Dielectrophoretically Assembled SWCNTs Networks on SU-8 Substrate for PEG/SWCNTs Composite Gas Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. CNTs/Polymers Composite Gas Sensor Architecture and Fabrication
- (a)
- A 4″ silicon wafer used as the supporting plate of the flexible SU-8 film was cleaned via the RCA process.
- (b)
- DC sputter deposition of adhesion layer (500 Å Ti) and sacrificial layer (3000 Å Cu) occurred.
- (c)
- Spin coating and curing of 17 μm SU-8 bottom cladding was performed.
- (d)
- Spin coating with positive photoresist AZ4620 (AZ Electronic Materials, Bridgewater, NJ, USA) was performed, followed by heating at 90 °C for 2 min, then the 1st mask and photolithography process was conducted. Then, we used a DC vacuum sputtering system to deposit 500 Å of titanium (Ti), 2000 Å of copper (Cu), and 500 Å of titanium (Ti). After deposition, we used a lift-off process for the pattern of the heater.
- (e)
- After spin coating with negative photoresist SU-8 3025 with 17 μm, we performed the 2nd mask and photolithography process. Spin-coating with a thickness of 5 μm of positive photoresist was performed, followed by heating to 90 °C for 2 min, and the silicon wafer was immersed in the developer (Propylene glycol methyl ether acetate, PGMEA) for 1 min.
- (f)
- After spin coating 5 μm positive photoresist AZ4620, we performed the 3rd mask and photolithography process. We used a DC vacuum sputtering system to deposit 500 Å of titanium (Ti), 2000 Å of gold (Au), and 500 Å of titanium (Ti). We used a lift-off process to remove excess metal and generate the designed electrode, wiring and pad.
- (g)
