Spray Coating of Wood with Nanoparticles from Lignin and Polylactic Glycolic Acid Loaded with Thyme Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil-Containing Nanoparticle Preparation
2.2. Spray Coating of Wood Samples
- CU—coating uptake in percent;
- w1—uncoated sample weight after drying at 40 °C for 2 h;
- w2—coated sample weight after drying at 40 °C for 2 h.
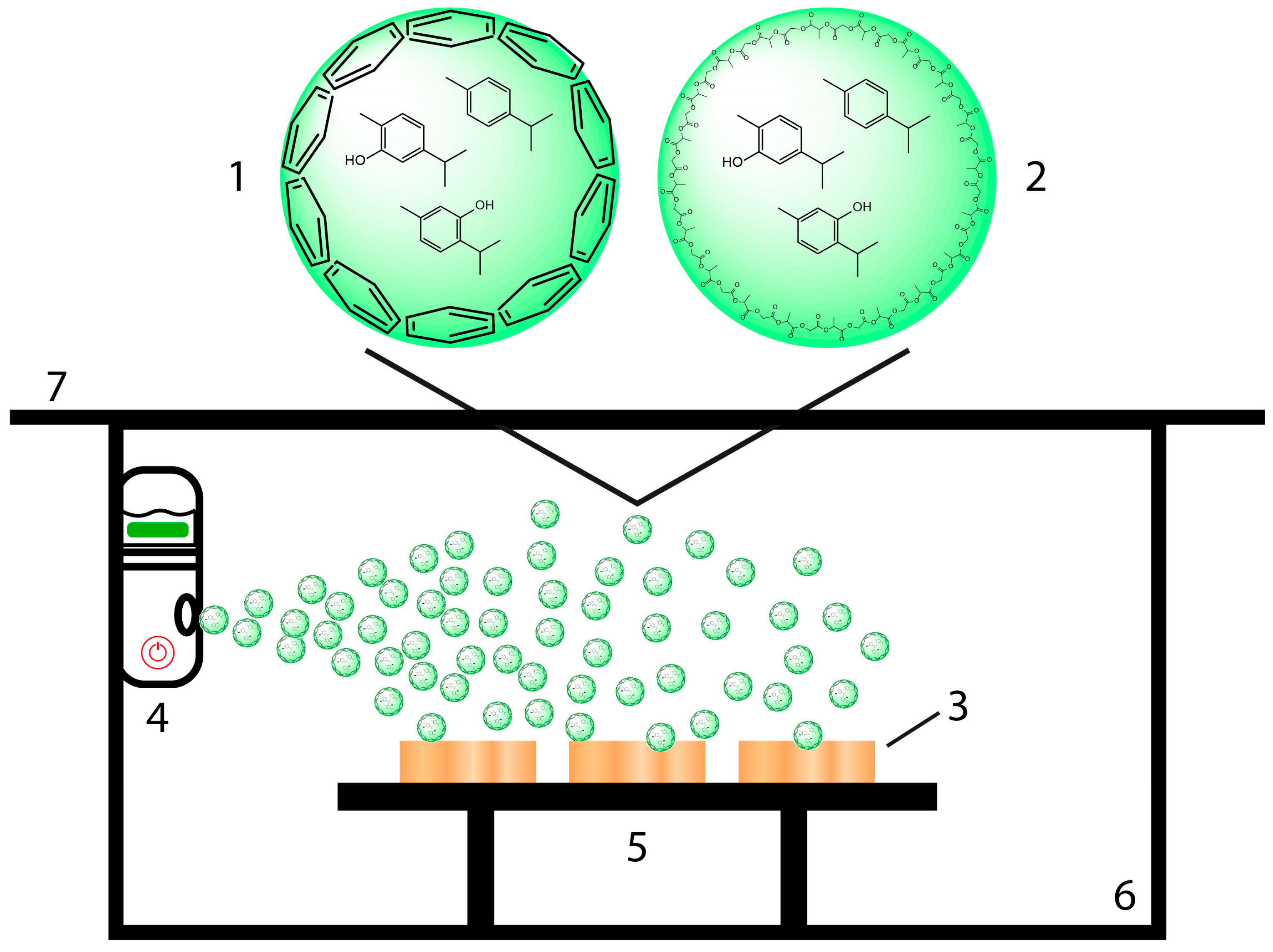
2.3. Release Experiments of EOs from Spray-Coated Wood Samples
2.4. FTIR Imaging
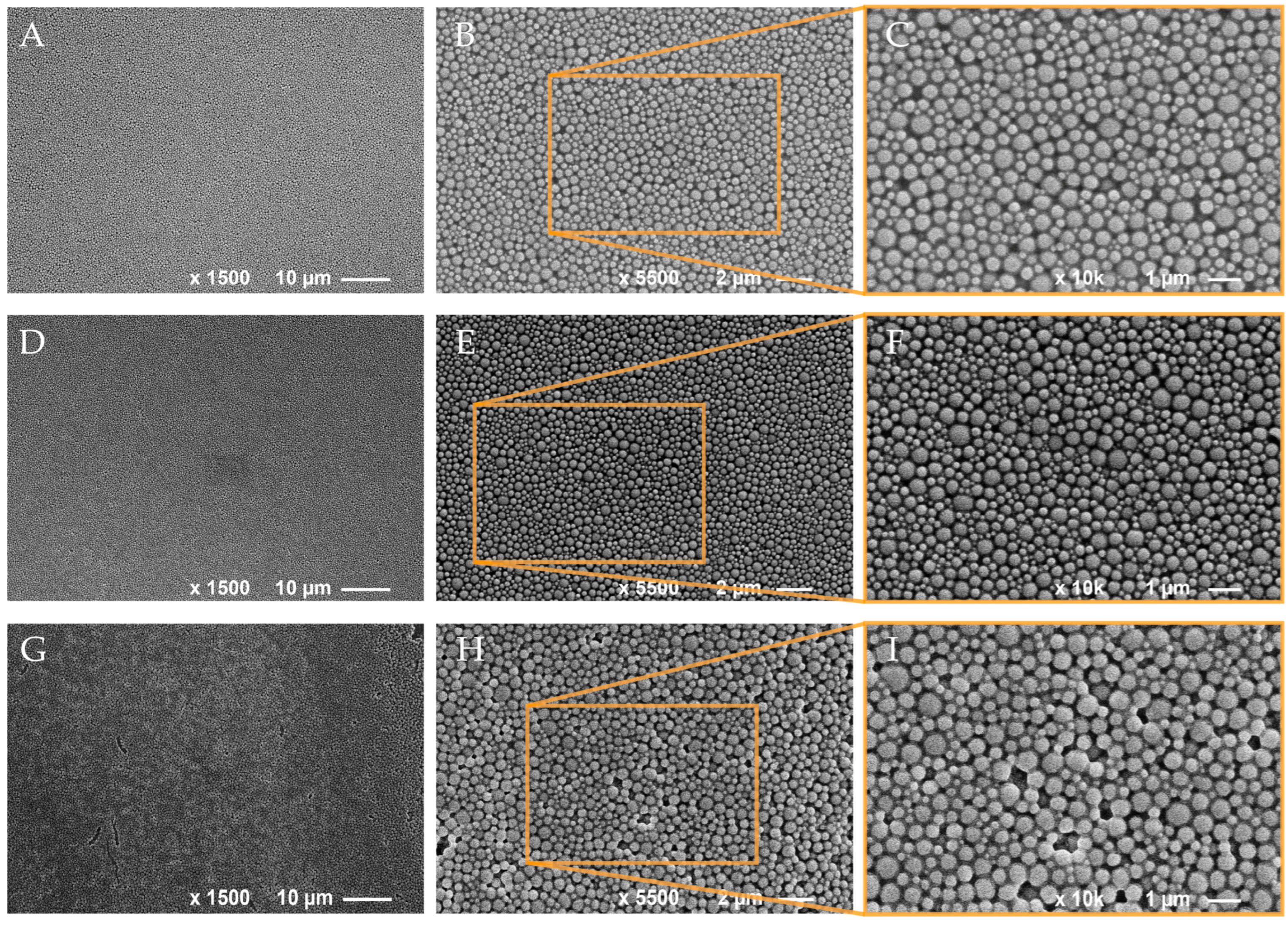
2.5. Scanning Electron Microscopy
2.6. Confocal Laser Scanning Microscopy
3. Results and Discussion
3.1. Characterization of Lignin Nanoparticles Loaded with Essential Oils (EOLs)
3.2. Spray Coating of NP Dispersions onto Wood Samples
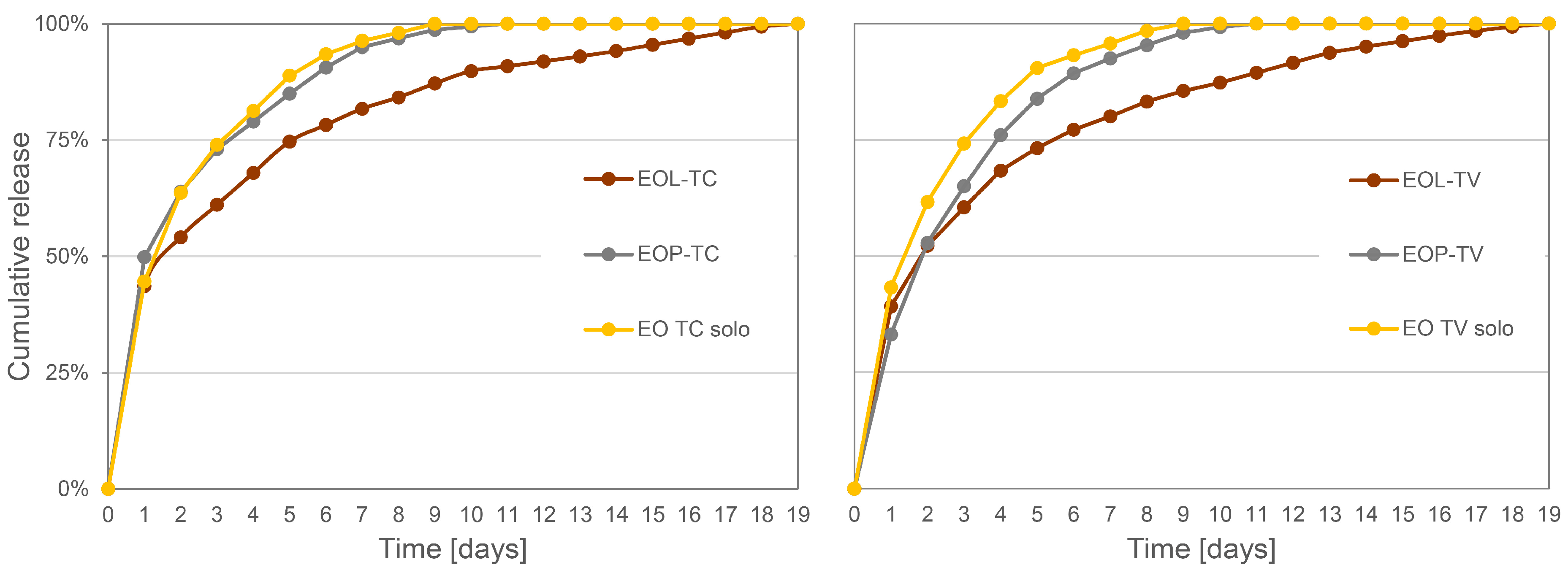
3.3. Release Experiments of Essential Oils from the Spray Coatings
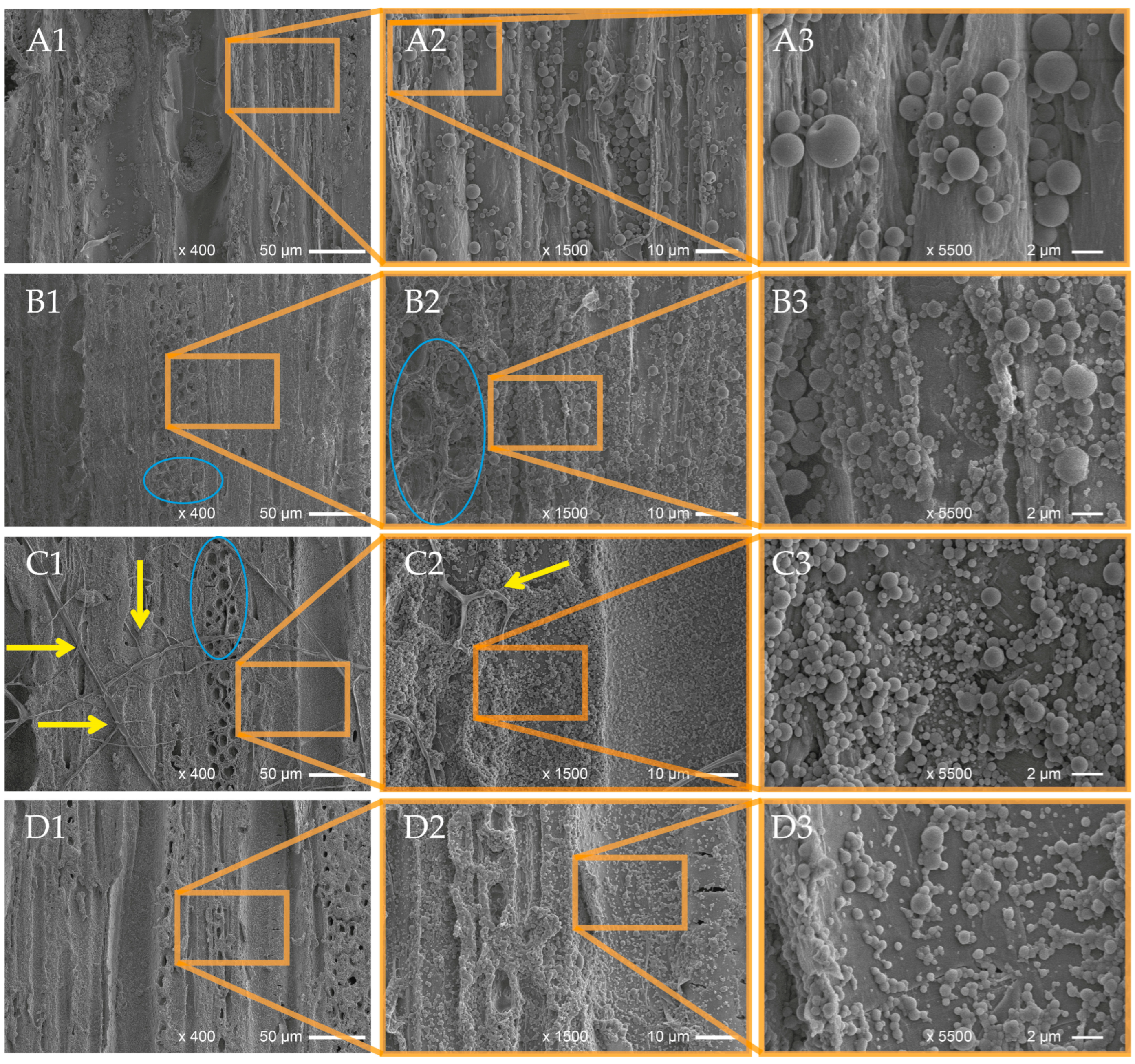
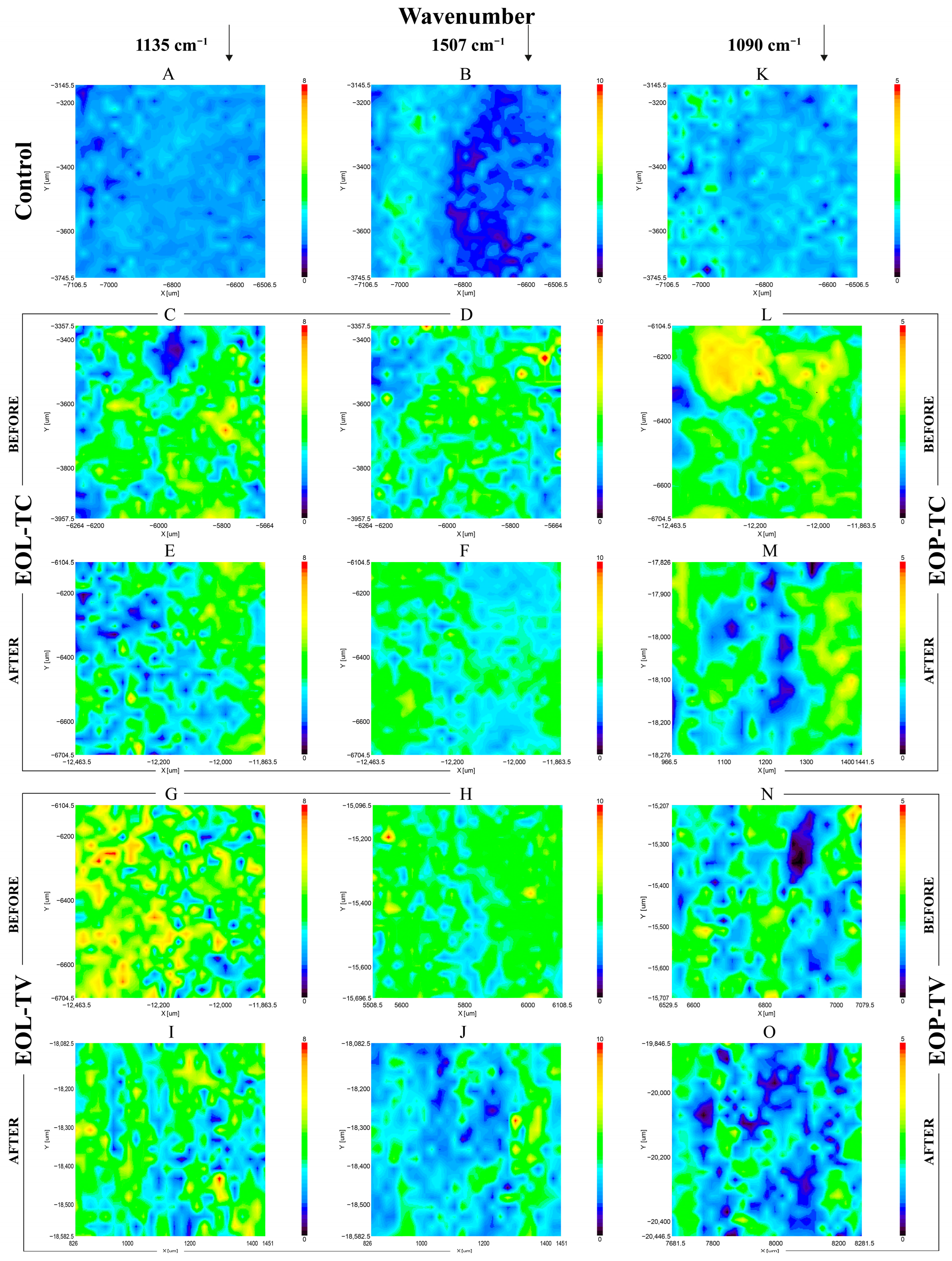
3.4. FTIR Imaging
3.5. Confocal Laser Scanning Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Romagnoli, M.; Fragiacomo, M.; Brunori, A.; Follesa, M.; Scarascia Mugnozza, G. Solid Wood and Wood Based Composites: The Challenge of Sustainability Looking for a Short and Smart Supply Chain. In Digital Wood Design: Innovative Techniques of Representation in Architectural Design; Bianconi, F., Filippucci, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 783–807. [Google Scholar] [CrossRef]
- Singh, T.; Arpanaei, A.; Elustondo, D.; Wang, Y.; Stocchero, A.; West, T.A.P.; Fu, Q. Emerging technologies for the development of wood products towards extended carbon storage and CO2 capture. Carbon Capture Sci. Technol. 2022, 4, 100057. [Google Scholar] [CrossRef]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Fagus sylvatica and other beeches in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 94–95. [Google Scholar]
- Sciomenta, M.; Spera, L.; Peditto, A.; Ciuffetelli, E.; Savini, F.; Bedon, C.; Romagnoli, M.; Nocetti, M.; Brunetti, M.; Fragiacomo, M. Mechanical characterization of homogeneous and hybrid beech-Corsican pine glue-laminated timber beams. Eng. Struct. 2022, 264, 114450. [Google Scholar] [CrossRef]
- Blanchet, P.; Pepin, S. Trends in Chemical Wood Surface Improvements and Modifications: A Review of the Last Five Years. Coatings 2021, 11, 1514. [Google Scholar] [CrossRef]
- Brosse, N.; El Hage, R.; Chaouch, M.; Pétrissans, M.; Dumarçay, S.; Gérardin, P. Investigation of the chemical modifications of beech wood lignin during heat treatment. Polym. Degrad. Stab. 2010, 95, 1721–1726. [Google Scholar] [CrossRef]
- De Angelis, M.; Humar, M.; Kržišnik, D.; Tamantini, S.; Romagnoli, M. Influence of Thermal Modification and Impregnation with Biocides on Physical Properties of Italian Stone Pine Wood (Pinus pinea L.). Appl. Sci. 2022, 12, 3801. [Google Scholar] [CrossRef]
- Esteves, B.; Pereira, H. Wood modification by heat treatment: A Review. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Järvinen, J.; Ilgın, H.E.; Karjalainen, M. Wood Preservation Practices and Future Outlook: Perspectives of Experts from Finland. Forests 2022, 13, 1044. [Google Scholar] [CrossRef]
