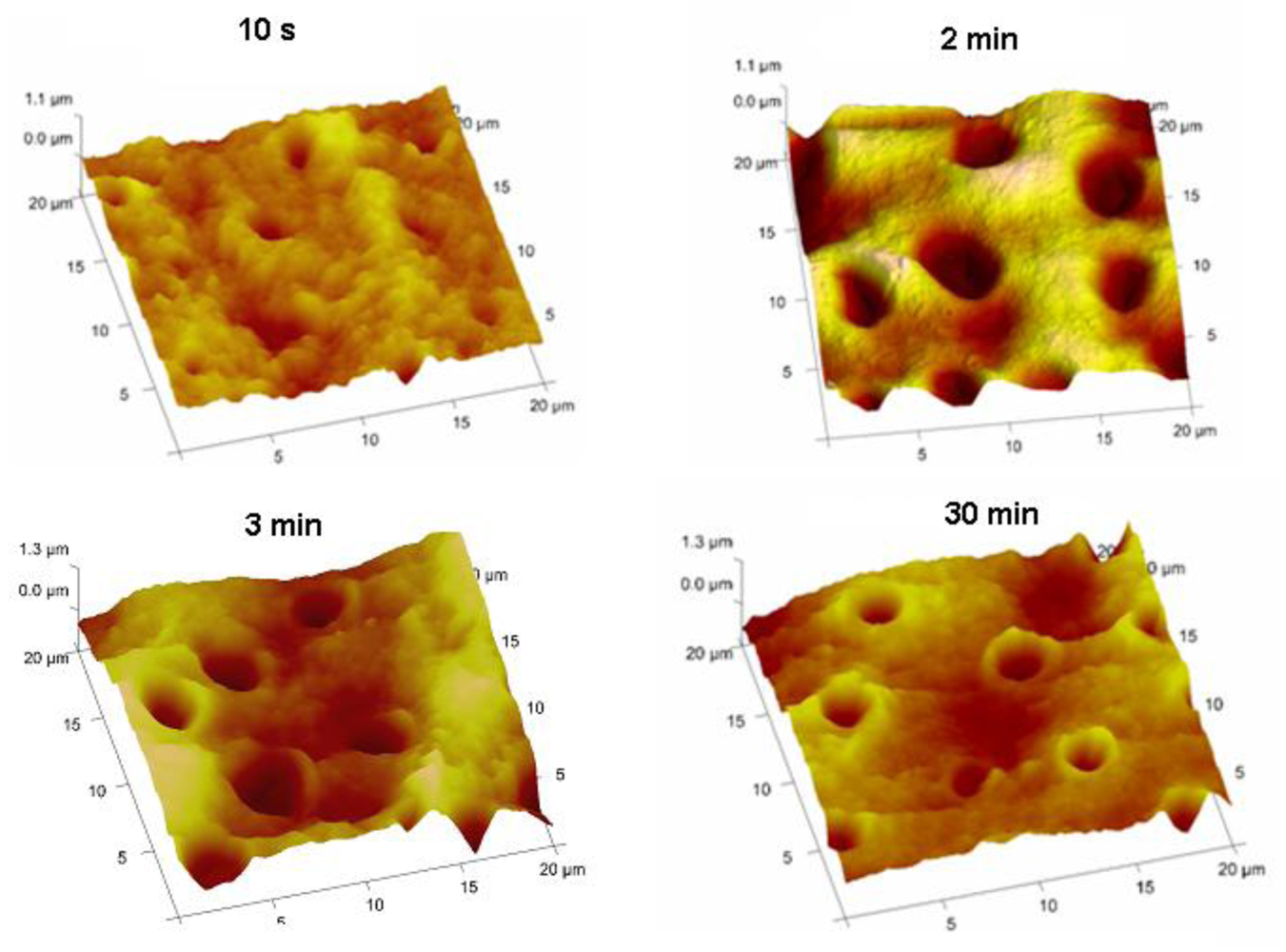
Typical SEM images of transverse sections of human and bovine dentine are shown in Figures 1(a) and Figures 1(b) and an AFM image from bovine dentine is shown in Figure 1(c). Tubule diameters in the bovine specimens (1.7–2.6 μm, c.f. lit. [30-33] 1.8–3.5 μm) were similar to those observed in human specimens (2.2–3.3 μm, c.f. lit. [30-33] 1.9–4.0 μm). Tubules in bovine specimens were less closely packed (3.1 × 104 mm −2, c.f. lit. [30-33] 2.1 × 104−3.5×104 mm −2) than those in human specimens (7.9 × 104 mm −2, c.f. lit. [30-33] 2.1 × 104–8.3 × 104 mm −2). AFM images, Figure 2, also established an optimum time of 2 min for exposure of freshly cut sections to citric acid (100 g/L), just sufficient for the removal of the smear layer and of debris within tubules. Longer exposures resulted in the formation of craters in the tubule structure whereas shorter exposures did not completely remove the smear layer.
