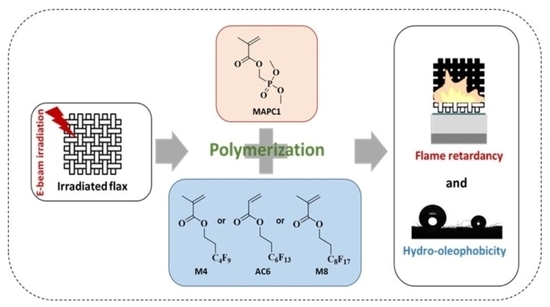
One-Step Multifunctionalization of Flax Fabrics for Simultaneous Flame-Retardant and Hydro-Oleophobic Properties Using Radiation-Induced Graft Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grafting Process
2.3. Measurements
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Scanning Electron Microscopy SEM
2.3.3. Measurement of Phosphorus and Fluorine Contents
Phosphorus Content
- Inductively coupled plasma atomic emission spectroscopy
- b.
- X-ray fluorescence (XRF)
Fluorine Content Measurement
2.3.4. Pyrolysis Combustion Flow Calorimetry (PCFC)
2.3.5. Cone Calorimetry
2.3.6. Contact Angle Measurements
2.3.7. Sliding Angle Measurements
3. Results and Discussion
3.1. Grafting of Fluorinated and Phosphorus Polymers onto Irradiated Flax Fabrics
3.1.1. FTIR Analysis
3.1.2. Reactivity of Monomers Used in Radiation-Induced Grafting Polymerization
3.1.3. Localization of the Fluorine and Phosphorus Elements in the Modified Flax Fibers
3.2. Hydro- and Oleophobic Properties and Water Repellency of Treated Flax Fabrics
3.3. Flame-Retardant Properties of Treated Flax Fabrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, M.; Chen, M.; Zong, Y.; Li, Z. Modification of fabric via co-grafted with fluorine-free carbene polymer and its hydrophobicity. Polymer 2022, 247, 124802. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Li, Z. Preparation of golf ball-shaped microspheres with fluorinated polycaprolactone via single-solvent electrospraying for superhydrophobic coatings. Prog. Org. Coat. 2019, 131, 276–284. [Google Scholar] [CrossRef]
- Li, W.; Zong, Y.; Liu, Q.; Sun, Y.; Li, Z.; Wang, H.; Li, Z. A highly stretchable and biodegradable superamphiphobic fluorinated polycaprolactone nanofibrous membrane for antifouling. Prog. Org. Coat. 2020, 147, 105776. [Google Scholar] [CrossRef]
- Li, W.; Liu, K.; Zhang, Y.; Guo, S.; Li, Z.; Tan, S.C. A facile strategy to prepare robust self-healable superhydrophobic fabrics with self-cleaning, anti-icing, UV resistance, and antibacterial properties. Chem. Eng. J. 2022, 446, 137195. [Google Scholar] [CrossRef]
- Sonnier, R.; Otazaghine, B.; Viretto, A.; Apolinario, G.; Ienny, P. Improving the flame retardancy of flax fabrics by radiation grafting of phosphorus compounds. Eur. Polym. J. 2015, 68, 313–325. [Google Scholar] [CrossRef]
- Hajj, R.; El Hage, R.; Sonnier, R.; Otazaghine, B.; Gallard, B.; Rouif, S.; Nakhl, M.; Lopez-Cuesta, J.-M. Grafting of phosphorus flame retardants on flax fabrics: Comparison between two routes. Polym. Degrad. Stab. 2018, 147, 25–34. [Google Scholar] [CrossRef]
- Hajj, R.; Otazaghine, B.; Sonnier, R.; El Hage, R.; Rouif, S.; Nakhl, M.; Lopez-Cuesta, J.-M. Influence of monomer reactivity on radiation grafting of phosphorus flame retardants on flax fabrics. Polym. Degrad. Stab. 2019, 166, 86–98. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, S.; Wang, L.; Zheng, Y. Lotus effect in wetting and self-cleaning. Biotribology 2016, 5, 31–43. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Shi, T.; Liang, J.; Li, X.; Zhang, C.; Yang, H. Improving the Corrosion Resistance of Aluminum Alloy by Creating a Superhydrophobic Surface Structure through a Two-Step Process of Etching Followed by Polymer Modification. Polymers 2022, 14, 4509. [Google Scholar] [CrossRef]