- After spin coating 17 μm negative photoresist SU-8 3025 and heating to 95 °C for 30 min, we immersed the silicon wafer in the developer (PGMEA) for 1 min, then heated it to 120 °C for 10 min. We performed the 4th mask and photolithography process process to complete the electrode pad opening. We used a Reactive Ion Etching (RIE) system for anisotropic dry etching of the top layer of titanium, defining the region where the electrode needed to be electrically isolated on the pad.
- (h)
- We immersed the component in copper etchant to release the sensor from the slicon wafer.
- (i)
- After the SU-8 based structure was fabricated, the PEG/SWCNTs bilayer sensing films were prepared via dielectrophoresis and drop casting method.
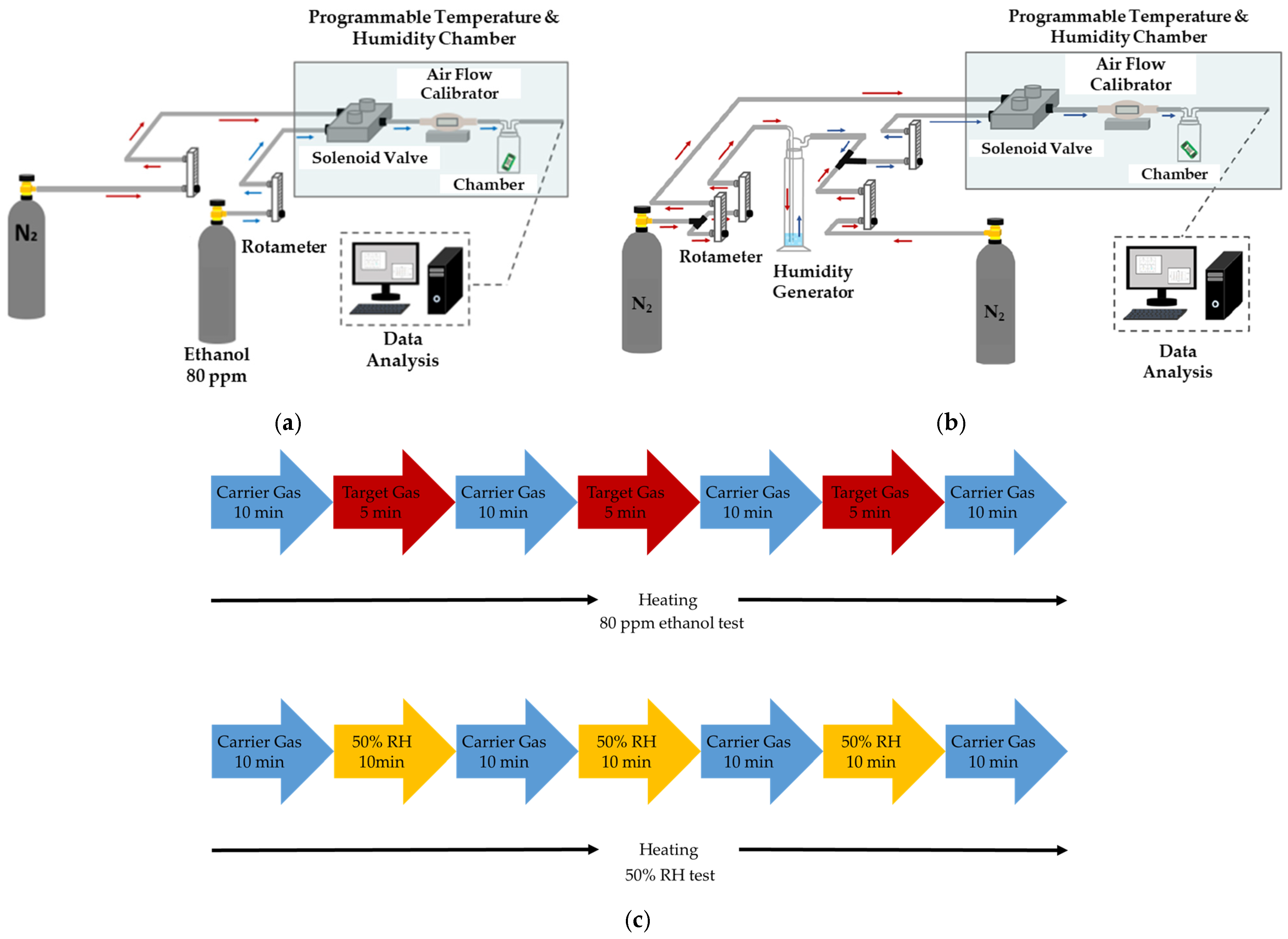
2.2. Experimental Scheme for Gas Sensor Evaluation
3. Results and Discussion
3.1. Dielectrophoresis Characterization
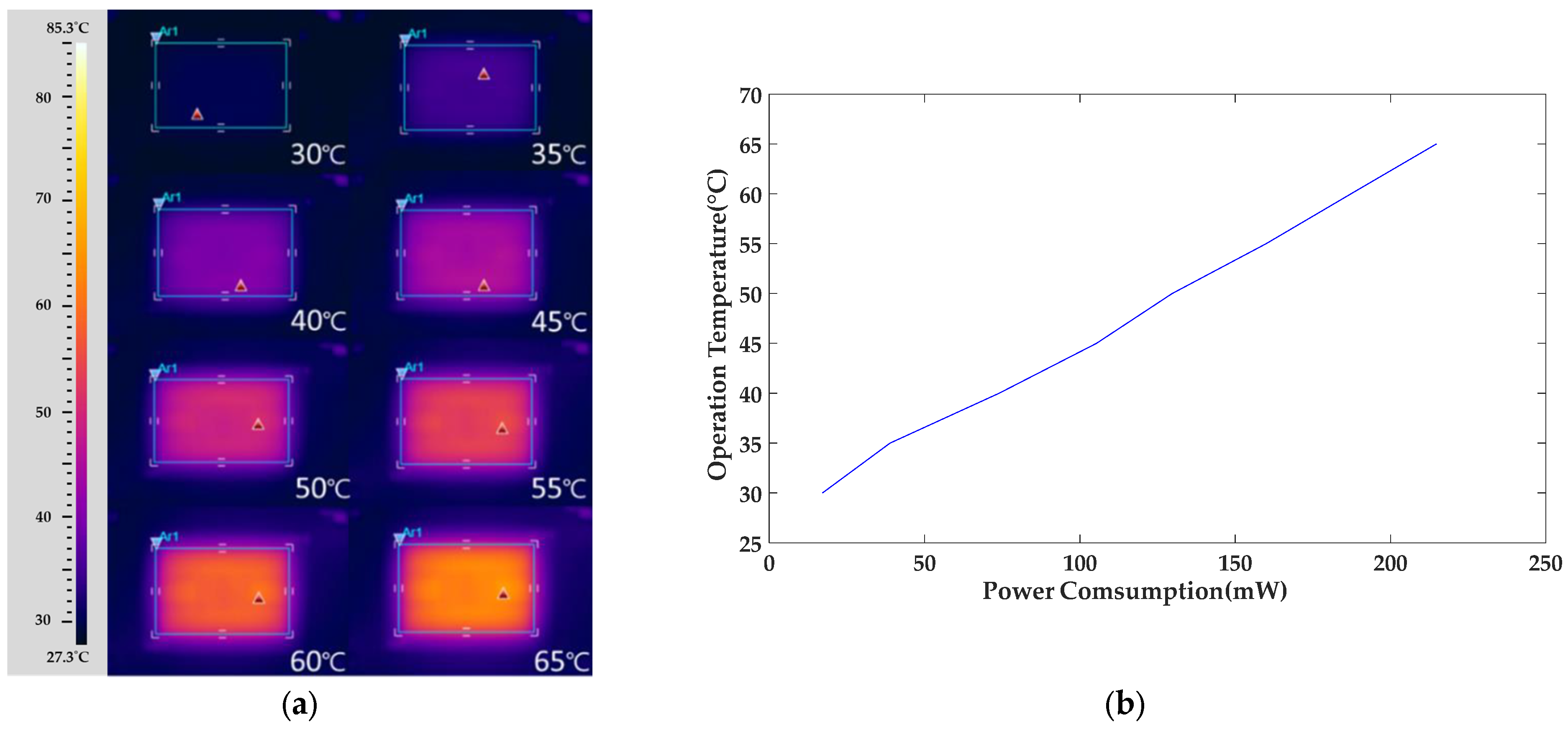
3.2. Heater Performance
3.3. Response of Target Gas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Zhu, Y.; Huang, J.; Zhang, J.; Pan, D.; Zhou, J.; Ryu, J.E.; Umar, A.; Guo, Z. Advances in Responsively Conductive Polymer Composites and Sensing Applications. Polym. Rev. 2021, 61, 157–193. [Google Scholar] [CrossRef]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yue, W.; Li, Y.; Gao, S.; Zhang, C.; Kan, H.; Niu, H.; Wang, W.; Guo, Y. Carbon-based nanomaterials for the detection of volatile organic compounds: A review. Carbon 2021, 180, 274–297. [Google Scholar] [CrossRef]
- Lu, J.; Kumar, B.; Castro, M.; Feller, J.-F. Vapour sensing with conductive polymer nanocomposites (CPC): Polycarbonate-carbon nanotubes transducers with hierarchical structure processed by spray layer by layer. Sens. Actuators B Chem. 2009, 140, 451–460. [Google Scholar] [CrossRef]
- Mangu, R.; Rajaputra, S.; Singh, V.P. MWCNT–polymer composites as highly sensitive and selective room temperature gas sensors. Nanotechnology 2011, 22, 215502. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ha, S.C.; Yang, Y.; Kim, Y.J.; Cho, S.M.; Yang, H.; Kim, Y.T. Portable Electronic Nose System Based on the Carbon Black-Polymer Composite Sensor Array. Sens. Actuators B-Chem. 2005, 108, 285–291. [Google Scholar] [CrossRef]