- Khademibami, L.; Bobadilha, G.S. Recent Developments Studies on Wood Protection Research in Academia: A Review. Front. For. Glob. Chang. 2022, 5, 28. [Google Scholar] [CrossRef]
- Repič, R.; Pondelak, A.; Kržišnik, D.; Humar, M.; Knez, N.; Knez, F.; Škapin, A.S. Environmentally friendly protection of European beech against fire and fungal decay using a combination of thermal modification and mineralisation. Wood Mater. Sci. Eng. 2023, 19, 33–44. [Google Scholar] [CrossRef]
- Romagnoli, M.; Cavalli, D.; Pernarella, R.; Zanuttini, R.; Togni, M. Physical and mechanical characteristics of poor-quality wood after heat treatment. iForest Biogeosci. For. 2015, 8, 884–891. [Google Scholar] [CrossRef]
- Todaro, L.; Dichicco, P.; Moretti, N.; D’Auria, M. Effect of combined steam and heat treatments on extractives and lignin in sapwood and heartwood of turkey oak (Quercus cerris L.) wood. BioResources 2013, 8, 1718–1730. [Google Scholar] [CrossRef]
- Broda, M. Natural Compounds for Wood Protection against Fungi—A Review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef] [PubMed]
- Humar, M.; Kržišnik, D.; Lesar, B.; Thaler, N.; Ugovšek, A.; Zupančič, K.; Žlahtič, M. Thermal modification of wax-impregnated wood to enhance its physical, mechanical, and biological properties. Holzforschung 2017, 71, 57. [Google Scholar] [CrossRef]
- Lozhechnikova, A.; Bellanger, H.; Michen, B.; Burgert, I.; Österberg, M. Surfactant-free carnauba wax dispersion and its use for layer-by-layer assembled protective surface coatings on wood. Appl. Surf. Sci. 2017, 396, 1273–1281. [Google Scholar] [CrossRef]
- Tondi, G.; Palanti, S.; Wieland, S.; Thevenon, M.F.; Petutschnigg, A.; Schnabel, T. Durability of tannin-boron-treated timber. BioResources 2012, 7, 5138–5151. [Google Scholar] [CrossRef]
- Vek, V.; Balzano, A.; Poljanšek, I.; Humar, M.; Oven, P. Improving Fungal Decay Resistance of Less Durable Sapwood by Impregnation with Scots Pine Knotwood and Black Locust Heartwood Hydrophilic Extractives with Antifungal or Antioxidant Properties. Forests 2020, 11, 1024. [Google Scholar] [CrossRef]
- Vek, V.; Keržič, E.; Poljanšek, I.; Eklund, P.; Humar, M.; Oven, P. Wood Extractives of Silver Fir and Their Antioxidant and Antifungal Properties. Molecules 2021, 26, 6412. [Google Scholar] [CrossRef]
- Elaieb, M.T.; Ayed, S.B.; Dumarçay, S.; Faria, B.D.F.H.D.; Thévenon, M.-F.; Gérardin, P.; Candelier, K. Natural durability of four Tunisian Eucalyptus spp. and their respective compositions in extractives. Holzforschung 2020, 74, 260–274. [Google Scholar] [CrossRef]
- Fidah, A.; Salhi, N.; Mohamed, R.; Kabouchi, B.; Ziani, M.; Aberchane, M.; Famiri, A. Natural durability of Cedrus atlantica wood related to the bioactivity of its essential oil against wood decaying fungi. Maderas Cienc. Tecnol. 2016, 18, 1–15. [Google Scholar] [CrossRef]
- Keržič, E.; Humar, M.; Oven, P.; Vek, V. Development of extraction methodology for identification of extractive-compounds indexing natural durability of selected wood species. Wood Mater. Sci. Eng. 2023, 18, 1940–1950. [Google Scholar] [CrossRef]
