Preparation and Characterization of Electrospun EVOH/Ti3C2 Composite Fibers
Abstract
:1. Introduction
2. Experiments
2.1. Experimental Materials
2.2. Preparation of the EVOH/Ti3C2 Fibers
2.3. Characterization
3. Results and discussion
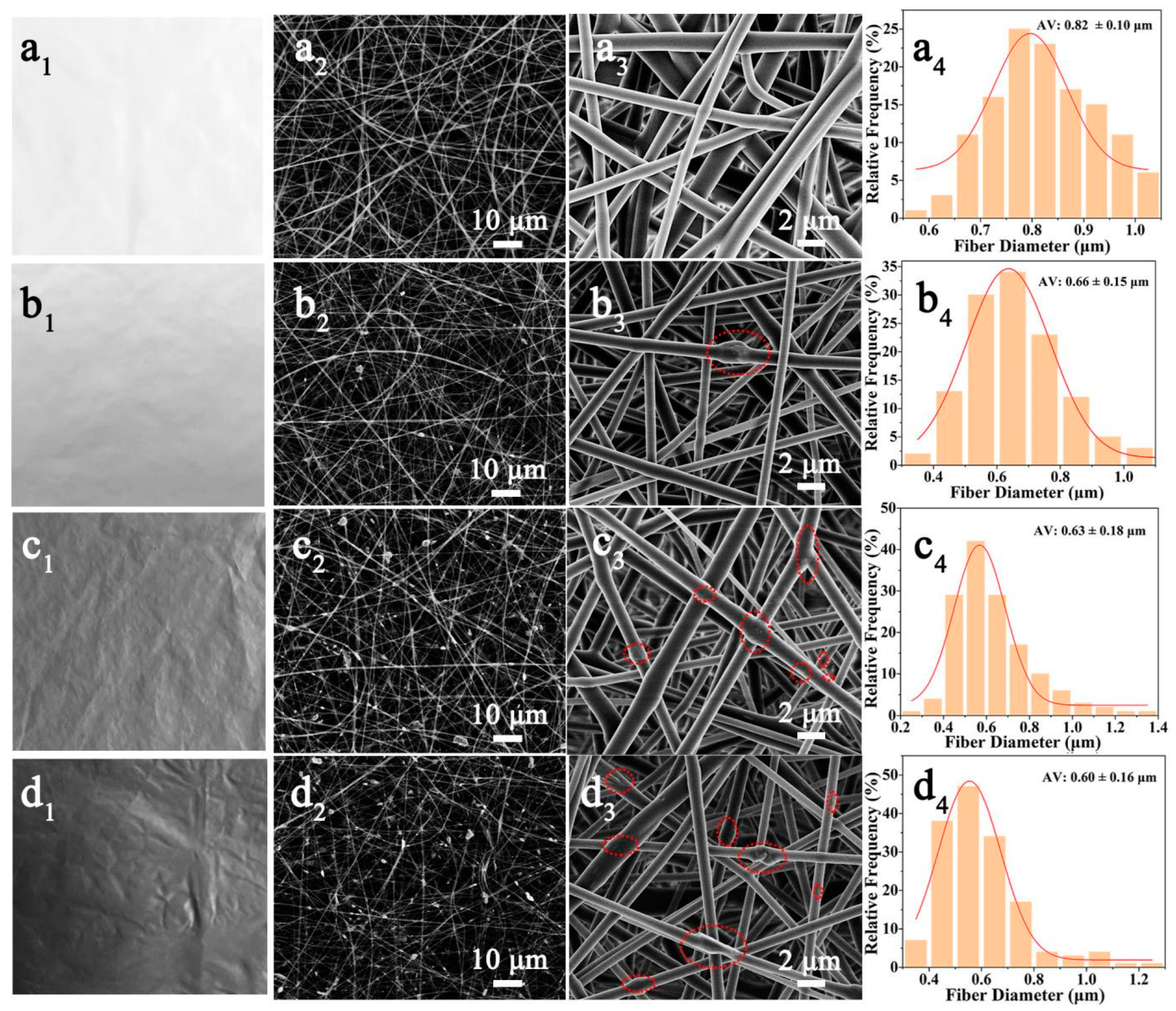
3.1. Morphologies of Electrospun EVOH/Ti3C2 Composite Fibers
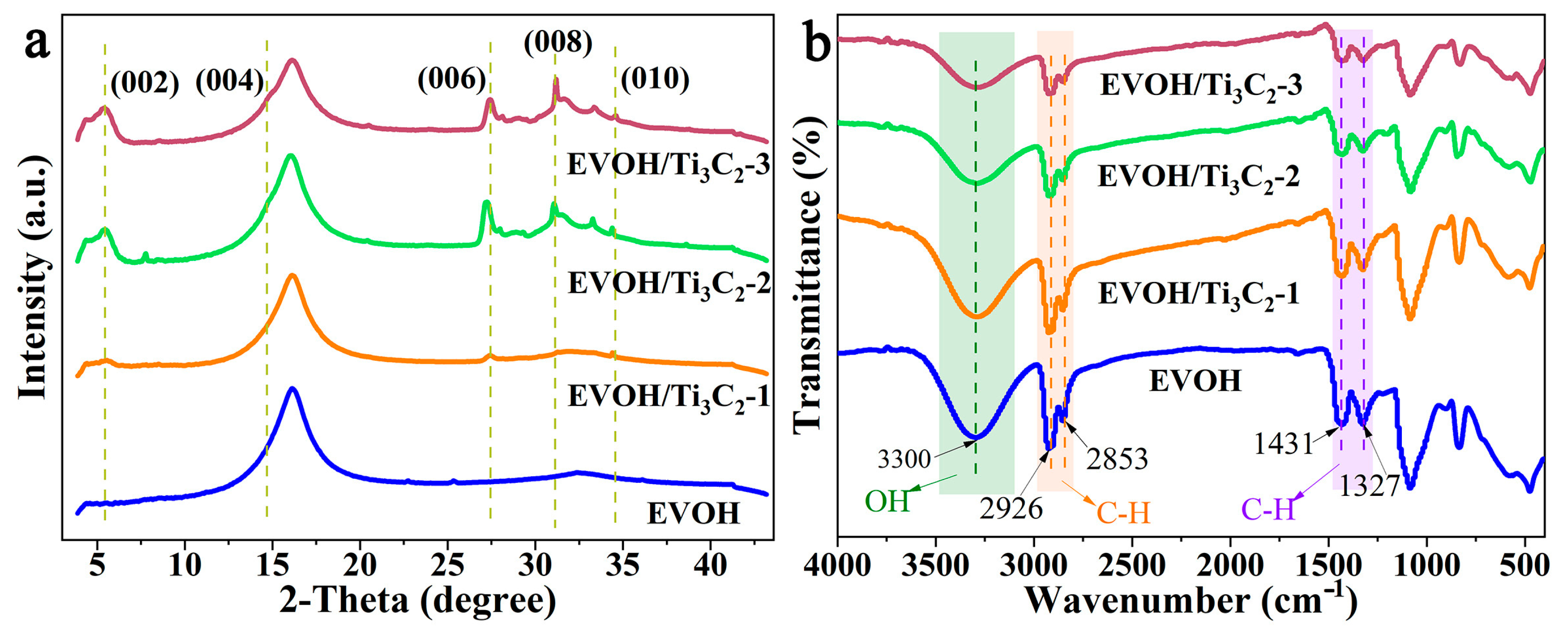
3.2. Structure of Electrospun EVOH/Ti3C2 Composite Fibers
3.3. Thermal Properties of Electrospun EVOH/Ti3C2 Composite Fibers
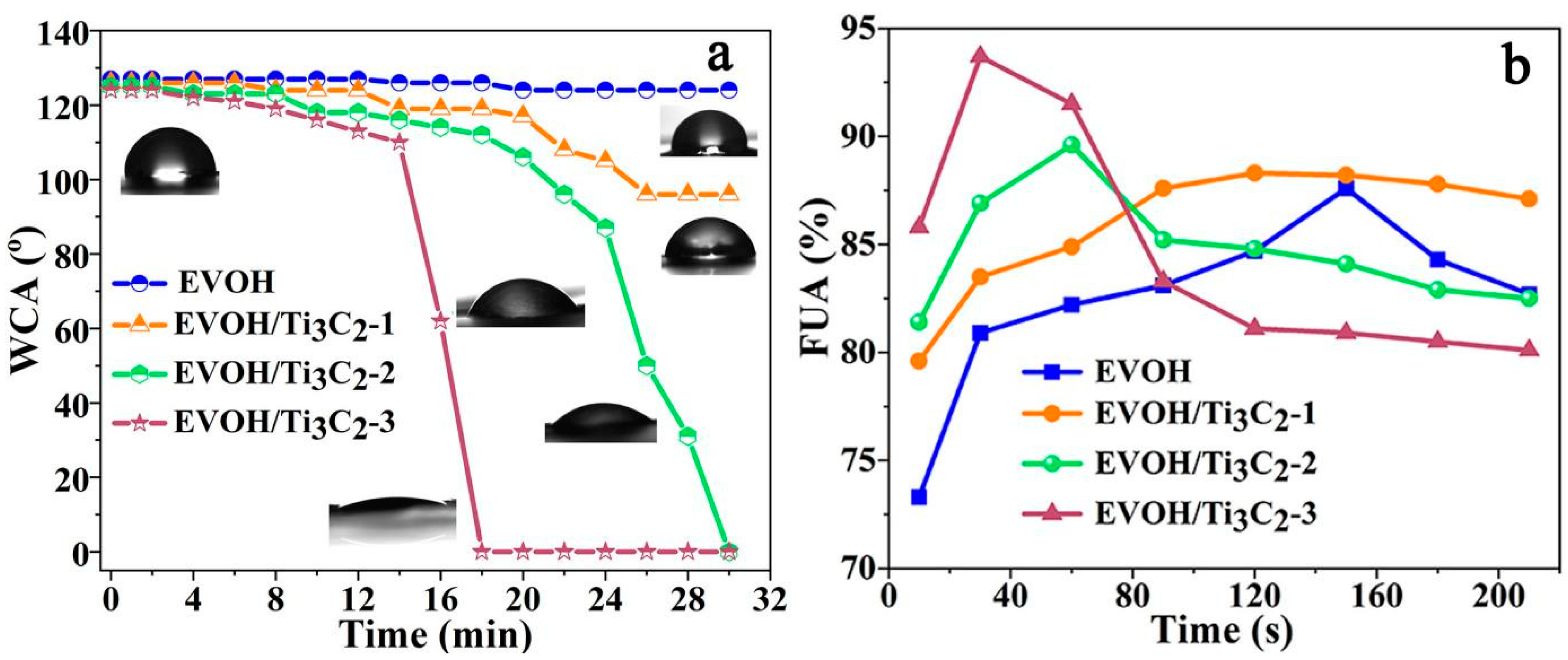
3.4. Wettability of the Fibrous Membranes
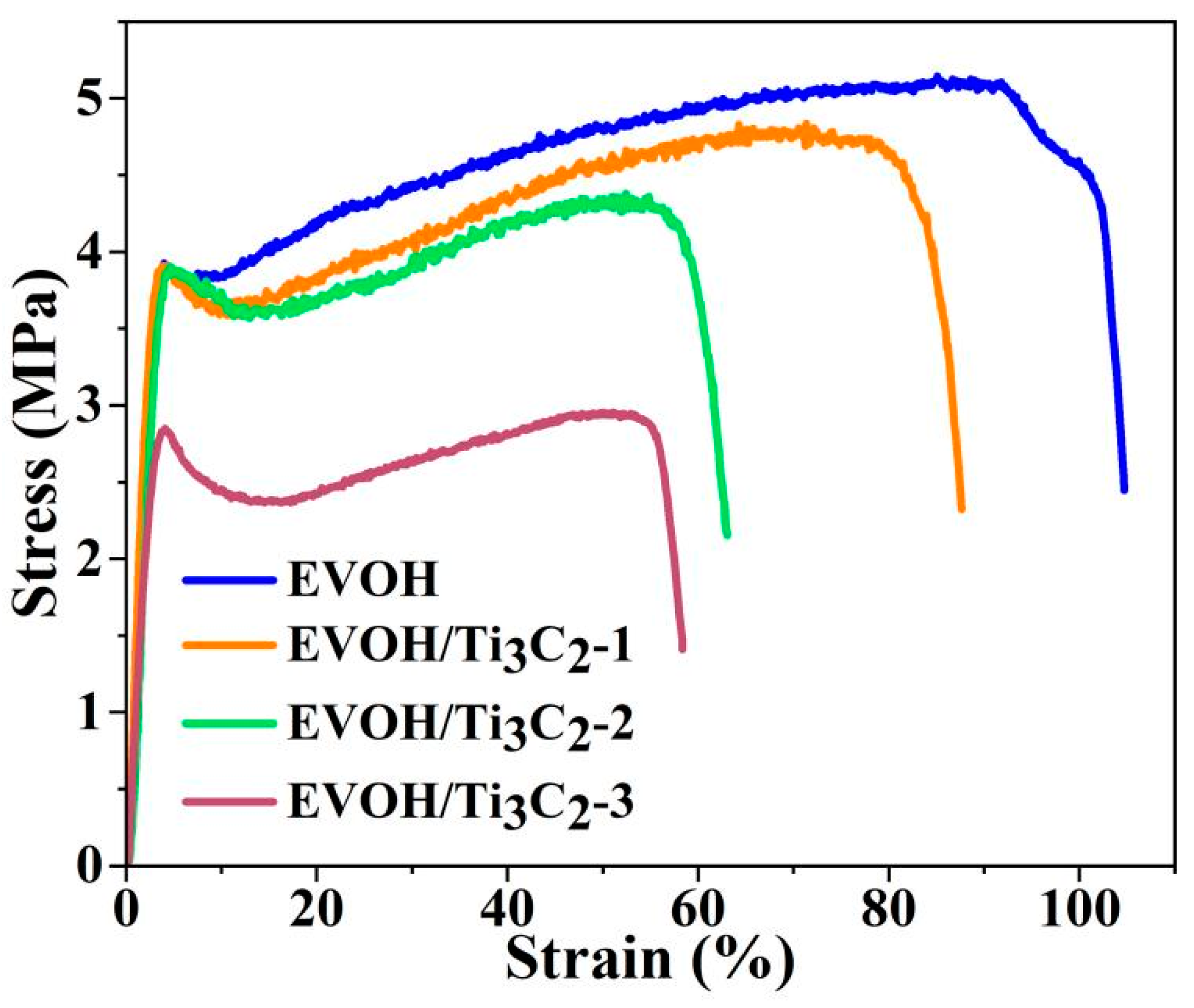
3.5. Mechanical Properties of Fibrous Membranes
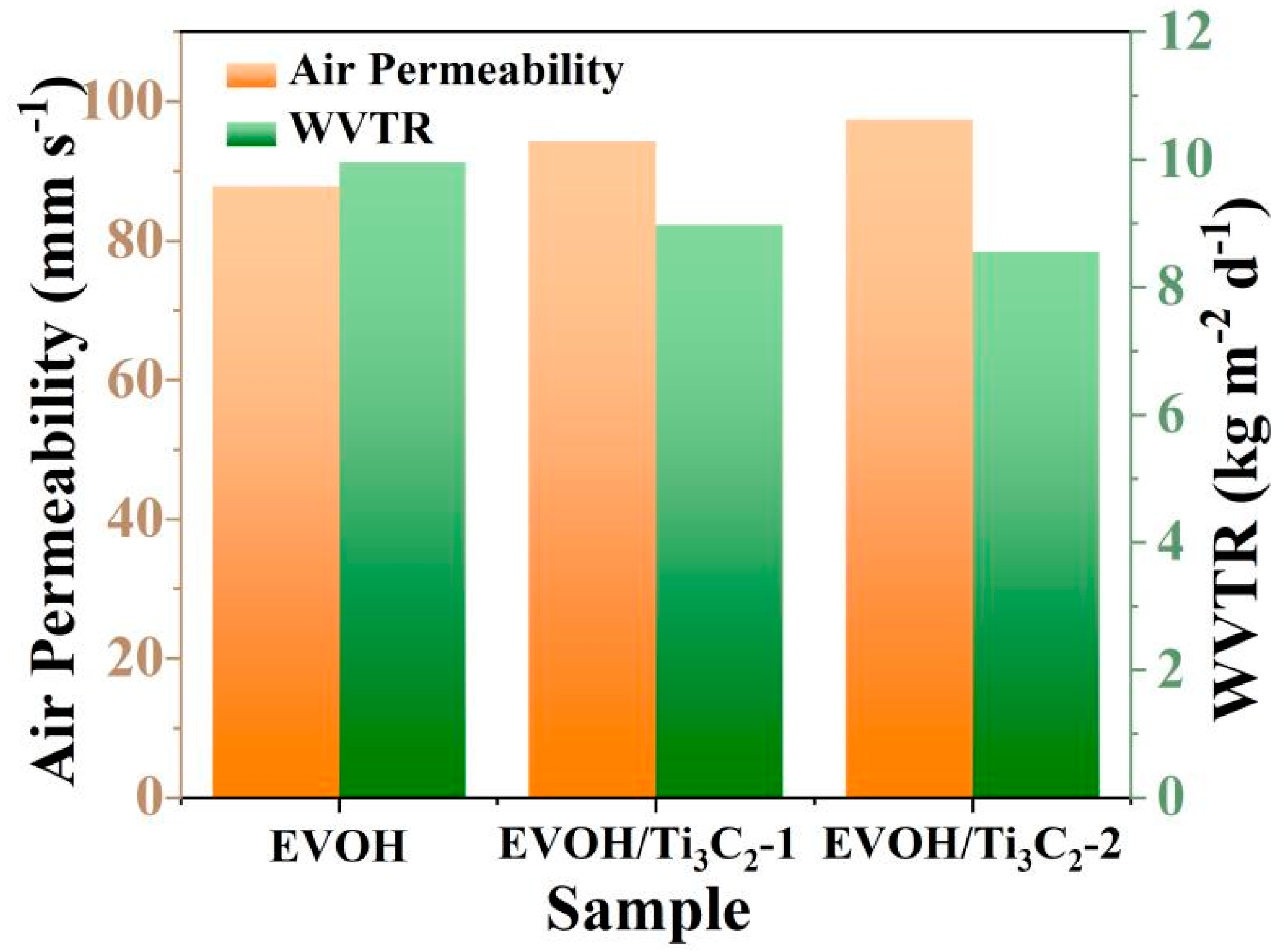
3.6. Air Permeability and Water Vapor Permeability of Fibrous Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun nanofibers for drug delivery and biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef]
- Zahra, F.T.; Quick, Q.; Mu, R. Electrospun PVA fibers for drug delivery: A review. Polymers 2023, 15, 3837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Jiang, Q.T.; Wang, L.; Qiu, Z.P.; Liu, Y.Y.; Zhou, J.; Liu, B. Facile preparation of nitrogen-enriched hierarchical porous carbon nanofibers by Mg(OAc)2-assisted electrospinning for flexible supercapacitors. Appl. Surf. Sci. 2018, 456, 827–834. [Google Scholar] [CrossRef]