- Xie, H.F.; Yang, Q.D.; Sun, X.X.; Yang, J.J.; Huang, Y.P. Gas sensor arrays based on polymer-carbon black to detect organic vapors at low concentration. Sens. Actuators B-Chem. 2006, 113, 887–891. [Google Scholar] [CrossRef]
- Chiou, J.C.; Wu, C.C.; Huang, Y.C.; Chang, S.C.; Lin, T.M. Effects of Operating Temperature on Droplet Casting of Flexible Polymer/Multi-Walled Carbon Nanotube Composite Gas Sensors. Sensors 2017, 17, 4. [Google Scholar] [CrossRef]
- Chiou, J.C.; Wu, C.C.; Lin, T.M. Sensitivity enhancement of acetone gas sensor using polyethylene glycol/multi-walled carbon nanotubes composite sensing film with thermal treatment. Polymers 2019, 11, 423. [Google Scholar] [CrossRef]
- Gong, S.; Zhu, Z.H.; Meguid, S.A. Carbon nanotube agglomeration effect on piezoresistivity of polymer nanocomposites. Polymer 2014, 55, 5488–5499. [Google Scholar] [CrossRef]
- Tamayo-Vegas, S.; Muhsan, A.; Liu, C.; Tarfaoui, M.; Lafdi, K. The Effect of Agglomeration on the Electrical and Mechanical Properties of Polymer Matrix Nanocomposites Reinforced with Carbon Nanotubes. Polymers 2022, 14, 1842. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, A.; Halim, M.M.; Halin, I.A. Dielectrophoretic Alignment of Carbon Nanotubes: Theory, Applications, and Future. Nanotechnology 2023, 34, 242001–242039. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, H.; Pan, M.; Chen, D. A Carbon Nanotube Gas Sensor Fabricated by Dielectrophoresis and Its Application for NH3 Detection; IEEE Computer Society: Minneapolis, MO, USA, 2009; pp. 6046–6049. [Google Scholar]