- Militz, H.; Busetto, D.; Hapla, F. Investigation on natural durability and sorption properties of Italian Chestnut (Castanea sativa Mill.) from coppice stands. Holz. Als. Roh. Werkst. 2003, 61, 133–141. [Google Scholar] [CrossRef]
- Antonelli, F.; Bartolini, M.; Plissonnier, M.-L.; Esposito, A.; Galotta, G.; Ricci, S.; Davidde Petriaggi, B.; Pedone, C.; Di Giovanni, A.; Piazza, S.; et al. Essential Oils as Alternative Biocides for the Preservation of Waterlogged Archaeological Wood. Microorganisms 2020, 8, 2015. [Google Scholar] [CrossRef] [PubMed]
- Pánek, M.; Reinprecht, L.; Hulla, M. Ten Essential Oils for Beech Wood Protection—Efficacy Against Wood-Destroying Fungi and Moulds, and Effect on Wood Discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Sadiki, M.; El Abed, S.; Balouiri, M.; Barkai, H.; El Bergadi, F.Z.; El Farricha, O.; Ibnsouda Koraichi, S. Combined effect of essential oils against bacteria associated with deterioration of historical wood. J. Mater. Environ. Sci. 2017, 8, 594–602. [Google Scholar]
- Salem, M.Z.M.; Zidan, Y.E.; Mansour, M.M.A.; El Hadidi, N.M.N.; Abo Elgat, W.A.A. Antifungal activities of two essential oils used in the treatment of three commercial woods deteriorated by five common mold fungi. Int. Biodeterior. Biodegrad. 2016, 106, 88–96. [Google Scholar] [CrossRef]
- Spagnoletti, A.; Guerrini, A.; Tacchini, M.; Vinciguerra, V.; Leone, C.; Maresca, I.; Simonetti, G.; Sacchetti, G.; Angiolella, L. Chemical Composition and Bio-Efficacy of Essential Oils from Italian Aromatic Plants: Mentha suaveolens, Coridothymus capitatus, Origanum hirtum and Rosmarinus officinalis. Nat. Prod. Commun. 2016, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Vettraino, A.M.; Zikeli, F.; Humar, M.; Biscontri, M.; Bergamasco, S.; Romagnoli, M. Essential oils from Thymus spp. as natural biocide against common brown- and white-rot fungi in degradation of wood products: Antifungal activity evaluation by in vitro and FTIR analysis. Eur. J. Wood Wood Prod. 2023, 81, 747–763. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Zikeli, F.; Scarascia Mugnozza, G.; Vinciguerra, V.; Tabet, D.; Romagnoli, M. Lignin nanoparticles containing essential oils for controlling Phytophthora cactorum diseases. For. Pathol. 2022, 52, e12739. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Rojas, F.; Tedesco, V.; Giusiano, G.; Angiolella, L. Chemical characterization and antifungal activity of Origanum vulgare, Thymus vulgaris essential oils and carvacrol against Malassezia furfur. Nat. Prod. Res. 2019, 33, 3273–3277. [Google Scholar] [CrossRef]
- Voda, K.; Boh, B.; Vrtačnik, M.; Pohleven, F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int. Biodeterior. Biodegrad. 2003, 51, 51–59. [Google Scholar] [CrossRef]
- Vrenna, G.; Artini, M.; Ragno, R.; Relucenti, M.; Fiscarelli, E.V.; Tuccio Guarna Assanti, V.; Papa, R.; Selan, L. Anti-Virulence Properties of Coridothymus capitatus Essential Oil against Pseudomonas aeruginosa Clinical Isolates from Cystic Fibrosis Patients. Microorganisms 2021, 9, 2257. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Chen, P.-F.; Chang, S.-T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crops Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Mi, N.; Wang, Y.; Li, G.; Wang, L.; Xie, Y. Antifungal activity of monoterpenes against wood white-rot fungi. Int. Biodeterior. Biodegrad. 2016, 106, 157–160. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; Sennato, S.; Scarascia Mugnozza, G.; Romagnoli, M. Preparation of Lignin Nanoparticles with Entrapped Essential Oil as a Bio-Based Biocide Delivery System. ACS Omega 2020, 5, 358–368. [Google Scholar] [CrossRef]