- Rasoolzadeh, M.; Sherafat, Z.; Vahedi, M.; Bagherzadeh, E. Structure dependent piezoelectricity in electrospun PVDF-SiC nanoenergy harvesters. J. Alloys Compd. 2022, 917, 165505. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zheng, M.; Dunne, F.; Liu, T.; Fu, X.W.; Kong, L.; Pan, S.Y.; Zhong, W.H. Morphology engineering of protein fabrics for advanced and sustainable filtration. J. Mater. Chem. A 2018, 6, 21585–21595. [Google Scholar] [CrossRef]
- Gao, Q.; Agarwal, S.; Greiner, A.; Zhang, T. Electrospun fiber-based flexible electronics: Fiber fabrication, device platform, functionality integration and applications. Prog. Mater. Sci. 2023, 137, 101139. [Google Scholar]
- Sarrami, P.; Karbasi, S.; Farahbakhsh, Z.; Bigham, A.; Rafienia, M. Fabrication and characterization of novel polyhydroxybutyrate-keratin/nanohydroxyapatite electrospun fibers for bone tissue engineering applications. Int. J. Biol. Macromol. 2022, 220, 1368–1389. [Google Scholar] [CrossRef]
- Liu, Q.X.; Guo, X.P.; Zhang, Y.F.; Wang, X.; Mo, M.; Wu, T. Progress in electrospun fibers for manipulating cell behaviors. Adv. Fiber Mater. 2023, 5, 1241–1272. [Google Scholar] [CrossRef]
- Zhao, R.; Tian, Y.Y.; Li, S.Y.; Ma, T.T.; Lei, H.T.; Zhu, G.S. An electrospun fiber based metal–organic framework composite membrane for fast, continuous, and simultaneous removal of insoluble and soluble contaminants from water. J. Mater. Chem. A 2019, 7, 22559–22570. [Google Scholar] [CrossRef]