- An, L.; Friedrich, C. Dielectrophoretic assembly of carbon nanotubes and stability analysis. Prog. Nat. Sci. Mater. Int. 2013, 23, 367–373. [Google Scholar] [CrossRef]
- Zhou, T.; Kropp, E.; Chen, J.; Kulinsky, L. Step-Wise Deposition Process for Dielectrophoretic Formation of Conductive 50-Micron-Long Carbon Nanotube Bridges. Micromachines 2020, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Hierlemann, A.; Zellers, E.T. Use of linear solvation energy relationships for modeling responses from polymer-coated acoustic-wave vapor sensors. Anal. Chem. 2001, 73, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.K.; Wang, L.C.; Kuo, C.T. Development of a high performance integrated sensor chip with a multi-walled carbon nanotube assisted sensing array. Thin Solid Film. 2013, 529, 205–208. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, H.; Yu, A.; Perea, D.; Haddon, R.C. Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers. J. Am. Chem. Soc. 2005, 127, 8197–8203. [Google Scholar] [CrossRef]
- Dimaki, M.; Bøggild, P. Dielectrophoresis of Carbon Nanotubes Using Microelectrodes: A Numerical Study. Nanotechnology 2004, 15, 1095–1102. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Yunus, N.A.M.; Halin, I.A.; Hamidon, M.N. Simulation of dielectrophoretic alignment for carbon nanotube on indium tin oxide. Mater. Today Pro. 2019, 7, 593–600. [Google Scholar] [CrossRef]
- Young, S.J.; Lin, Z.D. Ethanol gas sensors based on multi-wall carbon nanotubes on oxidized Si substrate. Microsyst. Technol. 2018, 24, 55–58. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.X.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Maijenburg, A.W.; Maas, M.G.; Rodijk, E.J.B.; Ahmed, W.; Kooij, E.S.; Carlen, E.T.; Blank, D.H.A.; ten Elshof, J.E. Dielectrophoretic alignment of metal and metal oxide nanowires and nanotubes: A universal set of parameters for bridging