Sargassum@magnetite Composite EDTA-Functionalized for the Potential Removal of Mercury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubilization of Sargassum spp. Polysaccharides
2.3. Synthesis of the Magnetic Sargassum Composite (Fe3O4@Sargassum) Functionalized with EDTA (Fe3O4@Sargassum@EDTA)
2.4. Determination of the Capacity of Adsorption of Hg2+ Using Fe3O4@Sargassum@EDTA
2.5. Effect of pH, Time, and Temperature on the Fe3O4@Sargassum@EDTA Adsorption of Hg2+
2.6. Reusability of Fe3O4@Sargassum@EDTA
2.7. Identification of Functional Groups, Thermal Property Analysis, Surface Roughness, and Magnetic Properties of the Composite
2.8. Statistical Analysis
3. Results and Discussion
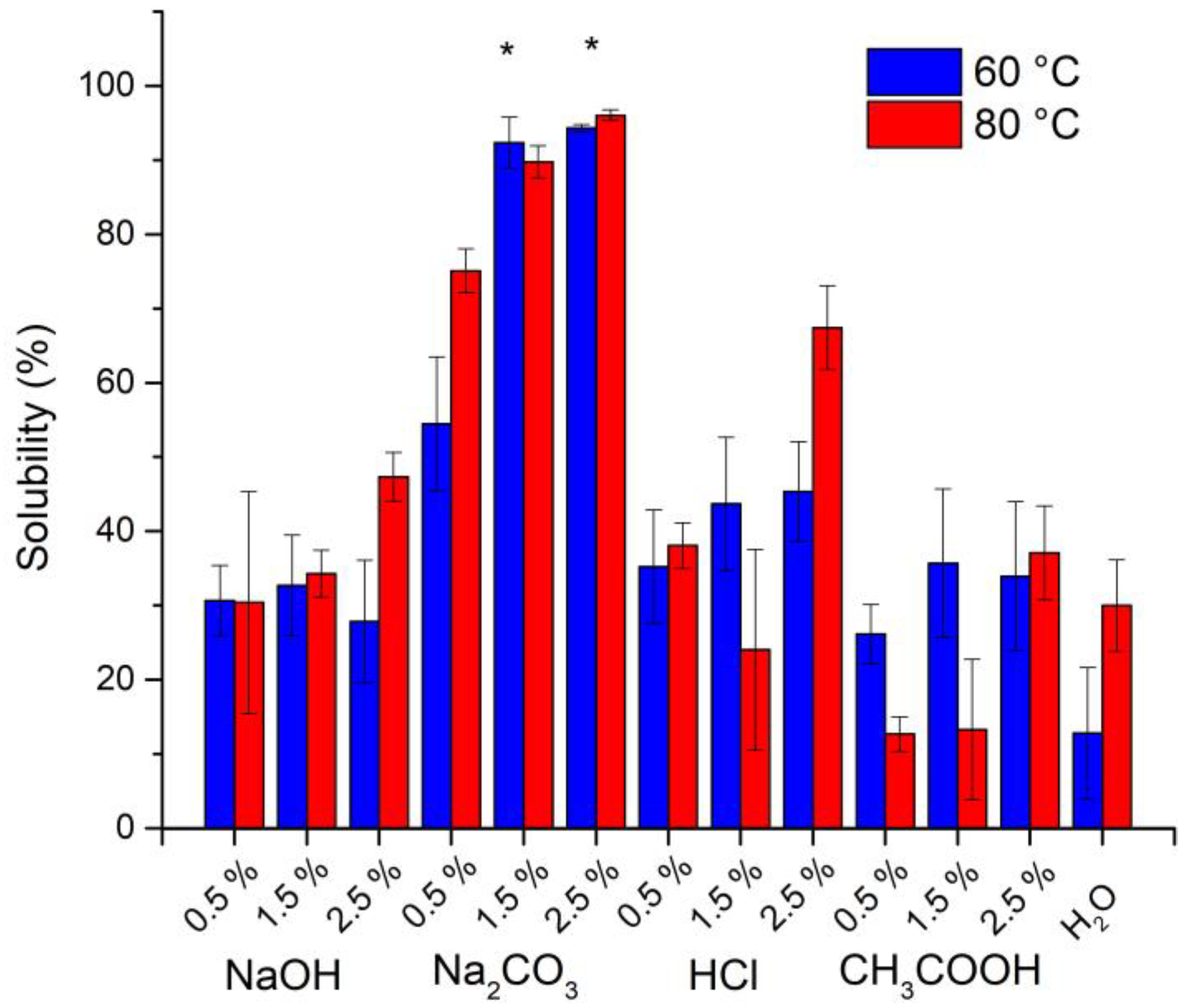
3.1. Solubilization of Sargassum spp. Polysaccharides
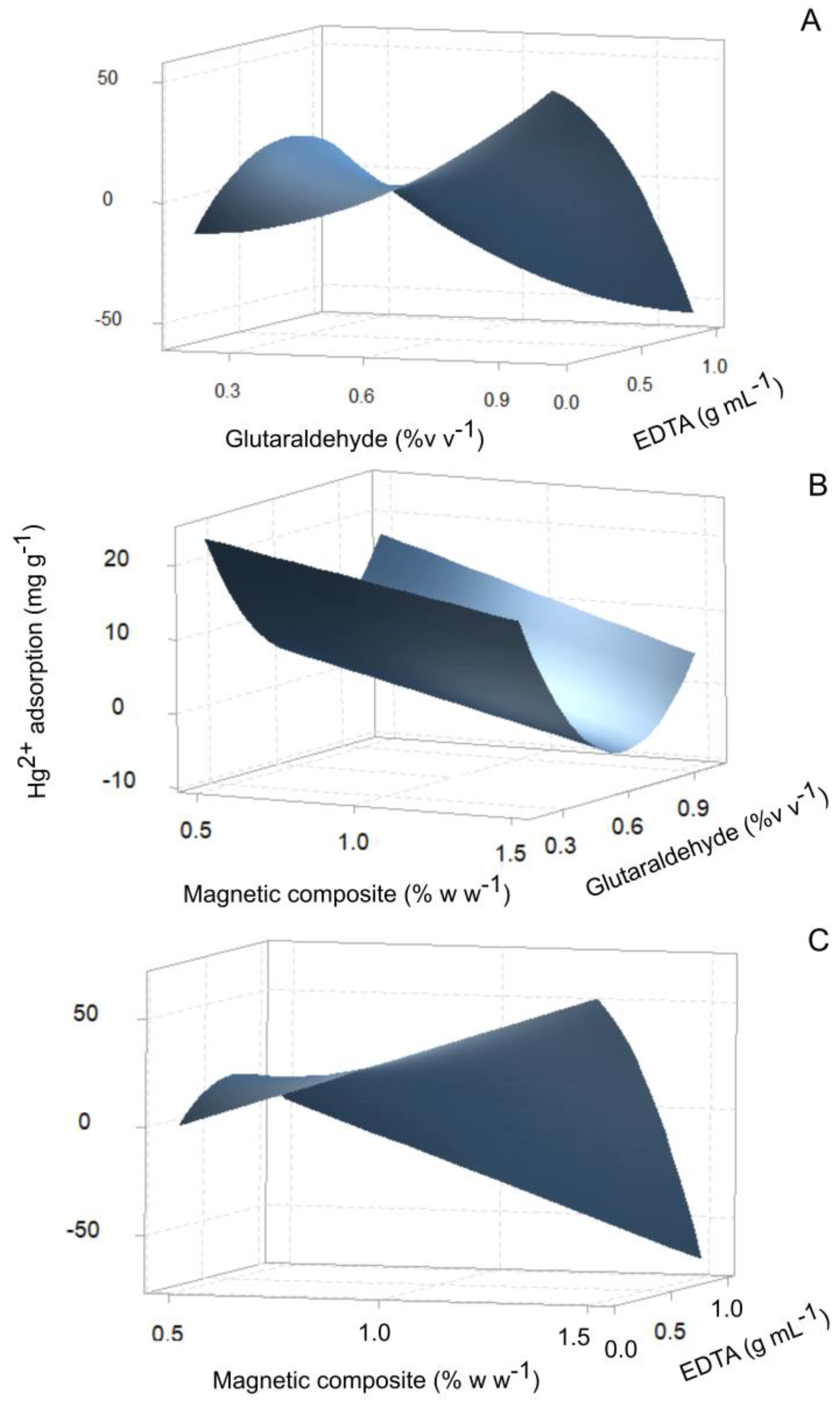
3.2. Synthesis of the Magnetic Sargassum Composite (Fe3O4@Sargassum) Functionalized with EDTA (Fe3O4@Sargassum@EDTA)
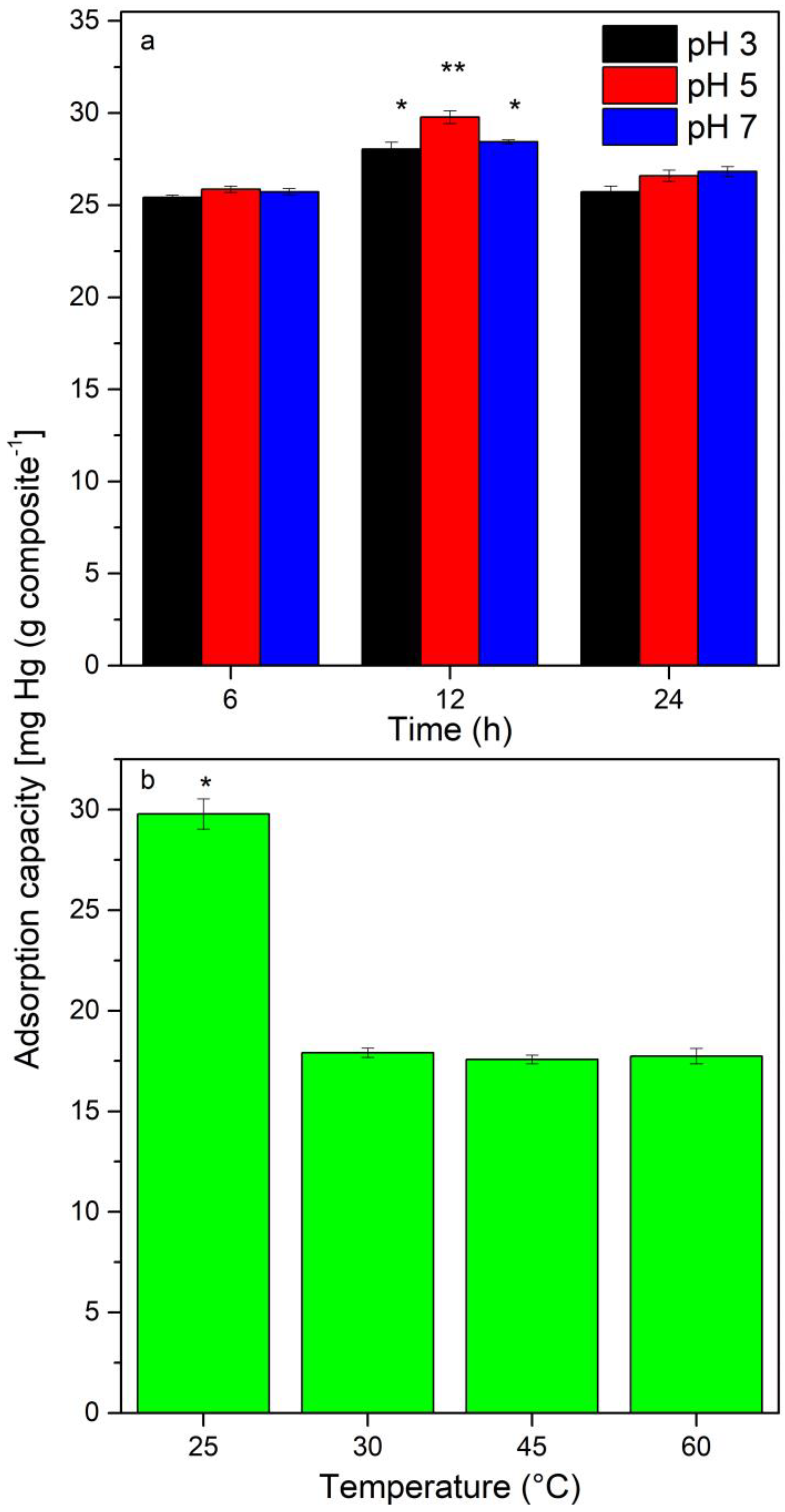
3.3. Effect of pH, Time, and Temperature on Fe3O4@Sargassum@EDTA Adsorption of Hg2+
3.4. Reusability of Fe3O4@Sargassum@EDTA
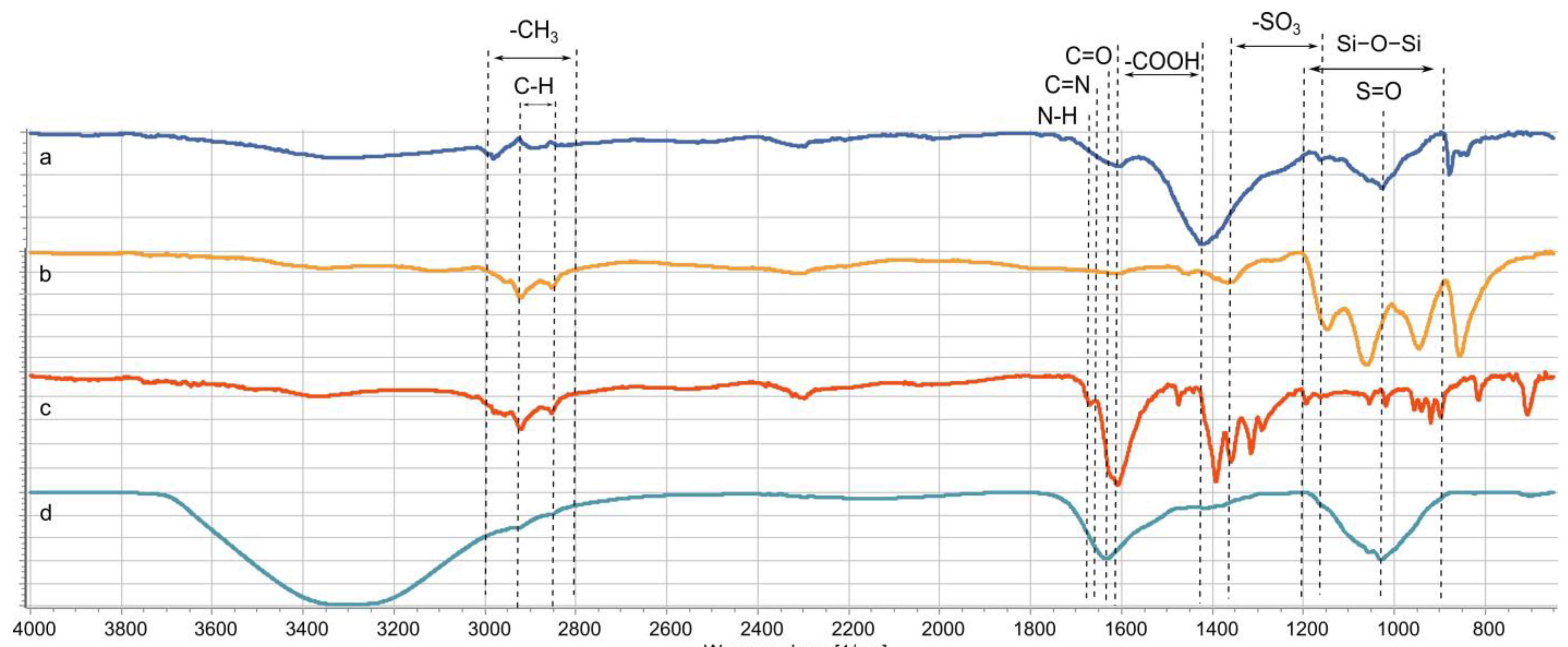