- Gao, J.F.; Li, B.; Wang, L.; Huang, X.W.; Xue, H.G. Flexible membranes with a hierarchical nanofiber/microsphere structure for oil adsorption and oil/water separation. J. Ind. Eng. Chem. 2018, 68, 416–424. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.W.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40. [Google Scholar] [CrossRef]
- Gao, Z.; Xiao, X.; Carlo, A.D.; Yin, J.; Wang, Y.; Huang, L.; Tang, J.; Chen, J. Advances in wearable strain sensors based on electrospun fibers. Adv. Funct. Mater. 2023, 33, 2214265. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, Y.; Wu, M.; Li, Y.; Ma, J.; Li, H.; Zheng, Y.; Zeng, Y. Highly efficient fabrication of biomimetic nanoscaled tendrils for high-performance PM0.3 air filters. Nano Lett. 2024, 24, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, D.; Zhao, Z.; Lin, L. Composite fibrous membrane comprising PLA and PCL fibers for biomedical application. Compos. Commun. 2022, 34, 101268. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, Z.; Jia, Z.; Xu, X.; Chen, Y.; Peng, W.; Zhang, J.; Wang, H.; Li, S.; Wen, J. Preparation of antimicrobial PVDF and EVOH membranes by grafting quaternary ammonium compounds. React. Funct. Polym. 2022, 170, 105117. [Google Scholar] [CrossRef]
- Jing, L.; Jia, Z.; Xu, X.; Chen, Y.; Peng, W.; Zhang, J.; Wang, H.; Li, S.; Wen, J. Preparation of antimicrobial poly (ethylene-co-vinyl alcohol) membrane by grafting with N-halamine. React. Funct. Polym. 2022, 172, 105187. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, K.; Zheng, X.; Xiao, R. Poly(ethylene-co-vinyl alcohol) functional nanofiber membranes for the removal of Cr(VI) from water. Ind. Eng. Chem. Res. 2015, 54, 6836–6844. [Google Scholar] [CrossRef]