- Campanella, L.; Angeloni, R.; Cibin, F.; Dell’Aglio, E.; Grimaldi, F.; Reale, R.; Vitali, M. Capsulated essential oil in gel spheres for the protection of cellulosic cultural heritage. Nat. Prod. Res. 2019, 35, 116–123. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Gao, B.; Zhao, Y.; Jiang, Y.; Zha, Z.; Xue, W.; Gong, L. Lignin Nanoparticles: Green Synthesis in a γ-Valerolactone/Water Binary Solvent and Application to Enhance Antimicrobial Activity of Essential Oils. ACS Sustain. Chem. Eng. 2020, 8, 714–722. [Google Scholar] [CrossRef]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potarniche, A.-V.; Timar, M.C.; Żychska, M.; Sabliov, C.M. Nanodelivery of essential oils as efficient tools against antimicrobial resistance: A review of the type and physical-chemical properties of the delivery systems and applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New Opportunities in the Valorization of Technical Lignins. ChemSusChem 2021, 14, 1016–1036. [Google Scholar] [CrossRef]
- Bergamasco, S.; Tamantini, S.; Zikeli, F.; Vinciguerra, V.; Scarascia Mugnozza, G.; Romagnoli, M. Synthesis and Characterizations of Eco-Friendly Organosolv Lignin-Based Polyurethane Coating Films for the Coating Industry. Polymers 2022, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- De Haro, J.C.; Allegretti, C.; Smit, A.T.; Turri, S.; D’Arrigo, P.; Griffini, G. Biobased Polyurethane Coatings with High Biomass Content: Tailored Properties by Lignin Selection. ACS Sustain. Chem. Eng. 2019, 7, 11700–11711. [Google Scholar] [CrossRef]
- De Hoyos-Martinez, P.L.; Charrier-El Bouhtoury, F.; Labidi, J. Biobased phenolic resins for wood protection against fire. In Proceedings of the COST FP1407 3rd Conference “Wood Modification Research and Applications”, Kuchl, Austria, 14–15 September 2017. [Google Scholar]
- Gordobil, O.; Herrera, R.; Llano-Ponte, R.; Labidi, J. Esterified organosolv lignin as hydrophobic agent for use on wood products. Prog. Org. Coat. 2017, 103, 143–151. [Google Scholar] [CrossRef]
- Henn, K.A.; Forsman, N.; Zou, T.; Österberg, M. Colloidal Lignin Particles and Epoxies for Bio-Based, Durable, and Multiresistant Nanostructured Coatings. ACS Appl. Mater. Interfaces 2021, 13, 34793–34806. [Google Scholar] [CrossRef]
- Herrera, R.; Poohphajai, F.; Sandak, A.; Gordobil, O. Simultaneous Improvement of Surface Wettability and UV Resistance of Wood with Lignin-Based Treatments. Polymers 2023, 15, 3409. [Google Scholar] [CrossRef] [PubMed]
- Jusic, J.; Tamantini, S.; Romagnoli, M.; Vinciguerra, V.; Di Mattia, E.; Zikeli, F.; Cavalera, M.; Scarascia Mugnozza, G. Improving sustainability in wood coating: Testing lignin and cellulose nanocrystals as additives to commercial acrylic wood coatings for bio-building. iForest Biogeosci. For. 2021, 14, 499–507. [Google Scholar] [CrossRef]