- Kozłowski, R.M.; Muzyczek, M. Improving the Flame Retardancy of Natural Fibres. In Handbook of Natural Fibres; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–391. [Google Scholar] [CrossRef]
- Gholampour, A.; Ozbakkaloglu, T. A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications. J. Mater. Sci. 2020, 55, 829–892. [Google Scholar] [CrossRef]
- Mokhothu, T.H.; John, M.J. Review on hygroscopic aging of cellulose fibres and their biocomposites. Carbohydr. Polym. 2015, 131, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jinjin, D. Improvement of hydrophobic properties of silk and cotton by hexafluoropropene plasma treatment. Appl. Surf. Sci. 2007, 253, 5051–5055. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.-H.; Kang, B.-K.; Uhm, H.S. Superhydrophobic CFx Coating via In-Line Atmospheric RF Plasma of He−CF4−H2. Langmuir 2005, 21, 12213–12217. [Google Scholar] [CrossRef] [PubMed]
- Kamlangkla, K.; Paosawatyanyong, B.; Pavarajarn, V.; Hodak, J.; Hodak, S.K. Mechanical strength and hydrophobicity of cotton fabric after plasma treatment. Appl. Surf. Sci. 2010, 256, 5888–5897. [Google Scholar] [CrossRef]
- Xue, C.-H.; Jia, S.-T.; Chen, H.-Z.; Wang, M. Superhydrophobic cotton fabrics prepared by sol–gel coating of TiO2and surface hydrophobization. Sci. Technol. Adv. Mater. 2008, 9, 035001. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, W.; Jiang, C.; He, S.; Xie, Y.; Wang, Z. Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process. Carbohydr. Polym. 2018, 197, 75–82. [Google Scholar] [CrossRef]
- Mahltig, B.; Böttcher, H. Modified Silica Sol Coatings for Water-Repellent Textiles. J. Sol-Gel Sci. Technol. 2003, 27, 43–52. [Google Scholar] [CrossRef]
- Miao, H.; Bao, F.; Cheng, L.; Shi, W. Cotton fabric modification for imparting high water and oil repellency using perfluoroalkyl phosphate acrylate via γ-ray-induced grafting. Radiat. Phys. Chem. 2010, 79, 786–790. [Google Scholar] [CrossRef]
- Deng, B.; Cai, R.; Yu, Y.; Jiang, H.; Wang, C.; Li, J.; Li, L.; Yu, M.; Li, J.; Xie, L.; et al. Laundering Durability of Superhydrophobic Cotton Fabric. Adv. Mater. 2010, 22, 5473–5477. [Google Scholar] [CrossRef]
- Cai, R.; Deng, B.; Jiang, H.; Yu, Y.; Yu, M.; Li, L.; Li, J. Radiation induced graft polymerization of a fluorinated acrylate onto fabric. Radiat. Phys. Chem. 2012, 81, 1354–1356. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; John, M.J.; Jacobs, V.; Luyt, A.S. Review on flammability of biofibres and biocomposites. Carbohydr. Polym. 2014, 111, 149–182. [Google Scholar] [CrossRef] [PubMed]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.-M. Towards Bio-based Flame Retardant Polymers; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Sonnier, R.; Ferry, L.; Lopez-Cuesta, J.M. Flame Retardancy of Phosphorus-Containing Polymers; The Royal Society of Chemistry: Oxford, UK, 2014; pp. 252–270. [Google Scholar]