- Wang, A.; Xu, C.; Zhang, C.; Gan, Y.; Wang, B. Experimental investigation of the properties of electrospun nanofibers for potential medical application. J. Nanomater. 2015, 2015, 418932. [Google Scholar] [CrossRef]
- Mondragón, M.; López-Villegas, O.; Sánchez-Valdés, S.; Rodríguez-González, F.J. Effect of thermoplastic starch and photocrosslinking on the properties and morphology of electrospun poly(ethylene-co-vinylalcohol) mats. Polym. Eng. Sci. 2019, 60, 474–480. [Google Scholar] [CrossRef]
- Liang, M.; Wang, F.; Liu, M.; Yu, J.; Si, Y.; Ding, B. N-Halamine functionalized Eeectrospun Pply(Vinyl Alcohol-co-Ethylene) nanofibrous membranes with rechargeable antibacterial activity for bioprotective applications. Adv. Fiber Mater. 2019, 1, 126–136. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Y.; Xiao, R.; Jacob, K.; Tao, L.; Li, S.; Guo, L. Chemical vapor deposition based superelastic and superhydrophoboic thermoplastic polymeric nanofibrous aerogels for water purification. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2975–2985. [Google Scholar] [CrossRef]
- Jun, B.M.; Kim, S.; Heo, J.; Park, C.M.; Her, N.; Jang, M.; Huang, Y.; Han, J.; Yoon, Y. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano Res. 2019, 12, 471–487. [Google Scholar] [CrossRef]