2.2. Polymer Emulsions and the Coating of Specimens
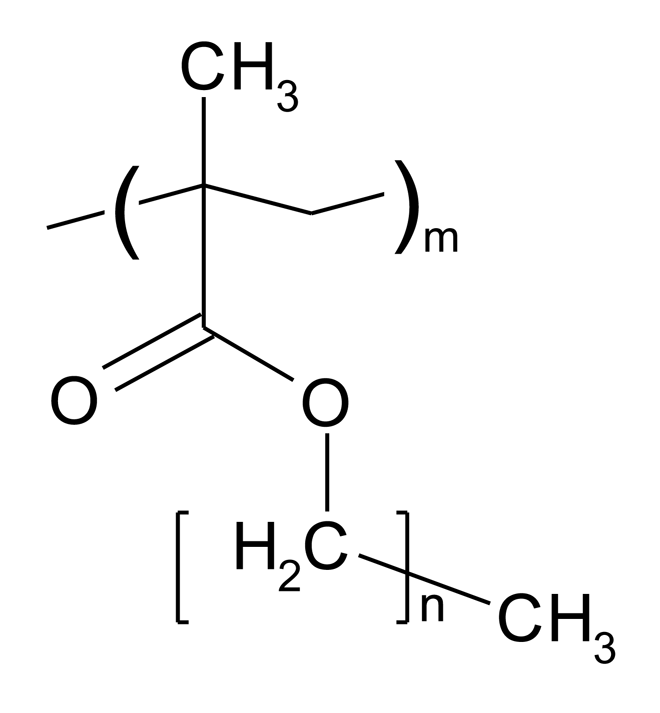
Poly(alkyl methacrylate)s were supplied by Sigma-Aldrich (Poole, Dorset, UK or Schnelldorf, Germany). The generic structure of these polymers and associated acronyms are shown in Figure 3, and their key physicochemical parameters summarised in Table 1. PBMA and PEMA were obtained as solids whilst POMA, PLMA, PHMA, PHxMA were supplied as solutions in toluene. Pure samples of solid polymers were recovered by repeated precipitation from cooled methanol.
Emulsions (28 g/kg) were prepared by adding each polymer (450 mg) in solution (dichloromethane (PEMA) or diethyl ether (PBMA) or low-boiling petroleum ether, 3.0 g) drop-wise (2 min) into aqueous sodium dodecyl sulphate (SDS, Acros Organics, 5 g/kg, 15 g) under sonication (Ultrasonic Processor, amplitude 37%, 7 min). The organic solvent was evaporated from each batch by stirring at ca. 5 K above the solvent boiling point for 4–6 h. To compensate for the concurrent evaporation of water, 500 μL aliquots of DI water were added at 30 min intervals (after cooling to ambient temperature) to give a final mass of 15.45 g. To determine the dry mass, the aqueous phase was removed following separation by centrifugation at 4,000 rpm for 5 min. Particle sizes were determined using a Coulter N4MD Sub-Micron Particle Analyser (Beckman Coulter, High Wycombe, Buckinghamshire, UK) with multiple scattering angle detection and size distribution analysis yielding the diameters and SDs indicated in Table 1. HA discs and bovine tooth specimens were coated by immersion (2 min) in the appropriate polymer-containing emulsion, followed by rinsing with DI water.
2.3. Acid Demineralisation In Vitro
The demineralisation experiments were performed using a 12-station synthesis carousel supplied by Radleys Discovery Technologies (Saffron Waldon, Essex, UK). Untreated pre-weighed HA discs or bovine enamel sections were individually placed on a wire mesh support at a fixed distance above the stirrer bar in a reaction tube containing 15.0 mL of 10 g/L citric acid adjusted to pH 3.75 thermostatted to 37 °C. The baskets were then lowered so that the specimens were fully immersed in the erosive medium; typically 4-6 specimens were employed for each experiment. Samples (100 μL aliquots) for the determination of dissolved phosphate were taken at 5 or 10 min intervals up to 40 min. Specimens were then removed, rinsed with DI water, and immersed in the test treatment (28 g/kg polymer latex; 120 s, 20 °C), DI water negative control or one of a range of benchmark control treatments comprising 250–2,000 mg/kg fluoride as NaF. In the latter case exposure times of both 30 s and 120 s were employed. Together with a fresh portion of erosion medium (15.0 mL), the treated specimens were then returned to a cleaned tube in the reaction carousel. Discontinuous sampling and subsequent analysis of phosphate and phosphorous by spectrophotometry or ICP-MS respectively were used to create plots of dissolution as a function of time and thus dissolution rate. The efficacy of the treatment to inhibit erosive demineralisation was determined by using each specimen as its own control. Using a one-way ANOVA with a post hoc Fisher test statistical analysis of the results was performed to compare the effects of the putative protective treatments with that of the fluoride control.
2.3.1. Determination of Dissolved Phosphate with Vanadomolybdate Reagent
Samples (100 μL) of phosphate-containing erosion medium were transferred to a 96-well microplate. Using a multipipette for simultaneous additions to all wells, samples were mixed with 100 μL of vanadomolybdate reagent (VWR, Lutterworth, Leicestershire, UK) and allowed to stand for 5 min, after which the absorbances were measured (Wallac Victor2 1420 Multilabel Counter, 450 nm, 1 well per second). Using the same procedure for standard solutions (KH2PO4 > 99%, dried) a calibration plot over the phosphorus concentration range 10–60 mg/L was linear (R2= 0.9996) but with a significant positive intercept (no reference cell). This calibration was used for calculating concentrations of phosphorus in the erosion media. A further calibration using a 1 cm pathlength cell with matched reference in a dual-beam spectrophotometer over the concentration range 0.1–2.3 mg/L was linear through the origin yielding values for A450 in the range 0.002 to 0.068; R2 = 0.9999. Allowing for a minimum net absorbance of 0.005, the lowest reliable estimation level was ca. 0.2 mg/L in 1 cm cells or 0.5 mg/L using the microplate reader.