- Teixeira, M.; Sonnier, R.; Otazaghine, B.; Ferry, L.; Aubert, M.; Tirri, T.; Wilén, C.-E.; Rouif, S. Radiation-grafting of flame retardants on flax fabrics—A comparison between different flame retardant structures. Radiat. Phys. Chem. 2018, 145, 135–142. [Google Scholar] [CrossRef]
- Taibi, J.; Rouif, S.; Clément, J.-L.; Ameduri, B.; Sonnier, R.; Otazaghine, B. Flame retardancy of flax fibers by pre-irradiation grafting of a phosphonate monomer. Ind. Crop. Prod. 2022, 176, 114334. [Google Scholar] [CrossRef]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [PubMed]
- Kousar, F.; Moratti, S.C. Synthesis of fluorinated phosphorus-containing copolymers and their immobilization and properties on stainless steel. RSC Adv. 2021, 11, 38189–38201. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Meng, W.-D.; Qing, F.-L. Synthesis and repellent properties of vinylidene fluoride-containing polyacrylates. J. Fluor. Chem. 2007, 128, 1469–1477. [Google Scholar] [CrossRef]
- Leng, B.; Shao, Z.; de With, G.; Ming, W. Superoleophobic Cotton Textiles. Langmuir 2009, 25, 2456–2460. [Google Scholar] [CrossRef]
- Zahid, M.; Mazzon, G.; Athanassiou, A.; Bayer, I.S. Environmentally benign non-wettable textile treatments: A review of recent state-of-the-art. Adv. Colloid Interface Sci. 2019, 270, 216–250. [Google Scholar] [CrossRef]
- Liagkouridis, I.; Awad, R.; Schellenberger, S.; Plassmann, M.M.; Cousins, I.T.; Benskin, J.P. Combined Use of Total Fluorine and Oxidative Fingerprinting for Quantitative Determination of Side-Chain Fluorinated Polymers in Textiles. Environ. Sci. Technol. Lett. 2021, 9, 30–36. [Google Scholar] [CrossRef]
- Schellenberger, S.; Jönsson, C.; Mellin, P.; Levenstam, O.A.; Liagkouridis, I.; Ribbenstedt, A.; Hanning, A.-C.; Schultes, L.; Plassmann, M.M.; Persson, C.; et al. Release of Side-Chain Fluorinated Polymer-Containing Microplastic Fibers from Functional Textiles During Washing and First Estimates of Perfluoroalkyl Acid Emissions. Environ. Sci. Technol. 2019, 53, 14329–14338. [Google Scholar] [CrossRef]
- Ameduri, B. Issues, Challenges, Regulations and Applications of Perfluoroalkyl Substances; Royal Society of Chemistry: Oxford, UK, 2022. [Google Scholar] [CrossRef]
- Taibi, J.; Rouif, S.; Ameduri, B.; Sonnier, R.; Otazaghine, B. Radiation induced graft polymerization of fluorinated monomers onto flax fabrics for the control of hydrophobic and oleophobic properties. Polymer 2023. under review. [Google Scholar]
- Huggett, C. Estimation of rate of heat release by means of oxygen consumption measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Vahabi, H.; Ferry, L.; Longuet, C.; Sonnier, R.; Negrell-Guirao, C.; David, G.; Lopez-Cuesta, J.-M. Theoretical and empirical approaches to understanding the effect of phosphonate groups on the thermal degradation for two chemically modified PMMA. Eur. Polym. J. 2012, 48, 604–612. [Google Scholar] [CrossRef]
