Tailoring Mechanical Properties of Collagen-Based Scaffolds for Vascular Tissue Engineering: The Effects of pH, Temperature and Ionic Strength on Gelation
Abstract
:1. Introduction
2. Experimental Section
| Factors | Levels | ||||||
|---|---|---|---|---|---|---|---|
| pH | 7 | 10 | |||||
| T (°C) | 4 (T1) | 21 (T2) | 37 (T3) | ||||
| Salt Conc. (mM) | 64.2 (c1) | 82.6 (c2) | 101 (c3) | 119 (c4) | 138 (c5) | 156 (c6) | 174 (c7) |
3. Results and Discussion
3.1. Turbidity Measurements
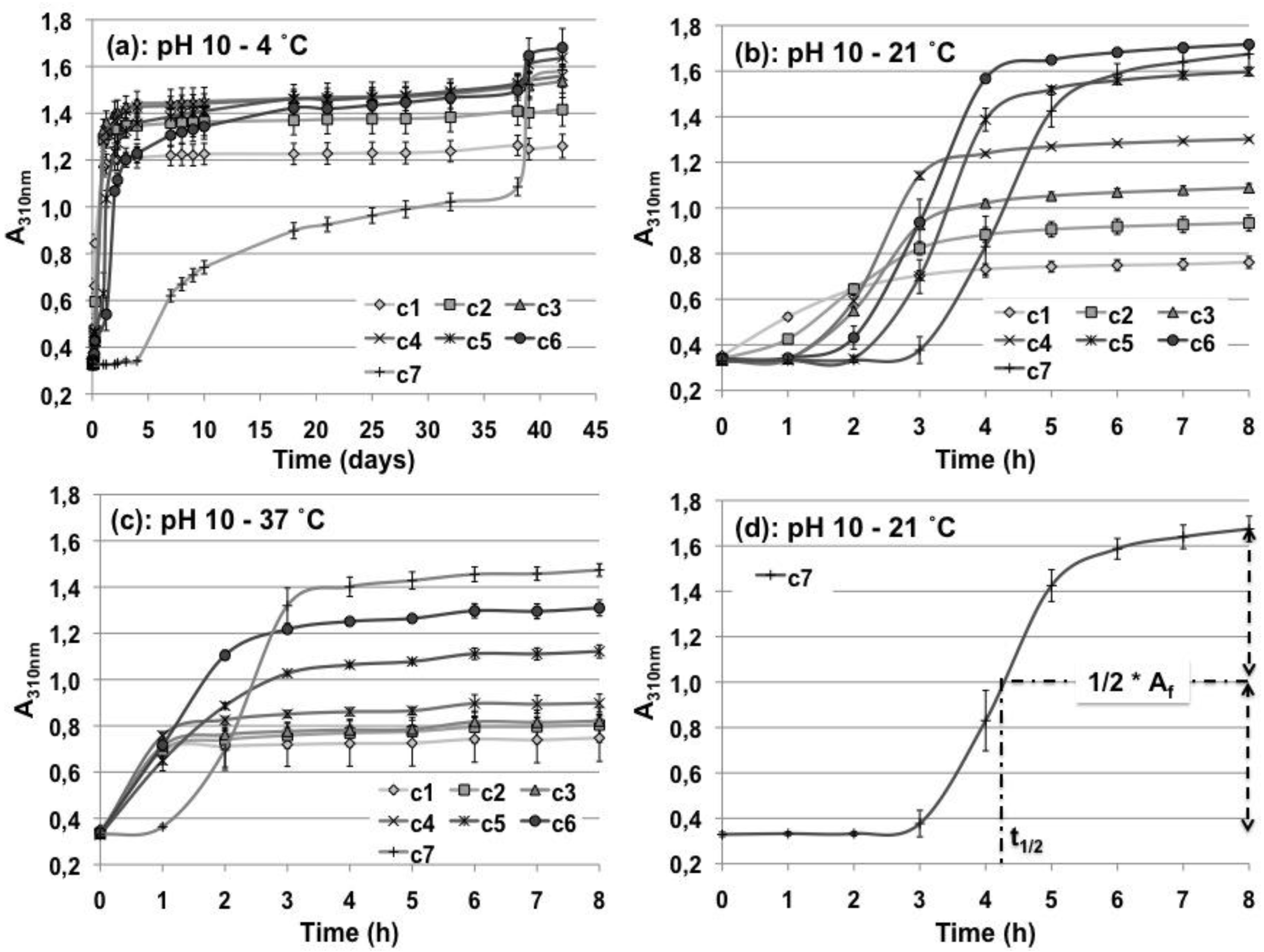
| pH 7 | pH 10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A310nm (T1) | A310nm (T2) | A310nm (T1*) | A310nm (T1) | t1/2 | A310nm (T2) | t1/2 | A310nm (T3) | t1/2 | |
| c1 | 1.5 ± 0.05 | 1.60 ± 0.07 | 1.26 ± 0.05 | 1.26 ± 0.04 | 5.6 h | 0.79 ± 0.03 | 1.4 h | 0.8 ± 0.12 | 21 min |
| c2 | 1.76 ± 0.02 | 1.83 ± 0.09 | 1.41 ± 0.07 | 1.41 ± 0.06 | 9 h | 0.97 ± 0.03 | 2 h | 0.88 ± 0.09 | 33 min |
| c3 | 1.87 ± 0.03 | 1.96 ± 0.03 | 1.53 ± 0.06 | 1.51 ± 0.05 | 13 h | 1.13 ± 0.02 | 2.4 h | 0.86 ± 0.01 | 28 min |
| c4 | 2.00 ± 0.02 | 2.06 ± 0.08 | 1.55 ± 0.04 | 1.52 ± 0.04 | 14 h | 1.35 ± 0.01 | 2.4 h | 0.94 ± 0.04 | 31 min |
| c5 | 2.02 ± 0.04 | 2.10 ± 0.02 | 1.64 ± 0.04 | 1.53 ± 0.04 | 29 h | 1.66 ± 0.02 | 3.4 h | 1.21 ± 0.03 | 1.5 h |
| c6 | 2.18 ± 0.03 | 2.04 ± 0.07 | 1.68 ± 0.08 | 1.5 ± 0.06 | 44 h | 1.78 ± 0.02 | 3.2 h | 1.41 ± 0.04 | 1.35 h |
| c7 | 2.16 ± 0.05 | 2.14 ± 0.05 | 1.59 ± 0.05 | 1.09 ± 0.04 | 24 d | 1.78 ± 0.06 | 4.34 h | 1.54 ± 0.03 | 2.3 h |
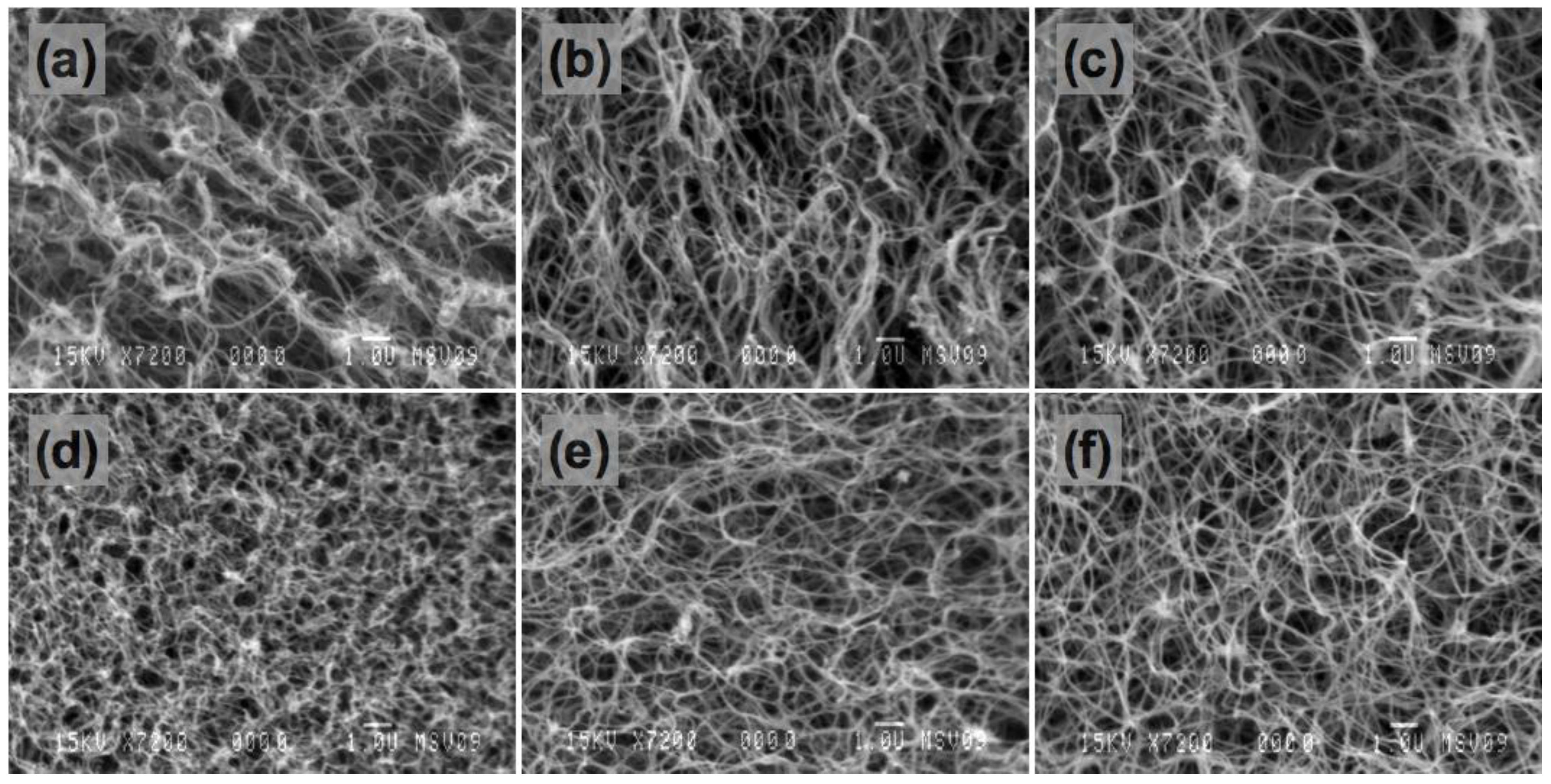
3.2. SEM
3.3. Mechanical Tests


| CM (kPa) | ||||||
|---|---|---|---|---|---|---|
| pH 7 | pH 10 | |||||
| Salt Conc. | T3 | T2 | T1 | T3 | T2 | T1 |
| c1 | 10.91± 0.63 | 10.40 ± 2.20 | 13.54 ± 0.004 | 15.72 ± 4.65 | 19.36 ± 1.70 | 29.25 ± 2.08 |
| c2 | 13.67 ± 0.63 | 11.14 ± 2.11 | 11.74 ± 4.93 | 18.16 ± 0.63 | 22.91 ± 3.67 | 26.08 ± 4.51 |
| c3 | 12.47 ± 0.31 | 11.94 ± 0.82 | 10.4 ± 4.42 | 17.71 ± 1.97 | 16.97 ± 3.67 | 27.1 ± 3.36 |