- Handoko, A.D.; Fredrickson, K.D.; Anasori, B.; Convey, K.W.; Johnson, L.R.; Gogotsi, Y.; Vojvodic, A.; Seh, Z.W. Tuning the basal plane functionalization of two-dimensional metal carbides (MXenes) to control hydrogen evolution activity. ACS Appl. Energy Mater. 2017, 1, 173–180. [Google Scholar] [CrossRef]
- Riazi, H.; Nemani, S.K.; Grady, M.C.; Anasori, B.; Soroush, M. Ti3C2 MXene–polymer nanocomposites and their applications. J. Mater. Chem. A 2021, 9, 8051. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasool, K.; Madhavan, V.E.; Aissa, B.; Gogotsi, Y.; Mahmoud, K.A. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533. [Google Scholar] [CrossRef]
- Fan, X.; Ding, Y.; Liu, Y.; Liang, J.; Chen, Y. Plasmonic Ti3C2Tx MXene enables highly efficient photothermal conversion for healable and transparent wearable device. ACS Nano 2019, 13, 8124–8134. [Google Scholar] [CrossRef]
- Zhang, J.; Seyedin, S.; Gu, Z.; Yang, W.; Wang, X.; Razal, J.M. MXene: A potential candidate for yarn supercapacitors. Nanoscale 2017, 9, 18604–18608. [Google Scholar] [CrossRef] [PubMed]
- Levitt, A.; Alhabeb, M.; Hatter, C.; Sarycheva, A.; Dionb, G.; Gogotsi, Y. Electrospun MXene/carbon nanofibers as supercapacitor electrodes. J. Mater. Chem. A 2019, 7, 296. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Urbanek, O.; McDaniel, R.M.; Street, R.M.; Barsoum, M.W.; Schauer, C.L. Preparation and characterization of polymer-Ti3C2Tx (MXene) composite nanofibers produced via electrospinning. J. Appl. Polym. Sci. 2017, 134, 45295. [Google Scholar] [CrossRef]