prepatterned microelectrodes. J. Colloid Interface Sci. 2011, 355, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, T.; Dong, Y.; Wang, X. A Room Temperature VOCs Gas Sensor Based on a Layer by Layer Multi-Walled Carbon Nanotubes/Poly-ethylene Glycol Composite. Sensors 2018, 18, 3113. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of humidity on gas sensing performance of carbon nanotube gas sensors operated at room temperature. IEEE Sens. J. 2021, 21, 5763–5770. [Google Scholar] [CrossRef]
- Shenogin, S.; Lee, J.; Voevodin, A.A.; Roy, A.K. The effect of molecular mobility on electronic transport in carbon nanotube-polymer composites and networks. J. Appl. Phys. 2014, 116, 233704. [Google Scholar] [CrossRef]






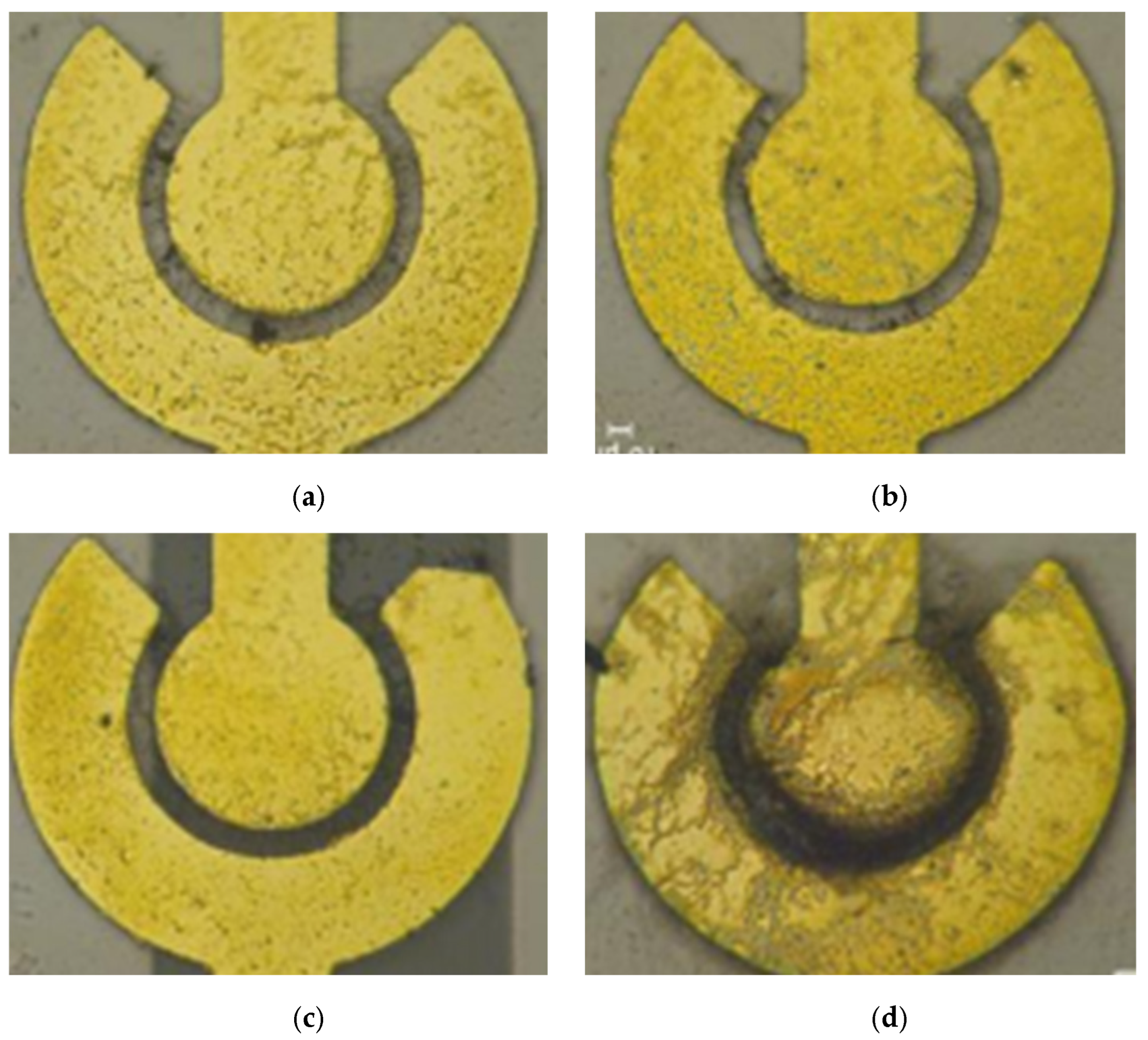




| DEP Voltage (Vpp) | Electrode Spacing | |||
|---|---|---|---|---|
| 10 μm | 15 μm | 18 μm | 20 μm | |
| 1 V | 44.8 K | 132.4 K | 1700 K | 2300 K |
| 2 V | 29.6 K | 69.8 K | 237.7 K | 330 K |
| 3 V | 16.6 K | 23.6 K | 57.8 K | 68.9 K |
| 4 V | 1.65 K | 11.3 K | 23.4 K | 33.1 K |
| 5 V | 0.991 K | 2.43 K | 7.1 K | 23.7 K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiou, J.-C.; Wu, C.-C.; Lin, T.-M.; Huang, Y.-C. Dielectrophoretically Assembled SWCNTs Networks on SU-8 Substrate for PEG/SWCNTs Composite Gas Sensor. Polymers 2024, 16, 74. https://doi.org/10.3390/polym16010074
Chiou J-C, Wu C-C, Lin T-M, Huang Y-C. Dielectrophoretically Assembled SWCNTs Networks on SU-8 Substrate for PEG/SWCNTs Composite Gas Sensor. Polymers. 2024; 16(1):74. https://doi.org/10.3390/polym16010074
Chicago/Turabian StyleChiou, Jin-Chern, Chin-Cheng Wu, Tse-Mei Lin, and Yu-Chieh Huang. 2024. "Dielectrophoretically Assembled SWCNTs Networks on SU-8 Substrate for PEG/SWCNTs Composite Gas Sensor" Polymers 16, no. 1: 74. https://doi.org/10.3390/polym16010074