2.3.2. Determination of 31P by ICP-MS
The analysis of dissolved phosphate as 31P by ICP-MS necessitated the elimination of [14N16O1H]+ and/or [15N16O]+ from the experimental protocol [35] by using HCl (analytical grade for trace analysis) rather than HNO3, and HPLC grade water for all procedures. All glassware and equipment was cleaned in 30 g/L HCl and subsequently rinsed ×3 with DI water. Each 100 μL sample was added to 14 g of 6 g/L HCl. Using an Agilent ICP-MS 7500 operating in standard mode with argon as carrier gas, a linear calibration (R2 = 1.000) was obtained for 31P using acidified (6 g/L HCl) phosphate solutions (Spex CertipreP 1,000 ppm 31P, Fisher Scientific; diluted to 1–110 μg/L). Allowing for the background response, the lowest reliable estimation level of 31P in the diluted samples was ca.5 μg/L.
3. Results
3.1. Comparison of Analytical Methods
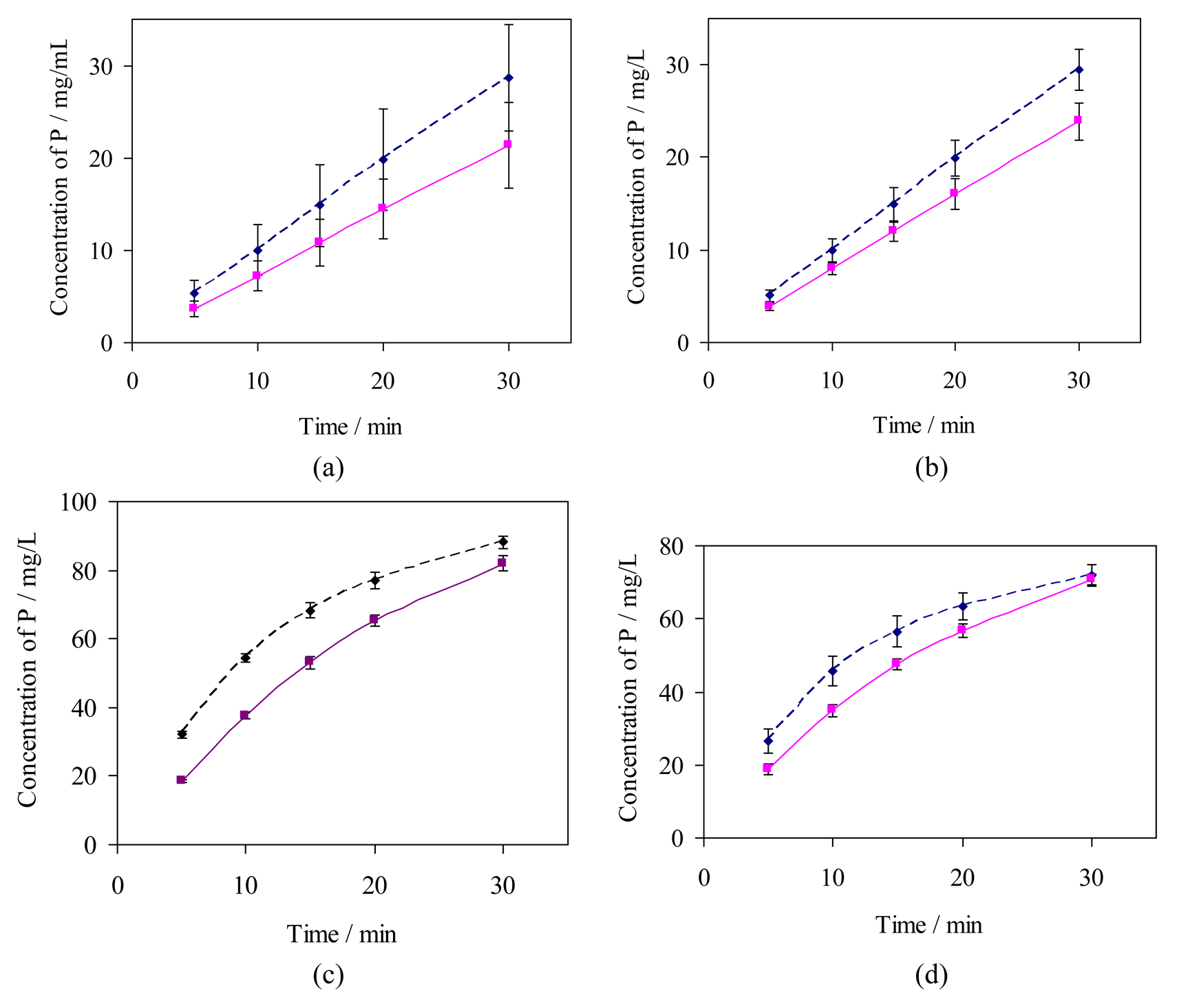
The rates of erosion of individual untreated dental specimens by citric acid (10 g/L, pH 3.75; 37 °C) have been measured by analysis of dissolved phosphorus, for comparison with rates obtained under the same conditions but after exposure of the same specimens to a putative protective treatment: the “in vitro demineralisation method”. These data have been used to assess the variability of specimens and to compare the two methods of analysis of aqueous samples for dissolved phosphate, the data are summarised graphically in Figure 4.
For HA discs, the initial rates of erosion were almost constant (0 to 10 min: as observed 1.24 ± 0.08 mg/L min, relative to the mass of the specimen 0.041 ± 0.002 mg/g min, relative to the area from the dimensions 5.6(±0.3) × 10−5 mg/mm2 min), decreasing by ca. 15% over each complete time profile (20 to 30 min: 1.06 ± 0.01 mg/L min, 0.035 ± 0.001 mg/g min, 4.8(±0.1) × 10−5 mg/mm2 min). For bovine tooth sections the initial rates of dissolution were higher (8.0 mg/L min estimated from a tangent to curve (i) at the origin, Figure 4; average over 0 to 5 min 5.8 mg/L min, 0.058 mg/g min, 1.6 × 10−4 mg/mm2 min). In all experiments the highest total amounts of dissolved phosphorus after 30 min were equivalent to 4.6(±0.9)% of the mass of the HA specimens and 2.5(±0.3)% of the mass of the of bovine tooth specimens. The corresponding proportions reacted of citric acid were estimated respectively as 6(±1)% and 11(±1)%.