- Mattos, B.D.; Tardy, B.L.; Magalhães, W.L.E.; Rojas, O.J. Controlled release for crop and wood protection: Recent progress toward sustainable and safe nanostructured biocidal systems. J. Control. Release 2017, 262, 139–150. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Basso, M.C.; Pizzi, A.; Delmotte, L. Polyurethanes from Kraft Lignin without Using Isocyanates. J. Renew. Mater. 2018, 6, 413–425. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2012, 46, 851–870. [Google Scholar] [CrossRef]
- Smit, A.T.; Bellinetto, E.; Dezaire, T.; Boumezgane, O.; Riddell, L.A.; Turri, S.; Hoek, M.; Bruijnincx, P.C.A.; Griffini, G. Tuning the Properties of Biobased PU Coatings via Selective Lignin Fractionation and Partial Depolymerization. ACS Sustain. Chem. Eng. 2023, 11, 7193–7202. [Google Scholar] [CrossRef]
- Tamantini, S.; Bergamasco, S.; Zikeli, F.; Humar, M.; Cavalera, M.; Romagnoli, M. Cellulose Nano Crystals (CNC) as Additive for a Bio-Based Waterborne Acrylic Wood Coating: Decay, Artificial Weathering, Physical and Chemical Tests. Nanomaterials 2023, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Teaca, C.-A.; Roşu, D.; Mustaţă, F.; Rusu, T.; Roşu, L.; Roşca, I.; Varganici, C.D. Natural bio-based products for wood coating and protection against degradation: A Review. BioResources 2019, 14, 4873–4901. [Google Scholar] [CrossRef]
- Vieira, F.R.; Magina, S.; Evtuguin, D.V.; Barros-Timmons, A. Lignin as a Renewable Building Block for Sustainable Polyurethanes. Materials 2022, 15, 6182. [Google Scholar] [CrossRef]
- Zikeli, F.; Vettraino, A.M.; Biscontri, M.; Bergamasco, S.; Palocci, C.; Humar, M.; Romagnoli, M. Lignin Nanoparticles with Entrapped Thymus spp. Essential Oils for the Control of Wood-Rot Fungi. Polymers 2023, 15, 2713. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; D’Annibale, A.; Capitani, D.; Romagnoli, M.; Scarascia Mugnozza, G. Preparation of Lignin Nanoparticles from Wood Waste for Wood Surface Treatment. Nanomaterials 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.J.; Puglia, D. Multifunctional lignin-based nanocomposites and nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Blindheim, F.H.; Chinga-Carrasco, G. Functional surfaces, films, and coatings with lignin—A critical review. RSC Adv. 2023, 13, 12529–12553. [Google Scholar] [CrossRef]
- Sreejaya, M.M.; Jeevan Sankar, R.; Ramanunni, K.; Pillai, N.P.; Ramkumar, K.; Anuvinda, P.; Meenakshi, V.S.; Sadanandan, S. Lignin-based organic coatings and their applications: A review. Mater. Today Proc. 2022, 60, 494–501. [Google Scholar] [CrossRef]
- Hult, E.-L.; Koivu, K.; Asikkala, J.; Ropponen, J.; Wrigstedt, P.; Sipilä, J.; Poppius-Levlin, K. Esterified lignin coating as water vapor and oxygen barrier for fiber-based packaging. Holzforschung 2013, 67, 899–905. [Google Scholar] [CrossRef]
- Kaya, C.; Stegmaier, T.; Gresser, G.T. Investigation of the Protective Function of a Lignin Coating of Natural Fiber Geotextiles against Biodegradation. Materials 2023, 16, 4849. [Google Scholar] [CrossRef] [PubMed]