- Lei, J.C.; Zhang, X.; Zhou, Z. Recent advances in MXene:Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Lin, X.P.; Li, Z.J.; Qiu, J.M.; Wang, Q.; Wang, J.X.; Zhang, H.; Chen, T.K. Fascinating MXene nanomaterials: Emerging opportunities in the biomedical field. Biomater. Sci. 2021, 9, 5437–5471. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Kandasubramanian, B. Advancements in MXene-polymer composites for various biomedical applications. Ceram. Int. 2020, 46, 8522–8535. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Li, S.; Jing, L.; Chen, P.; Xie, J.P. Synergistic antimicrobial titanium carbide (MXene) conjugated with gold nanoclusters. Adv. Healthc. Mater. 2020, 9, 2001007. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Xin, B.; Xu, Y. Coaxial electrospinning: Jet motion, core-shell fiber morphology, and structure as a function of material parameters. Ind. Eng. Chem. Res. 2020, 59, 6301–6308. [Google Scholar] [CrossRef]
- ASTM D737; Stardard Test Method for Air Permeability of Textile Fabrics, Subcommittee D 13.59 on Fabrics Test Meathods. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM E96/E96M; Standard Test Methods for Water Vapor Transmission of Materials, Subcommittee C 16.33 on Insulation Finishes and Moisture. ASTM International: West Conshohocken, PA, USA, 2016.