In comparing the erosion results from the two methods (15 HA specimens each), Figure 4 lines (ii) and (iii)), the errors were similar for results from both analytical methods (coefficients of variation, cv, 16–26% for ICP-MS and 18–25% for the phosphovanadomolybdate method). The mean values were consistent to within 5–11%, such that pairs of data at each time point did not differ significantly (‘t’ test for null hypothesis, p < 0.01). The errors in data were smaller for the results for bovine tooth specimens (cv 10–15%).
3.2. Effectiveness of Fluoride as Erosion-Inhibiting Agent
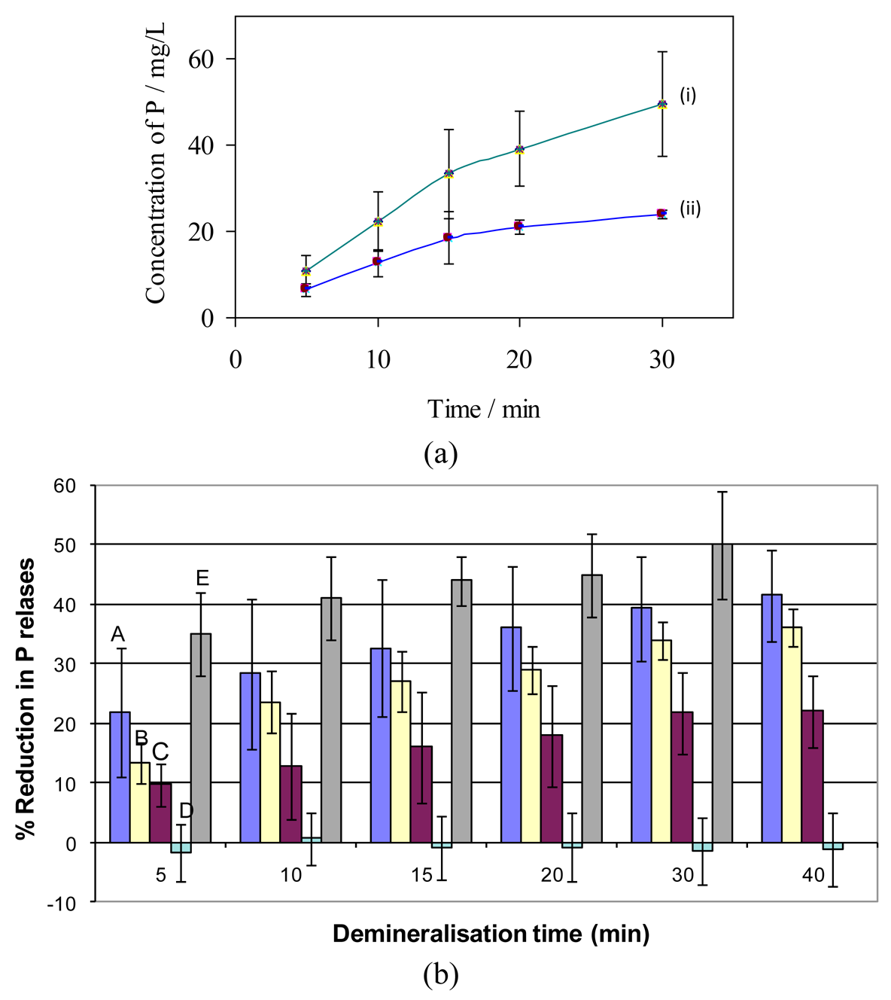
To compensate for the variability in the erosion rates of dental specimens under controlled conditions, the effects of protective treatments have been assessed in vitro by comparing measurements made before and after their application. Dissolution by citric acid (10 g/L, pH 3.75; 37 °C) was monitored by sampling at intervals over 30 min. The method was evaluated by using it to measure the effects of treating specimens with fluoride ion. Dose-response plots (n = 4 for each experiment) were constructed for HA discs that had been immersed (30 s or 120 s) in water or in aqueous sodium fluoride (fluoride ion concentrations 250-2,000 μg/g).
Treatment with fluoride ion (300 μg/g, 120 s) reduced the rate of erosion by 40 ± 10% over 0 to 5 min, increasing to 70 ± 20% over 20 to 30 min, Figure 5(a), reflecting the considerably reduced rate of erosion of the treated sample. Inhibition was increased by the use of higher concentrations of fluoride ion but the effect of using the longer treatment exposure time was much greater (Figure 5(b)). The proportional reductions in sampled phosphorus concentrations at each time point show lower variability (cv 8–22%) than the absolute values (cv 20–32% both before and after treatment).
3.3. Effectiveness of Poly(alkyl methacrylate) Coatings as Anti-Erosion Agents
Poly(alkyl methacrylate)s were selected as putative erosion-protection coatings because of their established good film-forming characteristics and of their affinity for the tooth surface [36]. For systematic investigation, the study employed a homologous series of polymers with glass transition temperatures in the range −100 to +65 ° C and surface energies in the range 17–34 mJ/m2, Table 1. For each specimen the inhibition of erosion was assessed from the relative initial rates of dissolution of phosphorus (average over 0–5 min) before and after coating. Relative rates of dissolution over 20–30 min were also obtained.