- Kopacic, S.; Ortner, A.; Guebitz, G.; Kraschitzer, T.; Leitner, J.; Bauer, W. Technical Lignins and Their Utilization in the Surface Sizing of Paperboard. Ind. Eng. Chem. Res. 2018, 57, 6284–6291. [Google Scholar] [CrossRef]
- Pinto, P.I.F.; Magina, S.; Fateixa, S.; Pinto, P.C.R.; Liebner, F.; Evtuguin, D.V. Modification of Paper Surface by All-Lignin Coating Formulations. Materials 2022, 15, 7869. [Google Scholar] [CrossRef] [PubMed]
- Boarino, A.; Wang, H.; Olgiati, F.; Artusio, F.; Özkan, M.; Bertella, S.; Razza, N.; Cagno, V.; Luterbacher, J.S.; Klok, H.-A.; et al. Lignin: A Sustainable Antiviral Coating Material. ACS Sustain. Chem. Eng. 2022, 10, 14001–14010. [Google Scholar] [CrossRef]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from Micro- to Nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef] [PubMed]
- Hussin, M.H.; Appaturi, J.N.; Poh, N.E.; Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Vahabi, H.; Syamani, F.A.; Fatriasari, W.; Solihat, N.N.; et al. A recent advancement on preparation, characterization and application of nanolignin. Int. J. Biol. Macromol. 2022, 200, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhuang, Y.; Chen, J.; Yang, G.; Dai, L. Research Progress on the Preparation and High-Value Utilization of Lignin Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7254. [Google Scholar] [CrossRef]
- Österberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef]
- Pereira, A.d.E.S.; Luiz de Oliveira, J.; Maira Savassa, S.; Barbara Rogério, C.; Araujo de Medeiros, G.; Fraceto, L.F. Lignin nanoparticles: New insights for a sustainable agriculture. J. Clean. Prod. 2022, 345, 131145. [Google Scholar] [CrossRef]
- Pylypchuk, I.; Sipponen, M.H. Organic solvent-free production of colloidally stable spherical lignin nanoparticles at high mass concentrations. Green Chem. 2022, 24, 8705–8715. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Ago, M.; Crestini, C. Understanding Lignin Aggregation Processes. A Case Study: Budesonide Entrapment and Stimuli Controlled Release from Lignin Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 9342–9351. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for Nano- and Microscaled Carrier Systems: Applications, Trends, and Challenges. ChemSusChem 2019, 12, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, M.; Klapiszewski, Ł.; Collins, M.N.; Jesionowski, T. Recent progress in biomedical and biotechnological applications of lignin-based spherical nano- and microstructures: A comprehensive review. Mater. Today Chem. 2022, 26, 101198. [Google Scholar] [CrossRef]
- Verdini, F.; Gaudino, E.C.; Canova, E.; Tabasso, S.; Behbahani, P.J.; Cravotto, G. Lignin as a Natural Carrier for the Efficient Delivery of Bioactive Compounds: From Waste to Health. Molecules 2022, 27, 3598. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Pylypchuk, I.; Okahisa, Y.; Sipponen, M.H. Urushi as a Green Component for Thermally Curable Colloidal Lignin Particles and Hydrophobic Coatings. ACS Macro Lett. 2023, 12, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Domenici, F.; Giantulli, S.; Brasili, F.; D’Errico, C.; Tsaouli, G.; Tortorella, E.; Bordi, F.; Morrone, S.; Palocci, C.; et al. PLGA based particles as “drug reservoir” for antitumor drug delivery: Characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2019, 180, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Haji Mansor, M.; Najberg, M.; Contini, A.; Alvarez-Lorenzo, C.; Garcion, E.; Jérôme, C.; Boury, F. Development of a non-toxic and non-denaturing formulation process for encapsulation of SDF-1α into PLGA/PEG-PLGA nanoparticles to achieve sustained release. Eur. J. Pharm. Biopharm. 2018, 125, 38–50. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Goudoulas, T.; Mehlhorn-Diehl, T.; Gestmann, F.; Fattahi, E.; Becker, T.; Germann, N. Encapsulation of Neem oil from Azadirachta indica into Poly (lactic-co-glycolic acid) as a novel sprayable miticide system with long-term storage stability and controlled release kinetic. Ind. Crops Prod. 2023, 201, 116954. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, C.; Frazao, C.J.R.; Krauβer, F.; Morin, N.; Walther, T.; François, J.M. A New Synthetic Pathway for the Bioproduction of Glycolic Acid from Lignocellulosic Sugars Aimed at Maximal Carbon Conservation. Front. Bioeng. Biotechnol. 2019, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Salusjärvi, L.; Havukainen, S.; Koivistoinen, O.; Toivari, M. Biotechnological production of glycolic acid and ethylene glycol: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Palocci, C.; Valletta, A.; Chronopoulou, L.; Donati, L.; Bramosanti, M.; Brasili, E.; Baldan, B.; Pasqua, G. Endocytic pathways involved in PLGA nanoparticle uptake by grapevine cells and role of cell wall and membrane in size selection. Plant Cell Rep. 2017, 36, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, G.; Badiali, C.; Chronopoulou, L.; Palocci, C.; Pasqua, G. Confocal Microscopy Investigations of Biopolymeric PLGA Nanoparticle Uptake in Arabidopsis thaliana L. Cultured Cells and Plantlet Roots. Plants 2023, 12, 2397. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Yoon, S.; Yang, X.; Kim, Y.J. Super-Tough and Biodegradable Poly(lactide-co-glycolide) (PLGA) Transparent Thin Films Toughened by Star-Shaped PCL-b-PDLA Plasticizers. Polymers 2023, 15, 2617. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.A.; Valle Iulianelli, G.C.; Bruno Tavares, M.I. Development and properties evaluation of bio-based PLA/PLGA blend films reinforced with microcrystalline cellulose and organophilic silica. Polym. Eng. Sci. 2017, 57, 464–472. [Google Scholar] [CrossRef]
- Mendez, O.E.; Astete, C.E.; Hermanová, S.; Boldor, D.; Orts, W.; Sabliov, C.M. Biobased films from amphiphilic lignin-graft-PLGA copolymer. BioResources 2023, 18, 5887–5907. [Google Scholar] [CrossRef]
- Mangiacapre, E.; Triolo, A.; Ramondo, F.; Lo Celso, F.; Russina, O. Unveiling the structural organisation of carvacrol through X-ray scattering and molecular Dynamics: A comparative study with liquid thymol. J. Mol. Liq. 2024, 394, 123778. [Google Scholar] [CrossRef]