| c4 | 10.78 ± 1.52 | 8.33 ± 4.68 | 9.50 ± 2.07 | 15.46 ± 1.89 | 12.45 ± 0.93 | 33.26 ± 1.75 |
| c5 | 7.41 ± 1.58 | 8.32 ± 0.48 | 7.93 ± 1.87 | 16.88 ± 1.46 | 16.33 ± 1.82 | 33.36 ± 1.30 |
| c6 | 7.73 ± 1.95 | 7.39 ± 0.51 | 6.13 ± 1.18 | 8.72 ± 4.97 | 10.30 ± 0.77 | 44.3 ± 7.27 |
| c7 | 7.15 ± 1.3 | 5.54 ± 0.30 | 8.09 ± 0.89 | 8.92 ± 1.30 | 12.59 ± 3.06 | 47.96 ± 1.88 |
| CSE (kPa) | ||||||
| c1 | 0.56 ± 0.03 | 0.55 ± 0.12 | 0.74 ± 0.13 | 0.68 ± 0.1 | 1.07 ± 0.04 | 1.80 ± 0.17 |
| c2 | 0.69 ± 0.01 | 0.59 ± 0.03 | 0.75 ± 0.19 | 0.84 ± 0.16 | 1.25 ± 0.05 | 1.84 ± 0.23 |
| c3 | 0.73 ± 0.03 | 0.76 ± 0.01 | 0.79 ± 0.05 | 0.81 ± 0.12 | 1.31 ± 0.17 | 1.91 ± 0.16 |
| c4 | 0.65 ± 0.04 | 0.69 ± 0.12 | 0.74 ± 0.03 | 0.8 ± 0.12 | 0.97 ± 0.01 | 2.14 ± 0.29 |
| c5 | 0.52 ± 0.11 | 0.56 ± 0.02 | 0.6 ± 0.08 | 0.90 ± 0.07 | 1.02 ± 0.07 | 2.53 ± 0.26 |
| c6 | 0.49 ± 0.06 | 0.58 ± 0.08 | 0.68 ± 0.01 | 0.65 ± 0.06 | 0.89 ± 0.08 | 2.78 ± 0.16 |
| c7 | 0.54 ± 0.03 | 0.51 ± 0.11 | 0.84 ± 0.03 | 0.69 ± 0.001 | 0.87 ± 0.12 | 2.8 ± 0.35 |

3.4. Viability Test

4. Conclusions
Acknowledgements
References
- WHO Fact Sheet N° 317—Cardiovascular Diseases. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed on 5 July 2010).
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Hamilton, G.; Seifalian, A.M. Current Status of Prosthetic Bypass Grafts: A Review. J. Biomed. Mat. Res. B Appl. Biomater. 2005, 74B, 570–581. [Google Scholar] [CrossRef]
- Ratcliffe, A. Tissue Engineering of Vascular Grafts. Matrix Biol. 2000, 19, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, B.C.; Williams, C.; Tranquillo, R.T. Small-Diameter Artificial Arteries Engineered in vitro. Circ. Res. 2006, 98, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kakisis, J.D.; Liapis, C.D.; Breuer, C.; Sumpio, B.E. Artificial Blood Vessel: The Holy Grail of Peripheral Vascular Surgery. J. Vasc. Surg. 2005, 41, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Chevallay, B.; Herbage, D. Collagen-Based Biomaterials as 3D Scaffold for Cell Cultures: Applications for Tissue Engineering and Gene Therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Habermehl, J.; Vesentini, S.; Mantovani, D. Biological Performances of Collagen-Based Scaffolds for Vascular Tissue Engineering. Biomaterials 2005, 26, 7410–7417. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voet, D.; Voet, J.G. Three-Dimensional Structures of Proteins—Fibrous Proteins—Collagen. In Biochemistry, 3rd ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2004; pp. 233–239. [Google Scholar]
- Rajan, N.; Habermehl, J.; Cote, M.-F.; Doillon, C.J.; Mantovani, D. Preparation of Ready-to-Use, Storable and Reconstituted Type I Collagen from Rat Tail Tendon for Tissue Engineering Applications. Nat. Protoc. 2007, 1, 2753–2758. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.W.; Seehra, G.P. Collagen Self-Assembly and the Development of Tendon Mechanical Properties. J. Biomech. 2003, 36, 1529–1553. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Rajan, N.; Habermehl, J.; Mantovani, D. Preparation and Characterization of a Scaffold for Vascular Tissue Engineering by Direct-Assembling of Collagen and Cells in a Cylindrical Geometry. Macromol. Biosci. 2007, 7, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Habermehl, J.; Skopinska, J.; Boccafoschi, F.; Sionkowska, A.; Kaczmarek, H.; Laroche, G.; Mantovani, D. Preparation of Ready-to-Use, Stockable and Reconstituted Collagen. Macromol. Biosci. 2005, 5, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Lanza, R.P. Methods of Tissue Engineering; Academic Press: London, UK, 2002. [Google Scholar]
- Seliktar, D.; Black, A.R.; Vito, R.P.; Nerem, R.M. Dynamic Mechanical Conditioning of Collagen-Gel Blood Vessel Constructs Induces Remodeling in vitro. Ann. Biomed. Eng. 2000, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Niklason, L.E.; Gao, J.; Abbott, W.M.; Hirschi, K.K.; Houser, S.; Marini, R.; Langer, R. Functional Arteries Grown in vitro. Science 1999, 284, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Beckman, M.J.; Shields, K.J.; Diegelmann, R.F. Collagen. In Encyclopedia of Biomaterials and Biomedical Engineering; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 324–334. [Google Scholar]