For HA specimens, erosion was slightly enhanced by pre-immersion in water, Table 2. Relative to this, significant inhibition of erosion was shown by specimens coated with PBMA (32% initially, decreasing to 22%) and PHxMA (24% initially, decreasing to 20%), Figure 6(a,b) and Table 2. Erosion also appeared to be inhibited by coating with PHMA (ca 9% initially) but the standard deviations were too large for the effect to be significant. The polymers PLMA and POMA formed coatings with very slight inhibiting effects while any effect of PEMA coatings was undetectable. For comparison, the inhibition by fluoride ion (300 μg/g) was 43% over 0–5 min increasing to 73% over 20–30 min, Figure 5(a).
In erosion tests using bovine tooth sections prior to treatment, Figure 6(c,d), dissolved phosphorus concentrations initially increased rapidly, reaching 40 mg/L after 10–15 min, but dissolution progressively slowed thereafter. Following measurements to establish baseline erosion rates [37], immersion of the specimens in water (120 s) appeared to inhibit erosion considerably, Table 2. The inhibition (fractional reduction in initial erosion rate) due to PBMA was similar to that given by fluoride ion (Table 2) and to that found using HA discs (F− 250 μg/g, 30 s; Figure 5(b)). Treatment with PHxMA also apparently inhibited erosion but the effect was not significantly greater than the inhibition by water. The effects of combined or sequential treatments with fluoride and PBMA or PHxMA were very close to those for single treatments with fluoride, Table 2. Longer term effects (10–30 min) could not be assessed from the measurements made.
4. Discussion
4.1. Comparison of Analytical Methods
The precision, consistency, the scatter of experimental data and the lower detection limits have been compared for the two analytical methods. In both methods samples were taken using 100 μL micropipettes. In the phosphovanadomolybdate method micropipettes were also used for adding the complexing reagent, whereas in ICP-MS each sample was diluted with a precise, relatively large, amount of aqueous HCl. Absorbances were measured to ±0.001, giving precision in the range 0.2–2%. In ICP-MS, counting rates were reproducible to <2%; other contributions to error arising from procedures are likely to be relatively small. The observed errors in the HA disc erosion experiments (cv, 16–26%; similar for the two methods) were much greater than 2%, while the differences (5–11%) between comparable mean analytical values were not significant. Therefore up to 8% of the variability may be attributed to the HA discs. For erosion experiments using bovine tooth specimens, the errors were smaller–indicating that these specimens were less variable than the HA discs. As used for these experiments, there was little to choose between the two analytical methods, which have different potential problems of interference. Samples for ICP-MS were, however, diluted by 1:140 whereas for the phosphovanadomolybdate method the dilution factor was 1:2. Therefore, allowing also for the minimum detection levels obtained, the ICP-MS method would be more sensitive by a conservative factor of ca. 40. This method could be used for measuring much lower levels of dissolved phosphate, for example from erosion experiments either over shorter time scales or using smaller dental samples.
4.2. Poly(alkyl methacrylate) Barrier Coatings
New agents or technologies incorporated into dentifrices and/or mouth-rinses in future may play an important role in enhancing the anti-erosion efficacy of the formulation. Ideally, these technologies should be fluoride-compatible since anti-caries activity is a key benefit of modern dentifrices and rinses, and the available in vitro and in situ studies suggest fluoride may be clinically relevant as an anti-erosion active. Although the polymeric materials investigated here are intended to form protective barrier coatings that protect the underlying mineral from acid attack, the established in vitro and in situ anti-erosion efficacy of fluoride effectively dictates its adoption as the positive/benchmark control in anti-erosion studies. The homologous series of poly(alkyl methacrylate)s investigated in the present study are characterised by a range of glass transition temperatures, and surface energies of deposited films; both exhibit decreasing trends with increasing pendent-chain length. With the exception of PEMA, the relative efficacy of each coating as an inhibitor of acid erosion of HA discs was also lower for the polymers with longer pendent chains, as indicated in Table 2. The behaviour of PEMA may be due to the poor film-forming characteristics of this polymer. Its Tg is higher than the temperature used for film deposition and its latex particle sizes exceeded 100 nm (Table 1), which is the maximum for the deposition of good quality films [36].
Data from the citric acid-mediated dissolution of untreated HA discs showed a progressive general decrease in erosion rate of 10–20% over each 30 min assay as shown in Figure 5(a). Since erosion might have been expected to accelerate due to a progressive increase in accessible area as the external surface became pitted, this decrease probably reflected the reducing aggressiveness of the challenge as the citric acid was consumed and calcium and phosphate were released. Throughout each assay the dissolution of HA discs after their treatment with PBMA or PHxMA was significantly less (p < 0.01) than that of the corresponding controls (before treatment). These consistent and statistically significant results identify PBMA or by PHxMA as potentially useful anti-erosion agents that may offer additional efficacy benefits to fluoride-based formulations, or new fluoride-free formulations.