- Zhu, Z.; Min, T.; Zhang, X.; Wen, Y. Microencapsulation of Thymol in Poly(lactide-co-glycolide) (PLGA): Physical and Antibacterial Properties. Materials 2019, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Cusola, O.; Rojas, O.J.; Roncero, M.B. Lignin Particles for Multifunctional Membranes, Antioxidative Microfiltration, Patterning, and 3D Structuring. ACS Appl. Mater. Interfaces 2019, 11, 45226–45236. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin Nanoparticle as a Novel Green Carrier for the Efficient Delivery of Resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Cusola, O.; Kivistö, S.; Vierros, S.; Batys, P.; Ago, M.; Tardy, B.L.; Greca, L.G.; Roncero, M.B.; Sammalkorpi, M.; Rojas, O.J. Particulate Coatings via Evaporation-Induced Self-Assembly of Polydisperse Colloidal Lignin on Solid Interfaces. Langmuir 2018, 34, 5759–5771. [Google Scholar] [CrossRef]
- Moroishi, H.; Sonotaki, S.; Murakami, Y. PLA- and PLA/PLGA-Emulsion Composite Biomaterial Sheets for the Controllable Sustained Release of Hydrophilic Compounds. Materials 2018, 11, 2588. [Google Scholar] [CrossRef]




| EO-TC | EO-TV | ||
|---|---|---|---|
| Component | % | Component | % |
| carvacrol | 68.6 | thymol | 47.9 |
| p-cymene | 7.7 | p-cymene | 15.8 |
| γ-terpinene | 6.8 | γ-terpinene | 10.0 |
| β-caryophyllene | 2.6 | carvacrol | 4.4 |
| β-myrcene | 1.8 | linalool | 4.1 |
| linalool | 1.5 | β-caryophyllene | 2.1 |
| α-thujene | 1.2 | β-myrcene | 2.0 |
| α-terpinene | 1.1 | borneol | 1.3 |
| α-pinene | 0.9 | α-terpinene | 1.3 |
| terpinene 4-ol | 0.7 | α-thujene | 1.2 |
| thymol | 0.6 | camphene | 1.1 |
| Sample | Solids [mg/mL] | EO [mg/mL] | DLE [%] | DLC [%] | Particle Size [nm] | Particle Size after Spray Coating [nm] | Particle Size after Release Experiment [nm] |
|---|---|---|---|---|---|---|---|
| LNP alone | 16.7 | - | - | - | 1325 ± 680 | n. d. | n. d. |
| EOL-TC | 14.6 | 5.1 | 51 | 35 | 1519 ± 814 | 1912 ± 870 | 1273 ± 608 |
| EOL-TV | 15.5 | 4.3 | 42 | 28 | 1414 ± 913 | 1535 ± 841 | 571 ± 313 |
| PLGA NPs alone | 11.4 | - | - | - | 426 ± 109 | n. d. | n. d. |
| EOP-TC | 15.4 | 3.3 | 32 | 22 | 370 ± 125 | 594 ± 207 | 568 ± 229 |
| EOP-TV | 13.8 | 4.0 | 39 | 29 | 351 ± 107 | n. d. | 581 ± 222 |
| Sample | Coating Uptake per Wood Sample | EO Uptake | |
|---|---|---|---|
| [mg] | [%] | [mg] | |
| EOL-TC | 54.7 | 2.70 | 19.1 |
| EOL-TV | 48.2 | 2.54 | 13.4 |
| EOP-TC | 24.1 | 1.30 | 5.2 |
| EOP-TV | 28.7 | 1.44 | 8.3 |
| EO-TC alone | 6.6 | 0.36 | 6.6 |
| EO-TV alone | 9.1 | 0.50 | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zikeli, F.; Jusic, J.; Palocci, C.; Mugnozza, G.S.; Romagnoli, M. Spray Coating of Wood with Nanoparticles from Lignin and Polylactic Glycolic Acid Loaded with Thyme Essential Oils. Polymers 2024, 16, 947. https://doi.org/10.3390/polym16070947
Zikeli F, Jusic J, Palocci C, Mugnozza GS, Romagnoli M. Spray Coating of Wood with Nanoparticles from Lignin and Polylactic Glycolic Acid Loaded with Thyme Essential Oils. Polymers. 2024; 16(7):947. https://doi.org/10.3390/polym16070947
Chicago/Turabian StyleZikeli, Florian, Jasmina Jusic, Cleofe Palocci, Giuseppe Scarascia Mugnozza, and Manuela Romagnoli. 2024. "Spray Coating of Wood with Nanoparticles from Lignin and Polylactic Glycolic Acid Loaded with Thyme Essential Oils" Polymers 16, no. 7: 947. https://doi.org/10.3390/polym16070947