- Hinchiranan, N.; Wannako, P.; Paosawatyanyong, B.; Prasassarakich, P. 2,2,2-Trifluoroethyl methacrylate-graft-natural rubber: Synthesis and application as compatibilizer in natural rubber/fluoroelastomer blends. Mater. Chem. Phys. 2013, 139, 689–698. [Google Scholar] [CrossRef]
- Illy, N.; Couture, G.; Auvergne, R.; Caillol, S.; David, G.; Boutevin, B. New prospects for the synthesis of N-alkyl phosphonate/phosphonic acid-bearing oligo-chitosan. RSC Adv. 2014, 4, 24042–24052. [Google Scholar] [CrossRef]
- Xu, A.-H.; Zhang, L.-Q.; Ma, J.-C.; Ma, Y.-M.; Geng, B.; Zhang, S.-X. Preparation and surface properties of poly(2,2,2-trifluoroethyl methacrylate) coatings modified with methyl acrylate. J. Coat. Technol. Res. 2016, 13, 795–804. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Chu, G.; Zhang, L.; Xu, A.; Li, H.; Geng, B. Synthesis, characterization and properties of a novel fluorinated methacrylate polymer. J. Fluor. Chem. 2011, 132, 915–919. [Google Scholar] [CrossRef]
- Xue, D.; Wang, X.; Ni, H.; Zhang, W.; Xue, G. Surface Segregation of Fluorinated Moieties on Random Copolymer Films Controlled by Random-Coil Conformation of Polymer Chains in Solution. Langmuir 2009, 25, 2248–2257. [Google Scholar] [CrossRef]
- Guyot, B.; Améduri, B.; Boutevin, B.; Sidéris, A. Etude de la Copolymérisation de Monomères Acryliques Fluorés avec le Méthacrylate de Morpholinoéthyle. Macromol. Chem. Phys. 1996, 197, 937–946. [Google Scholar] [CrossRef]
- Huo, M.; Li, D.; Song, G.; Zhang, J.; Wu, D.; Wei, Y.; Yuan, J. Semi-Fluorinated Methacrylates: A Class of Versatile Monomers for Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2018, 39, e1700840. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E. Preparation of micro- and nanospheres with superamphiphobic surfaces by dispersion polymerization. Colloid Polym. Sci. 2011, 290, 525–530. [Google Scholar] [CrossRef]
- Khan, M.Z.; Militky, J.; Petru, M.; Tomková, B.; Ali, A.; Tören, E.; Perveen, S. Recent advances in superhydrophobic surfaces for practical applications: A review. Eur. Polym. J. 2022, 178, 111481. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Chapple, S.; Anandjiwala, R. Flammability of Natural Fiber-reinforced Composites and Strategies for Fire Retardancy: A Review. J. Thermoplast. Compos. Mater. 2010, 23, 871–893. [Google Scholar] [CrossRef]
- El Gazi, M.; Sonnier, R.; Giraud, S.; Batistella, M.; Basak, S.; Dumazert, L.; Hajj, R.; El Hage, R. Fire Behavior of Thermally Thin Materials in Cone Calorimeter. Polymers 2021, 13, 1297. [Google Scholar] [CrossRef]












| Monomers Combination | Monomers Ratio (F/P) | Dose (kGy) | F-Content (wt%) a | P-Content (wt%) b | F-Monomer Concentration (10−4 mol/g) c | P-Monomer Concentration (10−4 mol/g) d | Initial F/P Monomers Molar Ratio | Final F/P Monomers Molar Ratio |
|---|---|---|---|---|---|---|---|---|
| MAPC1 | 0:100 | 20 | - | 0.22 | - | 0.71 | - | - |
| 0:100 | 100 | - | 1.73 | - | 5.58 | - | - | |
| M4 | 100:0 | 20 | 0.15 | - | 0.09 | - | - | - |