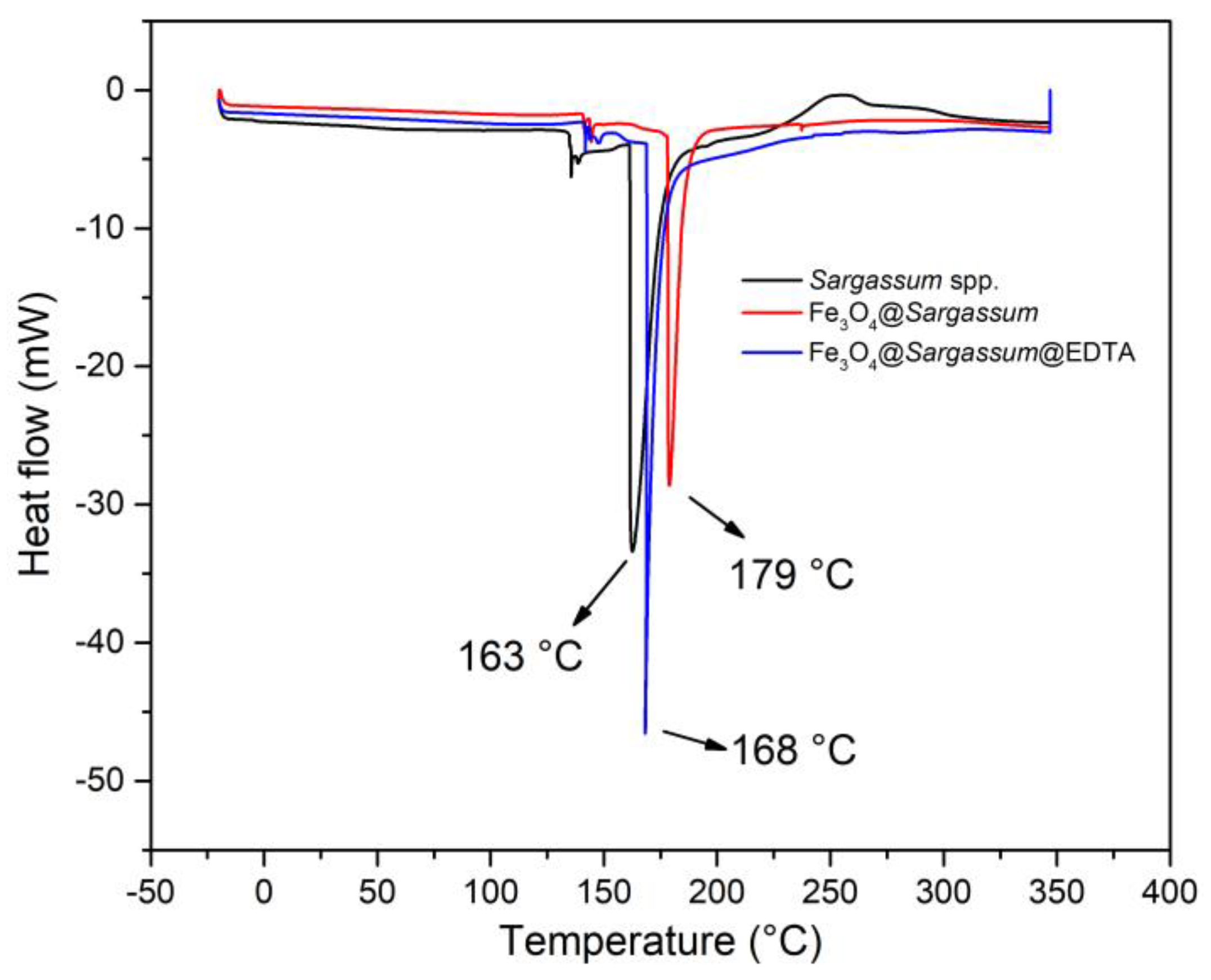
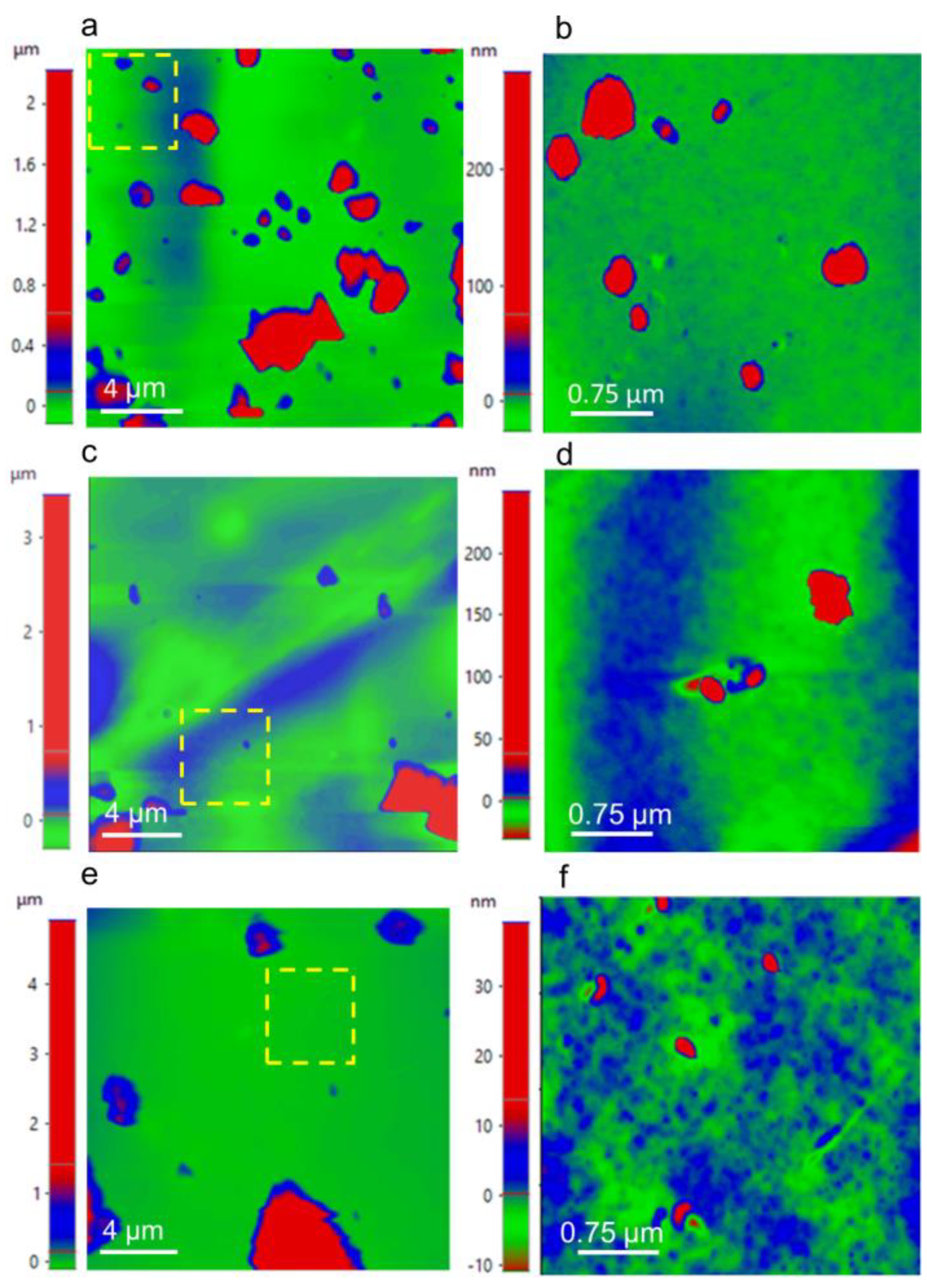
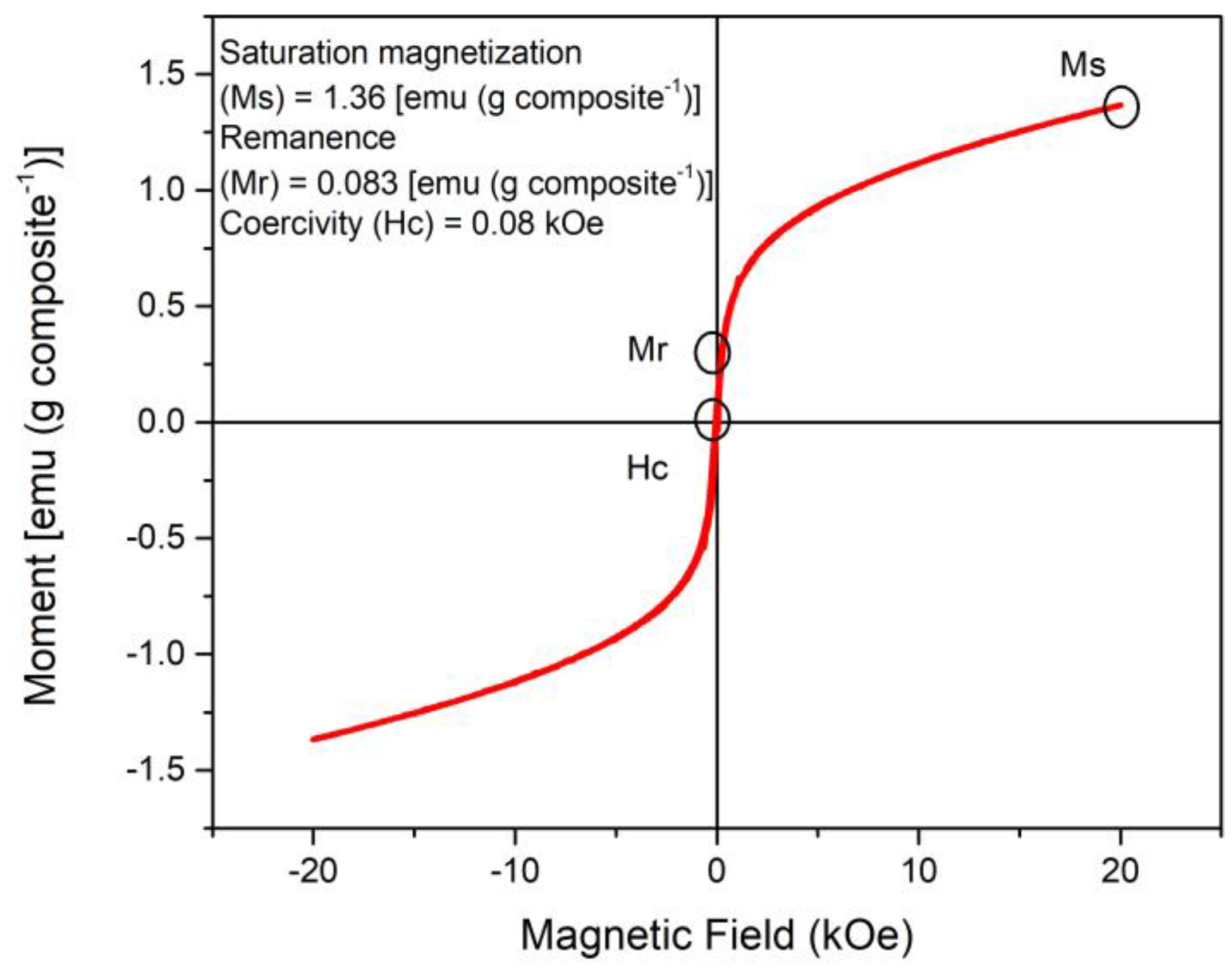
3.5. Identification of Functional Groups, Thermal Property Analysis, the Surface Roughness of the Composite, and its Magnetic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoramzadeh, E.; Nasernejad, B.; Halladj, R. Mercury biosorption from aqueous solutions by sugarcane bagasse. J. Taiwan Inst. Chem. Eng. 2013, 44, 266–269. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.H. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.G.; Silva, E.A. Biosorption of nickel(II) and copper(II) ions by Sargassum sp. in nature and alginate extraction products. Bioresour. Technol. Rep. 2019, 5, 43–50. [Google Scholar] [CrossRef]
- Husein, D.Z. Adsorption and removal of mercury ions from aqueous solution using raw and chemically modified Egyptian mandarin peel. Desalin. Water Treat. 2013, 51, 6761–6769. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, clinical and environmental toxicicology volume 3: Environmental toxicology. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- NMX-AA, Mexican Standar; Official Gazette of the Federation. Official Mexican Standar NOM-001-ECOL-1996; Que Which Establishes the Maximum Permissible Limits of Pollutants in Wastewater Discharges in National waters and Assets. 1997. Available online: http://dof.gob.mx/nota_detalle.php?codigo=4863829&fecha=6/01/1997 (accessed on 18 December 2021). (In Spanish)
- Nathan, R.J.; Jain, A.K.; Rosengren, R.J. Biosorption of heavy metals from water: Mechanism, critical evaluation and translatability of methodology. Environ. Technol. Rev. 2022, 11, 91–117. [Google Scholar] [CrossRef]
- Li, C.; Yu, Y.; Fang, A.; Feng, D.; Du, M.; Tang, A.; Chen, S.; Li, A. Insight into biosorption of heavy metals by extracellular polymer substances and the improvement of the efficacy: A review. Lett. Appl. Microbiol 2021, 75, 1064–1073. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sreekumari, S.S. Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J. Environ. Sci. 2011, 23, 1989–1998. [Google Scholar] [CrossRef]
- Zaidi, N.A.H.M.; Lim, B.; Usman, A.; Kooh, M.R.R. Efficient adsorption of malachite green dye using Artocarpus odoratissimus leaves with artificial neural network modelling. Desal. Water Treat. 2018, 101, 313–324. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Thotagamuge, R.; Chau, Y.F.C.; Mahadi, A.H.; Lim, C.M. Machine learning approaches to predict adsorption capacity of Azolla pinnata in the removal of methylene blue. J. Taiwan Inst. Chem. Eng. 2022, 132, 104134. [Google Scholar] [CrossRef]
- Mohammed, C.; Mahabir, S.; Mohammed, K.; John, N.; Lee, K.-Y.; Ward, K. Calcium alginate thin films derived from Sargassum natans for the selective adsorption of Cd2+, Cu2+ and Pb2+ ions. Ind. Eng. Chem. Res. 2018, 58, 1417–1425. [Google Scholar] [CrossRef]
- Monroy-Velázquez, L.V.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Aguiar, T.; Solís-Weiss, V.; Briones-Fourzán, P. Motile macrofauna associated with pelagic Sargassum in a Mexican reef lagoon. J. Environ. Manage 2019, 252, 109650. [Google Scholar] [CrossRef]
- Coração, A.C.D.S.; dos Santos, F.S.; Duarte, J.A.D.; Lopes-Filho, E.A.P.; De-Paula, J.C.; Rocha, L.M.; Krepsky, N.; Fiaux, S.B.; Teixeira, V.L. What do we know about the utilization of the Sargassum species as biosorbents of trace metals in Brazil? J. Environ. Chem. Eng. 2020, 8, 103941. [Google Scholar] [CrossRef]