- Yang, Y.-L.; Kaufman, L.J. Rheology and Confocal Reflectance Microscopy as Probes of Mechanical Properties and Structure during Collagen and Collagen/Hyaluronan Self-Assembly. Biophys. J. 2009, 96, 1566–1585. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.C. The Formation of Fibrils from Collagen Solutions. A Mechanism of Collagen-Fibril Formation. Biochem. J. 1960, 75, 598–605. [Google Scholar] [PubMed]
- Rosenblatt, J.; Devereux, B.; Wallace, D.G. Injectable Collagen as a pH-Sensitive Hydrogel. Biomaterials 1994, 15, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Roeder, B.A.; Kokini, K.; Sturgis, J.E.; Robinson, J.P.; Voytik-Harbin, S.L. Tensile Mechanical Properties of Three-Dimensional Type I Collagen Extracellular Matrices with Varied Microstructure. J. Biomed. Eng. 2002, 124, 214–222. [Google Scholar]
- Gobeaux, F.; Mosser, G.; Anglo, A.; Panine, P.; Davidson, P.; Giraud-Guille, M.M.; Belamie, E. Fibrillogenesis in Dense Collagen Solutions: A Physicochemical Study. J. Mol. Biol. 2008, 376, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.C.; Keech, M.K. The Formation of Fibrils from Collagen Solutions-The Effect of Experimental Conditions: Kinetic and Electron-Microscope Studies. Biochem. J. 1960, 75, 588–598. [Google Scholar]
- Williams, B.R.; Gelman, R.A.; Poppke, D.C.; Piez, K.A. Collagen Fibril Formation. Optimal in vitro Conditions and Preliminary Kinetic Results. J. Biol. Chem. 1978, 253, 6578–6585. [Google Scholar] [PubMed]
- Achilli, M.; Lagueux, J.; Mantovani, D. On the Effects of UV-C and pH on the Mechanical Behavior, Molecular Conformation and Cell Viability of Collagen-Based Scaffold for Vascular Tissue Engineering. Macromol. Biosci. 2010, 10, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Couet, F.; Rajan, N.; Mantovani, D. Macromolecular Biomaterials for Scaffold-based Vascular Tissue Engineering. Macromol. Biosci. 