That the initial rate of erosion relative to dimensions was approximately three times higher for bovine tooth sections than for HA discs is consistent with the tooth sections' greater exposed surface area, which was associated with the corrugated surface of a cut dentine surface together with the porosity associated with the tubules (Figure 2). Pre-etching of the enamel is also likely to have increased its surface area. Additional factors are possible, however, since the dissolution of biological HA is dependent on the molar ratio of calcium to phosphorus in the sample [38] and also because biological apatite is both more soluble and susceptible to partial transformation to other phosphate phases [39]. Further, the size and perfection of HA crystals may influence the rate of erosion, since smaller or less perfect crystals are more susceptible to dissolution [40]. Finally, the presence in biological apatite of carbonate ions and of other impurities may impact upon solubility [41,42]. The greater susceptibility of dentine to erosive demineralisation is well known and has been attributed to a combination of its porosity and its relatively small crystallites of HA [29]. Although the experiments showed that ca. 11% of the available citric acid had been consumed over the course of each assay (30 min), the decrease in the rate of erosion during the control measurements appeared to exceed what might have been expected from the changes in composition of the liquid phase. The rapidly declining rate of erosion of dentine (Figure 6(c,d)) may be due to the gradual build up of a collagen mat on the surface. This would explain the apparent inhibiting effect of water: after the control measurements the specimens would have become partially protected. The suitability of bovine- tooth specimens to act as their own controls in these experiments therefore becomes questionable. As a consequence, data obtained using these substrates may only be treated as indicative of trends in dissolution behaviour. Nevertheless, significant inhibition by PBMA of citric acid mediated erosion is confirmed. Treatments with both fluoride and a polymer coating produced similar inhibition to that with fluoride alone. Therefore although enhanced inhibition was not shown, neither was there any indication of incompatibility between the treatments.
5. Conclusions
Towards the development of an in vitro technique for the time-resolved quantification of acid-mediated tooth demineralisation, a method that monitors the release of phosphate from dental specimens has been used to evaluate, in comparison with the standard fluoride treatment, the relative efficiencies of protective coatings formed from a series of poly(alkyl methacrylate)s. To facilitate reproducibility and the control of experimental conditions, up to 12 dissolution assays were performed at the same time using a thermostatted carousel with automated sampling. Using HA discs and sections of bovine tooth, significant inhibition of demineralisation was shown by coatings of PBMA and PHxMA. The degree of protection offered by these two coatings was lower than that conferred by 300 mg/kg fluoride, and the present study indicated no significant improvement in erosion protection associated with combining the two treatments.
 ) bovine dentine (ICP-MS), (ii,
) bovine dentine (ICP-MS), (ii,
 ) hydroxyapatite discs (ICP-MS), and (iii,
) hydroxyapatite discs (ICP-MS), and (iii,
 ) hydroxyapatite discs (phosphovanadomolybdate complex absorbance); n = 15, error bars represent standard deviations.
) hydroxyapatite discs (phosphovanadomolybdate complex absorbance); n = 15, error bars represent standard deviations.
 ) bovine dentine (ICP-MS), (ii,
) bovine dentine (ICP-MS), (ii,
 ) hydroxyapatite discs (ICP-MS), and (iii,
) hydroxyapatite discs (ICP-MS), and (iii,
 ) hydroxyapatite discs (phosphovanadomolybdate complex absorbance); n = 15, error bars represent standard deviations.
) hydroxyapatite discs (phosphovanadomolybdate complex absorbance); n = 15, error bars represent standard deviations.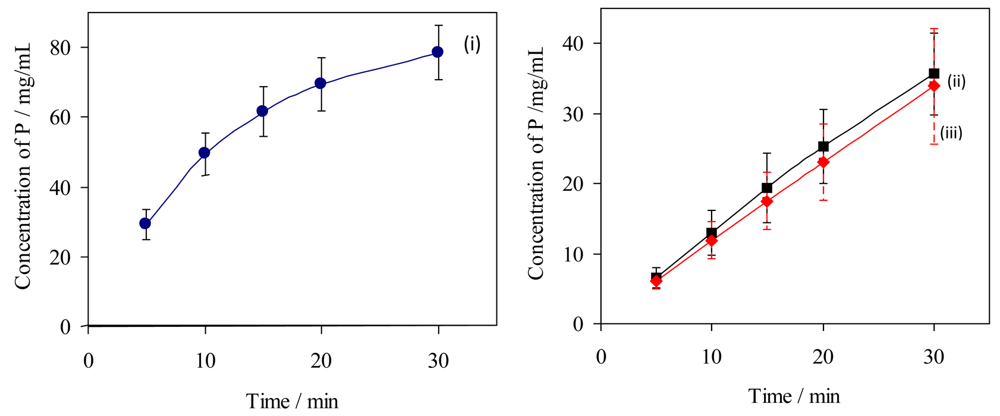
| Polymer | Code | RMM | γ/mJ/m2 | Tg/°C | d (sd)/nm |
|---|---|---|---|---|---|
| Poly(ethyl methacrylate) | PEMA | 515,000 | 33.6 | 65 | 197 (8) |
| Poly(butyl methacrylate) | PBMA | 337,000 | 28.8 | 20 | 74 (12) |
| Poly(hexyl methacrylate) | PHxMA | 400,000 | 23.1 | −5 | 94 (3) |
| Poly(lauryl methacrylate) | PLMA | 576,400 | 19.1 | −70 | 91 (7) |
| Poly(hexadecyl methacrylate) | PHMA | 200,000 | - | 15 | 91 (5) |
| Poly(octadecyl methacrylate) | POMA | 170,000 | 17.6 | −100 | 73 (14) |
| Specimen | Treatment (120 s) | Fractional reduction in initial rate of erosion (SD) |
|---|---|---|
| HA | 300 μg/g F− | 0.38 (0.07) a |
| Water | −0.05 (0.07) | |
| PEMA | −0.02 (0.07) | |
| PBMA | 0.27 (0.07)a | |
| PHxMA | 0.19 (0.08)a | |
| PLMA | 0.05 (0.10) | |
| PHMA | 0.09 (0.10) | |
| POMA | 0.02 (0.08) | |
| Bovine tooth section | 300 μg/g F− | 0.37 (0.04) |
| Water | 0.19 (0.02) | |
| PBMA | 0.35 (0.02)b | |
| PHxMA | 0.23 (0.05)c | |
| 300 μg/g F− + PBMA | 0.35 (0.01)b | |
| (i) PBMA (ii) 300 μg/g F− | 0.35 (0.02)b | |
| 300 μg/g F− + PHxMA | 0.33 (0.06)b | |
For HA specimensadenotes that the mean value is significantly different (p < 0.01) from that of the water control; for bovine tooth sectionsbdenotes that the mean value is not statistically different (p < 0.05) from the fluoride control;cdenotes that the mean value is not statistically different (p < 0.05) from the water-treated control (one-way ANOVA with a post hoc Fisher's test).