- Carpio, R.B.; Zhang, Y.; Kuo, C.T.; Chen, W.-T.; Schideman, L.C.; de Leon, L.R. Characterization and thermal decomposition of demineralized wastewater algae biomass. Algal. Res. 2019, 38, 101399. [Google Scholar] [CrossRef]
- Znad, H.; Awual, M.dR.; Martini, S. The utilization of algae and seaweed biomass for bioremediation of heavy metal-contaminated wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Wan Aida, W.M. Extraction of sulfated polysaccharides (fucoidan) from brown seaweed In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications, 1st ed.; Venkatesan, J., Anil, S., Kim, S.-K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–46. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs. 2020, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, H.; Chen, S.; Long, F.; Huang, B.; Yang, B.; Pan, X. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem. Eng. J. 2019, 356, 69–80. [Google Scholar] [CrossRef]
- Camacho, O.; Hernández-Carmona, G. Phenology and alginates of two Sargassum species from the Caribbean coast of Colombia. Cienc. Mar. 2012, 38, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Hernández, A.; Gracida, J.; García-Almendárez, B.E.; Regalado, C.; Núñez, R.; Amaro-Reyes, A. Characterization of magnetic nanoparticles coated with chitosan: A potential approach for enzyme immobilization. J. Nanomater. 2018, 2018, 9468574. [Google Scholar] [CrossRef]
- Khan, H.; Ahmed, M.J.; Bhanger, M.I. A simple spectrophotometric determination of trace level mercury using 1,5-diphenylthiocarbazone solubilized in micelle. Anal. Sci. 2005, 21, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Gai, K.; Avellan, A.; Hoelen, T.P.; Lopez-Linares, F.; Hatakeyama, E.S.; Lowry, G.V. Impact of mercury speciation on its removal from water by activated carbon and organoclay. Water Res. 2019, 157, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Llanes, F.; Volesky, B.; Mucci, A. Metal selectivity of Sargassum spp. and their alginates in relation to their alpha-L-guluronic acid content and conformation. Environ. Sci. Technol. 