- Gorji, M.; Jeddi, A.A.A.; Gharehaghaji, A.A. Fabrication and characterization of polyurethane electrospun nanofiber membranes for protective clothing applications. J. Appl. Polym. Sci. 2012, 125, 4135. [Google Scholar] [CrossRef]
- Wang, M.; Hsieh, A.J.; Rutledge, G.C. Electrospinning of poly(MMA-co-MAA) copolymers and their layered silicate nanocomposites for improved thermal properties. Polymer 2005, 46, 3407. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Lai, C.; Reneker, D.H.; Qiu, H.; Ye, Y.; Hou, H. Electrospun polymer nanofibres with small diameters. Nanotechnology 2006, 17, 1558. [Google Scholar] [CrossRef]
- Zhao, W.; Yalcin, B.; Cakmak, M. Dynamic assembly of electrically conductive PEDOT:PSS nanofbers in electrospinning process studied by high speed video. Synth. Met. 2015, 203, 107–116. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, X.; Liu, C.; Song, S.; Zhang, J.; Shen, J. FeS@LAB-35@Ti3C2 as a high-efficiency nanozyme for near infrared light induced photothermal enhanced chemodynamic antibacterial activity and wound healing. Nano Res. 2023, 16, 2840–2850. [Google Scholar]
- Jiang, Y.; Lu, J.; Guo, L. Fabrication of highly carboxylated thermoplastic nanofibrous membranes for efficient absorption and separation of protein. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131203. [Google Scholar] [CrossRef]
- Dai, L.; Ying, L. Infrared Spectroscopic Investigation of Hydrogen Bonding in EVOH Containing PVA Fibers. Macromol. Mater. Eng. 2002, 287, 509–514. [Google Scholar] [CrossRef]
- Siva, R.; Mobithis, M.; Ravichandran, R.; Valarmathi, T.; Jeya Jeevahan, J.; Sangeetha, M. Characterization of mechanical, chemical properties and microstructure of untreated and treated Cissus Quadrangularis fiber. Mater. Today Proc. 2021, 47, 4479–4483. [Google Scholar] [CrossRef]