References
- Hannig, C.; Hamkens, A.; Becker, K.; Attin, R.; Attin, T. Erosive effects of different acids on bovine enamel: release of calcium and phosphate in vitro. Arch. Oral Biol. 2005, 50, 541–552. [Google Scholar]
- Hughes, J.A.; West, N.X.; Parker, D.M.; van den Braak, M.H.; Addy, M. Effects of pH and concentration of citric, malic and lactic acids on enamel in vitro. J. Dent. 2000, 28, 147–152. [Google Scholar]
- Koulourides, T.A.; Bouonocore, M.G. Effect of organic ions on solubility of enamel and dentine in acid buffers. J. Dent. Res. 1961, 40, 578–593. [Google Scholar]
- Meurman, J.H.; Rytomaa, I.; Kari, K.; Laakso, T.; Murtomaa, H. Salivary pH and glucose after consuming various beverages, including sugar-containing drinks. Caries Res. 1987, 21, 353–359. [Google Scholar]
- Hannig, M.; Balz, M. Protective properties of salivary pellicle from two different intraoral sites on enamel erosion. Caries Res. 2001, 35, 142–148. [Google Scholar]
- Nekrashevych, Y.; Hannig, M.; Stosser, L. Assessment of enamel erosion and protective effect of salivary pellicle by surface roughness analysis and scanning electron microscopy. Oral Health Prev. Dent. 2004, 2, 5–11. [Google Scholar]
- Clarkson, J.J.; McLoughlin, J. Role of fluoride in oral health promotion. Int. Dent. J. 2000, 50, 119–128. [Google Scholar]
- Reich, E. Trends in caries and periodontal health epidemiology in Europe. Int. Dent. J. 2001, 51, 392–398. [Google Scholar]
- Jaeggi, T.; Lussi, A. Prevalence, incidence and distribution of erosion. Monographs in Oral Science 2006, 20, 44–65. [Google Scholar]
- El Aidi, H.; Bronkhorst, E.M.; Huysmans, M.C.; Truin, G.J. Dynamics of tooth erosion in adolescents: A 3-year longitudinal study. J. Dent. 2010, 38, 131–137. [Google Scholar]
- Nunn, J.H. Prevalence of dental erosion and implications for oral health. Eur. J. Oral Sci. 1996, 204, 156–161. [Google Scholar]
- Barbour, M.E.; Rees, G.D. The role of erosion, abrasion and attrition in tooth wear. J. Clin. Dent. 2006, 17, 88–93. [Google Scholar]
- Magalhães, A.C.; Wiegand, A.; Rios, D.; Honório, H.M.; Buzalaf, M.A. Insights into preventive measures for dental erosion. J. Appl. Oral Sci. 2009, 17, 75–86. [Google Scholar]
- Moss, S.J. Dental erosion. Int. Dent. J. 1998, 48, 529–539. [Google Scholar]
- Venasakulchai, A.; Williams, N.A.; Gracia, L.H.; Rees, G.D. A comparative evaluation of fluoridated and non-fluoridated mouthrinses using a 5-day cycling enamel erosion model. J. Dent. 2010, 38, S21–S29. [Google Scholar]
- Chen, Y.; Miao, X. Thermal and chemical stability of fluorohydroxyapatite ceramics with different fluorine contents. Biomaterials 2005, 26, 1205–1210. [Google Scholar]
- Diarra, M.; Pourroy, G.; Boymond, C.; Muster, D. Fluoride controlled release tablets for intrabuccal use. Biomaterials 2003, 24, 1293–1300. [Google Scholar]
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological factors in dental caries enamel structure and the caries process in the dynamic process of demineralization and remineralization (part 2). J. Clin. Pediatr. Dent. 2004, 28, 119–124. [Google Scholar]
- ten Cate, J.M.; Featherstone, J.D. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit. Rev. Oral Biol. Med. 1991, 2, 283–296. [Google Scholar]
- Petersson, G.H.; Bratthall, D. The caries decline: A review of reviews. Eur. J. Oral Sci. 1996, 104, 436–443. [Google Scholar]
- Mason, S.C.; Shirodaria, S.; Sufi, F.; Rees, G.D.; Birkhed, D. Evaluation of salivary fluoride retention from a new high fluoride mouthrinse. J. Dent. 2010, 38, S30–S36. [Google Scholar]
- Ganss, C.; Klimek, J.; Schaffer, U.; Spall, T. Effectiveness of two fluoridation measures on erosion progression in human enamel and dentine in vitro. Caries Res. 2001, 35, 325–330. [Google Scholar]
- Ganss, C.; Klimek, J.; Brune, V.; Schürmann, A. Effects of two fluoridation measures on erosion progression in human enamel and dentine in situ. Caries Res. 2004, 38, 561–566. [Google Scholar]
- Mellberg, J.R. Hard-tissue substrates for the evaluation of cariogenic and anti-cariogenic activity in situ. J. Dent. Res. 1992, 71, 913–919. [Google Scholar]