2007, 7, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.L.; Gawlitta, D.; Nerem, R.M.; Stegemann, J.P. Properties of Engineered Vascular Constructs Made from Collagen, Fibrin, and Collagen-Fibrin Mixtures. Biomaterials 2004, 25, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.D.; Mohseni, M.M.; Nerem, R.M.; Sambanis, A. A Biological Hybrid Model for Collagen-Based Tissue Engineered Vascular Constructs. Biomaterials 2003, 24, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Koob, T.J. Collagen Fixation. In Encyclopedia of Biomaterials and Biomedical Engineering; Marcel Dekker: New York, NY, USA, 2004; pp. 335–347. [Google Scholar]
- Helseth, D.L., Jr; Veis, A. Collagen Self-Assembly in vitro. Differentiating Specific Telopeptide-Dependent Interactions Using Selective Enzyme Modification and the Addition of Free Amino Telopeptide. J. Biol. Chem. 1981, 256, 7118–7128. [Google Scholar] [PubMed]
- Na, G.; Butz, L.; Carroll, R. Mechanism of in vitro Collagen Fibril Assembly. Kinetic and Morphological Studies. J. Biol. Chem. 1986, 261, 12290–12299. [Google Scholar] [PubMed]
- Cooper, A. Thermodynamic Studies of the Assembly in vitro of Native Collagen Fibrils. Biochem. J. 1970, 118, 355–365. [Google Scholar] [PubMed]
- Yang, Y.-L.; Leone, L.M.; Kaufman, L.J. Elastic Moduli of Collagen Gels Can Be Predicted from Two-Dimensional Confocal Microscopy. Biophys. J. 2009, 97, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Raub, C.B.; Suresh, V.; Krasieva, T.; Lyubovitsky, J.; Mih, J.D.; Putnam, A.J.; Tromberg, B.J.; George, S.C. Noninvasive Assessment of Collagen Gel Microstructure and Mechanics Using Multiphoton Microscopy. Biophys. J. 2007, 92, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Raub, C.B.; Unruh, J.; Suresh, V.; Krasieva, T.; Lindmo, T.; Gratton, E.; Tromberg, B.J.; George, S.C. Image Correlation Spectroscopy of Multiphoton Images Correlates with Collagen Mechanical Properties. Biophys. J. 2008, 94, 2361–2373. [Google Scholar] [PubMed]