| 100:0 | 100 | 0.22 | - | 0.13 | - | - | - | |
| AC6 | 100:0 | 20 | 0.31 | - | 0.13 | - | - | - |
| 100:0 | 100 | 1.09 | - | 0.44 | - | - | - | |
| M8 | 100:0 | 20 | 1.97 | - | 0.61 | - | - | - |
| 100:0 | 100 | 8.05 | - | 2.49 | - | - | - | |
| M4/MAPC1 | 20:80 | 20 | 0.26 | 0.45 | 0.15 | 1.45 | 0.16 | 0.10 |
| 50:50 | 0.27 | 0.10 | 0.16 | 0.32 | 0.63 | 0.50 | ||
| 80:20 | 0.24 | 0.07 | 0.14 | 0.23 | 2.50 | 0.63 | ||
| 20:80 | 100 | 0.26 | 0.23 | 0.15 | 0.74 | 0.16 | 0.21 | |
| 50:50 | 0.33 | 0.26 | 0.19 | 0.84 | 0.63 | 0.23 | ||
| 80:20 | 0.40 | 0.28 | 0.24 | 0.90 | 2.50 | 0.26 | ||
| AC6/MAPC1 | 20:80 | 20 | 0.16 | 1.22 | 0.06 | 3.94 | 0.12 | 0.02 |
| 50:50 | 0.18 | 0.45 | 0.07 | 1.45 | 0.50 | 0.05 | ||
| 80:20 | 0.37 | 0.35 | 0.15 | 1.13 | 1.99 | 0.13 | ||
| 20:80 | 100 | 0.21 | 0.33 | 0.08 | 1.06 | 0.12 | 0.08 | |
| 50:50 | 0.72 | 0.17 | 0.29 | 0.55 | 0.50 | 0.53 | ||
| 80:20 | 0.63 | 0.13 | 0.25 | 0.42 | 1.99 | 0.61 | ||
| M8/MAPC1 | 20:80 | 20 | 0.22 | 0.46 | 0.07 | 1.48 | 0.10 | 0.05 |
| 50:50 | 3.82 | 0.56 | 1.18 | 1.81 | 0.39 | 0.65 | ||
| 80:20 | 4.55 | 0.29 | 1.41 | 0.94 | 1.56 | 1.51 | ||
| 20:80 | 100 | 0.53 | 0.31 | 0.16 | 1.00 | 0.10 | 0.16 | |
| 50:50 | 8.04 | 0.77 | 2.99 | 2.48 | 0.39 | 1.00 | ||
| 80:20 | 6.63 | 0.24 | 2.05 | 0.77 | 1.56 | 2.65 |
| Monomers Combination | Monomers Ratio (F/P) | Dose (kGy) | F-Content (wt%) | P-Content (wt%) | WCA (°) | DCA (°) | SA (°) |
|---|---|---|---|---|---|---|---|
| Pristine flax | - | 0 | 0.00 | 0.00 | - | - | - |
| MAPC1 | 0:100 | 20 | 0.00 | 0.22 | - | - | - |
| 0:100 | 100 | 0.00 | 1.73 | - | - | - | |
| M4 | 100:0 | 20 | 0.15 | 0.00 | 138 ± 1 | 132 ± 2 | 43.5 ± 4.7 |
| 100:0 | 100 | 0.22 | 0.00 | 130 ± 2 | 125 ± 5 | 90.0 ± 0.0 | |
| AC6 | 100:0 | 20 | 0.31 | 0.00 | 145 ± 4 | 128 ± 2 | 38.0 ± 1.6 |
| 100:0 | 100 | 1.09 | 0.00 | 147 ± 3 | 134 ± 3 | 28.0 ± 3.7 | |
| M8 | 100:0 | 20 | 1.97 | 0.00 | 150 ± 2 | 133 ± 1 | 9.5 ± 1.3 |
| 100:0 | 100 | 8.05 | 0.00 | 150 ± 1 | 136 ± 2 | 9.8 ± 1.3 | |
| M4/MAPC1 | 20:80 | 20 | 0.26 | 0.45 | 0 ± 0 | 0 ± 0 | - |
| 50:50 | 0.27 | 0.10 | 124 ± 9 | 74 ± 10 | >90 | ||
| 80:20 | 0.24 | 0.07 | 133 ± 4 | 125 ± 2 | >90 | ||
| 20:80 | 100 | 0.26 | 0.23 | 137 ± 4 | 110 ± 13 | >90 | |
| 50:50 | 0.33 | 0.26 | 140 ± 2 | 129 ± 3 | 46.5 ± 5.5 | ||
| 80:20 | 0.40 | 0.28 | 142 ± 1 | 130 ± 2 | 37.0 ± 4.0 | ||
| AC6/MAPC1 | 20:80 | 20 | 0.16 | 1.22 | 0 ± 0 | 0 ± 0 | - |
| 50:50 | 0.18 | 0.45 | 129 ± 7 | 121 ± 3 | 45.0 ± 3.5 | ||
| 80:20 | 0.37 | 0.35 | 145 ± 2 | 130 ± 4 | 35.0 ± 3.0 | ||
| 20:80 | 100 | 0.21 | 0.33 | 94 ± 23 | 88 ± 13 | >90 | |
| 50:50 | 0.72 | 0.17 | 135 ± 9 | 125 ± 8 | 42.0 ± 4.5 | ||
| 80:20 | 0.63 | 0.13 | 142 ± 1 | 131 ± 3 | 31.0 ± 5.5 | ||
| M8/MAPC1 | 20:80 | 20 | 0.22 | 0.46 | 0 ± 0 | 0 ± 0 | - |
| 50:50 | 3.82 | 0.56 | 146 ± 2 | 134 ± 1 | 12.8 ± 2.8 | ||
| 80:20 | 4.55 | 0.29 | 147 ± 2 | 131 ± 1 | 13.5 ± 1.5 | ||
| 20:80 | 100 | 0.53 | 0.31 | 95 ± 3 | 62 ± 4 | - | |
| 50:50 | 8.04 | 0.77 | 151 ± 1 | 131 ± 3 | 11.3 ± 1.4 | ||
| 80:20 | 6.63 | 0.24 | 149 ± 2 | 132 ± 2 | 13.5 ± 1.5 |