- Attin, T.; Wegehaupt, F.; Gries, D.; Wiegand, A. The potential of deciduous and permanent bovine enamel as substitute for deciduous and permanent human enamel: Erosion-abrasion experiments. J. Dent. 2007, 35, 773–777. [Google Scholar]
- Barbour, M.E.; Rees, J.S. The laboratory assessment of enamel erosion: A review. J. Dent. 2004, 32, 591–602. [Google Scholar]
- Churchley, D.; Rees, G.D.; Barbu, E.; Nevell, T.G.; Tsibouklis, J. Fluoropolymers as low-surface-energy tooth coatings for oral care. Int. J. Pharm. 2008, 352, 44–49. [Google Scholar]
- Finke, M.; Jandt, K.D.; Parker, D.M. The early stages of native enamel dissolution studied with atomic force microscopy. J. Colloid Interface Sci. 2000, 232, 156–164. [Google Scholar]
- Hara, A.T.; Ando, M.; Gonzalez-Cabezas, C.; Cury, J.A.; Serra, M.C.; Zero, D.T. Protective effect of the dental pellicle against erosive challenges in situ. J. Dent. Res. 2006, 85, 612–616. [Google Scholar]
- Esser, M.; Trinschert, J.; Marx, R. Material characteristics of the hard tissues of bovine versus human teeth. Dtsch. Zahnarztl. Z. 1998, 53, 731–717. [Google Scholar]
- Garberoglio, R.; Brannstrom, M. Scanning electron microscopic investigation of human dentinal tubules. Arch. Oral Biol. 1976, 21, 355–362. [Google Scholar]
- Ketterl, W. The dentine in permanent human teeth. Stoma 1961, 14, 79–96. [Google Scholar]
- Tagami, J.; Tao, L.; Pashley, D.H.; Horner, J.A. The permeability of dentine from bovine incisors in vitro. Arch. Oral Biol. 1989, 34, 773–777. [Google Scholar]
- Wulf, M.; Grundke, K.; Kwok, D.Y.; Neumann, A.W. The influence of different alkyl side chains on the solid surface tension of polymethacrylates. J. Appl. Polym. Sci. 2000, 77, 2493–2504. [Google Scholar]
- Mason, S.; Hamon, R.; Nolan, A.; Zhang, H.; Davison, W. Performance of a mixed binding layer for measuring anions and cations in a single assay using the diffusive gradients in thin films technique. Anal. Chem. 2005, 77, 6339–6346. [Google Scholar]
- Nielsen, B.V.; Nevell, T.G.; Barbu, E.; Rees, G.D.; Tsibouklis, J. A thermogravimetric method for assessing the substantivity of polymer films on dentally relevant substrates. J. Therm. Anal. Calorim. 2010, 102, 121–126. [Google Scholar]
- Hara, A.T.; Ando, M.; Cury, J.A.; Serra, M.C.; Gonzalez-Cabezas, C.; Zero, D.T. Influence of the organic matrix on root dentine erosion by citric acid. Caries Res. 2005, 39, 134–138. [Google Scholar]
- Mavropoulos, E.; Rossi, A.M.; da Rocha, N.C.C.; Soares, G.A.; Moreira, J.C.; Moure, G.T. Dissolution of calcium-deficient hydroxyapatite synthesized at different conditions. Mater. Charact. 2003, 50, 203–207. [Google Scholar]
- Sun, R.; Li, M.; Lu, Y.; Li, S. Dissolution behavior of hollow hydroxyapatite microspheres immersed in deionised water. Mater. Res. Bull. 2006, 41, 1138–1145. [Google Scholar]
- Pleshko, N.; Boskey, A.; Mendelsohn, R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 1991, 60, 786–793. [Google Scholar]
- Nikcevic, I.; Jokanovic, V.; Mitric, M.; Nedic, Z.; Makovec, D.; Uskokovic, D. Mechanochemical synthesis of nanostructured fluorapatite/fluorhydroxyapatite and carbonated fluorapatite/fluorhydroxyapatite. J. Solid State Chem. 2004, 177, 2565–2574. [Google Scholar]
- Shellis, R.P.; Wilson, R.M. Apparent solubility distributions of hydroxyapatite and enamel apatite. J. Colloid Interface Sci. 2004, 278, 325–332. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nielsen, B.V.; Nevell, T.G.; Barbu, E.; Smith, J.R.; Rees, G.D.; Tsibouklis, J. Poly(alkyl methacrylate) Tooth Coatings for Dental Care: Evaluation of the Demineralisation-Protection Benefit Using a Time-Resolved In Vitro Method. Polymers 2011, 3, 314-329. https://doi.org/10.3390/polym3010314
Nielsen BV, Nevell TG, Barbu E, Smith JR, Rees GD, Tsibouklis J. Poly(alkyl methacrylate) Tooth Coatings for Dental Care: Evaluation of the Demineralisation-Protection Benefit Using a Time-Resolved In Vitro Method. Polymers. 2011; 3(1):314-329. https://doi.org/10.3390/polym3010314
Chicago/Turabian StyleNielsen, Birthe V., Thomas G. Nevell, Eugen Barbu, James R. Smith, Gareth D. Rees, and John Tsibouklis. 2011. "Poly(alkyl methacrylate) Tooth Coatings for Dental Care: Evaluation of the Demineralisation-Protection Benefit Using a Time-Resolved In Vitro Method" Polymers 3, no. 1: 314-329. https://doi.org/10.3390/polym3010314