- Tsai, P.P.; Schreuder-Gibson, H.; Gibson, P. Different electrostatic methods for making electret filter. J. Electrostat. 2002, 54, 333–341. [Google Scholar] [CrossRef]
- Wang, B.; Chao, X.; Li, Y.; Reid, S.R. Tensile strength of electrospun Poly(ethylene-co-vinyl alcohol) nanofibre sheets. Key Eng. Mater. 2013, 535, 215–218. [Google Scholar] [CrossRef]
- Richard-Lacroix, M.; Pellerin, C. Orientation and Partial Disentanglement in Individual Electrospun Fibers: Diameter Dependence and Correlation with Mechanical Properties. Macromolecules 2015, 48, 4511–4519. [Google Scholar] [CrossRef]
- Pai, C.L.; Boyce, M.C.; Rutledge, G.C. Mechanical properties of individual electrospun PA 6(3)T fibers and their variation with fiber diameter. Polymer 2011, 52, 2295–2301. [Google Scholar] [CrossRef]
- Sheng, J.L.; Xu, Y.; Yu, J.Y.; Ding, B. Robust fluorine-free superhydrophobic amino-silicone Oil/SiO2 modification of electrospun polyacrylonitrile membranes for waterproof-breathable application. ACS Appl. Mater. Interfaces 2017, 17, 15139–15147. [Google Scholar] [CrossRef] [PubMed]

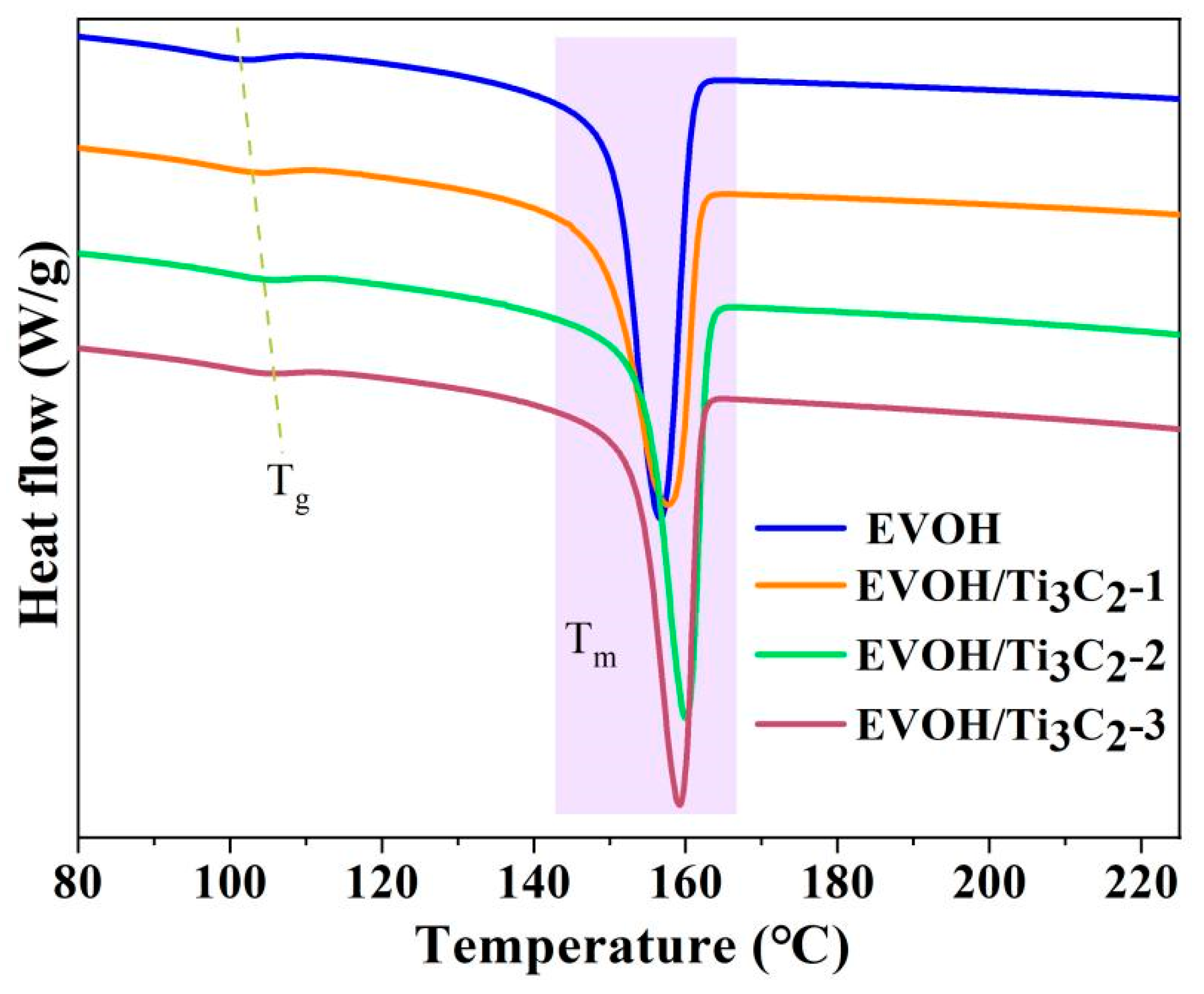
| Samples | Tg (°C) | Tm (°C) |
|---|---|---|
| EVOH | 101 | 156 |
| EVOH/Ti3C2-1 | 104 | 158 |
| EVOH/Ti3C2-2 | 105 | 160 |
| EVOH/Ti3C2-3 | 107 | 159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, Q. Preparation and Characterization of Electrospun EVOH/Ti3C2 Composite Fibers. Polymers 2024, 16, 630. https://doi.org/10.3390/polym16050630
Li X, Xu Q. Preparation and Characterization of Electrospun EVOH/Ti3C2 Composite Fibers. Polymers. 2024; 16(5):630. https://doi.org/10.3390/polym16050630
Chicago/Turabian StyleLi, Xiang, and Qiao Xu. 2024. "Preparation and Characterization of Electrospun EVOH/Ti3C2 Composite Fibers" Polymers 16, no. 5: 630. https://doi.org/10.3390/polym16050630