| Monomers Combination | Ratio (F:P) | Dose (kGy) | F-Content (wt%) | P-Content (wt%) | pHRR (W/g) | T-pHRR (°C) | THR (kJ/g) | Residue (wt%) |
|---|---|---|---|---|---|---|---|---|
| Pristine flax | - | 0 | 0.00 | 0.00 | 230 | 370 | 9.0 | 11 |
| MAPC1 | 0:100 | 20 | 0.00 | 0.22 | 131 | 312 | 6.8 | 21 |
| 0:100 | 100 | 0.00 | 1.73 | 47 | 255 | 2.9 | 40 | |
| M4 | 100:0 | 20 | 0.15 | 0.00 | 199 | 367 | 9.7 | 13 |
| 100:0 | 100 | 0.22 | 0.00 | 206 | 365 | 9.5 | 13 | |
| AC6 | 100:0 | 20 | 0.31 | 0.00 | 183 | 368 | 8.4 | 14 |
| 100:0 | 100 | 1.09 | 0.00 | 210 | 363 | 9.6 | 11 | |
| M8 | 100:0 | 20 | 1.97 | 0.00 | 207 | 375 | 9.9 | 8 |
| 100:0 | 100 | 8.05 | 0.00 | 210 | 364 | 9.1 | 8 | |
| M4/MAPC1 | 20:80 | 20 | 0.26 | 0.45 | 95 | 302 | 4.5 | 26 |
| 50:50 | 0.27 | 0.10 | 139 | 323 | 6.7 | 17 | ||
| 80:20 | 0.24 | 0.07 | 208 | 329 | 9.5 | 13 | ||
| 20:80 | 100 | 0.26 | 0.23 | 121 | 312 | 5.6 | 22 | |
| 50:50 | 0.33 | 0.26 | 106 | 304 | 5.4 | 24 | ||
| 80:20 | 0.40 | 0.28 | 122 | 302 | 6.9 | 18 | ||
| AC6/MAPC1 | 20:80 | 20 | 0.16 | 1.22 | 46 | 271 | 2.8 | 37 |
| 50:50 | 0.18 | 0.45 | 102 | 300 | 4.9 | 28 | ||
| 80:20 | 0.37 | 0.35 | 106 | 302 | 5.3 | 24 | ||
| 20:80 | 100 | 0.21 | 0.33 | 83 | 288 | 4.6 | 28 | |
| 50:50 | 0.72 | 0.17 | 157 | 306 | 7.9 | 16 | ||
| 80:20 | 0.63 | 0.13 | 200 | 323 | 9.2 | 14 | ||
| M8/MAPC1 | 20:80 | 20 | 0.22 | 0.46 | 84 | 288 | 4.4 | 27 |
| 50:50 | 3.82 | 0.56 | 63 | 289 | 5.4 | 24 | ||
| 80:20 | 4.55 | 0.29 | 85 | 298 | 8.1 | 11 | ||
| 20:80 | 100 | 0.53 | 0.31 | 90 | 299 | 4.4 | 23 | |
| 50:50 | 8.04 | 0.77 | 53 | 279 | 6.1 | 28 | ||
| 80:20 | 6.63 | 0.24 | 106 | 307 | 6.8 | 19 |
| Sample Weight (g) | P-Content (wt%) | F-Content (wt%) | Cone Calorimetry | |||||
|---|---|---|---|---|---|---|---|---|
| TTI (s) | pHRR (kW/m2) | THR * (kJ/g) | EHC * (kJ/g) | Residue * (wt%) | ||||
| Pristine flax fabric | 1.9 (1.8–1.9) | 0.00 | 0.00 | 28 (25–31) | 102 (98–105) | 15.7 (15.1–16.4) | 15.7 (15.1–16.4) | 0 |
| F-100 kGy-M4/MAPC1 (50:50) | 2.2 (2.2–2.2) | 0.77 | 0.49 | 14 (13–15) | 98 (98–98) | 10.7 (10.4–10.9) | 12.5 (11.9–13.1) | 15 (13–17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taibi, J.; Rouif, S.; Améduri, B.; Sonnier, R.; Otazaghine, B. One-Step Multifunctionalization of Flax Fabrics for Simultaneous Flame-Retardant and Hydro-Oleophobic Properties Using Radiation-Induced Graft Polymerization. Polymers 2023, 15, 2169. https://doi.org/10.3390/polym15092169
Taibi J, Rouif S, Améduri B, Sonnier R, Otazaghine B. One-Step Multifunctionalization of Flax Fabrics for Simultaneous Flame-Retardant and Hydro-Oleophobic Properties Using Radiation-Induced Graft Polymerization. Polymers. 2023; 15(9):2169. https://doi.org/10.3390/polym15092169
Chicago/Turabian StyleTaibi, Jamila, Sophie Rouif, Bruno Améduri, Rodolphe Sonnier, and Belkacem Otazaghine. 2023. "One-Step Multifunctionalization of Flax Fabrics for Simultaneous Flame-Retardant and Hydro-Oleophobic Properties Using Radiation-Induced Graft Polymerization" Polymers 15, no. 9: 2169. https://doi.org/10.3390/polym15092169