- Gentleman, E.; Lay, A.N.; Dickerson, D.A.; Nauman, E.A.; Livesay, G.A.; Dee, K.C. Mechanical Characterization of Collagen Fibers and Scaffolds for Tissue Engineering. Biomaterials 2003, 24, 3805–3813. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Reiber, A. Influence of Saline and pH on Collagen Type I Fibrillogenesis in vitro: Fibril Polymorphism and Colloidal Gold Labelling. Micron 2007, 38, 513–521. [Google Scholar] [PubMed]
- Rosenblatt, J.; Devereux, B.; Wallace, D.G. Effect of Electrostatic Forces on the Dynamic Rheological Properties of Injectable Collagen Biomaterials. Biomaterials 1992, 13, 878–886. [Google Scholar] [PubMed]
- Couet, F.; Mantovani, D. How to Optimize Maturation in a Bioreactor for Vascular Tissue Engineering: Focus on a Decision Algorithm for Experimental Planning. Ann. Biomed. Eng. 2010, 38, 2877–2884. [Google Scholar] [CrossRef]
- Bilodeau, K.; Couet, F.; Boccafoschi, F.; Mantovani, D. Design of a Perfusion Bioreactor Specific to the Regeneration of Vascular Tissues Under Mechanical Stresses. Artif. Organs 2005, 29, 906–922. [Google Scholar] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Achilli, M.; Mantovani, D. Tailoring Mechanical Properties of Collagen-Based Scaffolds for Vascular Tissue Engineering: The Effects of pH, Temperature and Ionic Strength on Gelation. Polymers 2010, 2, 664-680. https://doi.org/10.3390/polym2040664
Achilli M, Mantovani D. Tailoring Mechanical Properties of Collagen-Based Scaffolds for Vascular Tissue Engineering: The Effects of pH, Temperature and Ionic Strength on Gelation. Polymers. 2010; 2(4):664-680. https://doi.org/10.3390/polym2040664
Chicago/Turabian StyleAchilli, Matteo, and Diego Mantovani. 2010. "Tailoring Mechanical Properties of Collagen-Based Scaffolds for Vascular Tissue Engineering: The Effects of pH, Temperature and Ionic Strength on Gelation" Polymers 2, no. 4: 664-680. https://doi.org/10.3390/polym2040664