2003, 37, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Product and Process Optimization Using Designed Experiments, 2nd ed.; Wiley: New York, NY, USA, 2002; pp. 1–12. [Google Scholar]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Zhao, J.; Luan, L.; Li, Z.; Duan, Z.; Li, Y.; Zheng, S.; Xue, Z.; Xu, W.; Niu, Y. The adsorption property and mechanism for Hg(II) and Ag(I) by Schiff base functionalized magnetic Fe3O4 from aqueous solution. J. Alloys Compd. 2020, 825, 154051. [Google Scholar] [CrossRef]
- Carro, L.; Barriada, J.L.; Herrero, R.; de Vicente, M.E.S. Surface modifications of Sargassum muticum algal biomass for mercury removal: A physicochemical study in batch and continuous flow conditions. Chem. Eng. J. 2013, 229, 378–387. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Delacroix, A. Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J. Hazard Mater. 2003, 105, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Angelova, R.; Baldikova, E.; Pospiskova, K.; Maderova, Z.; Safarikova, M.; Safarik, I. Magnetically modified Sargassum horneri biomass as an adsorbent for organic dye removal. J. Clean. Prod. 2016, 137, 189–194. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Sikandar, M. Biosorption of Hg (II) from aqueous solution using algal biomass: Kinetics and isotherm studies. Heliyon 2020, 6, e03321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabaraki, R.; Nateghi, A.; Ahmady-Asbchin, S. Biosorption of lead (II) ions on Sargassum ilicifolium: Application of response surface methodology. Int. Biodeterior. Biodegr. 2014, 93, 145–152. [Google Scholar] [CrossRef]
- Awual, M.R. Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem. Eng. J. 2017, 307, 456–465. [Google Scholar] [CrossRef]
- Suess, E.; Berg, M.; Bouchet, S.; Cayo, L.; Hug, S.J.; Kaegi, R.; Voegelin, A.; Winkel, L.H.E.; Tessier, E.; Amouroux, D.; et al. Mercury loads and fluxes from wastewater: A nationwide survey in Switzerland. Water Res. 2020, 175, 115708. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jing, W.; Kang, P.; Xiaoming, Z.; Zhouping, W.; Wenshui, X. Preparation and characterization of Core/Shell-type Ag/chitosan nanoparticles with antibacterial activity. Bull. Korean Chem. Soc. 2011, 32, 1277–1281. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghamdi, Y.O.; Alamry, K.A.; Hussein, M.A.; Marwani, H.M.; Asiri, A.M. Sulfone-modified chitosan as selective adsorbent for the extraction of toxic Hg(II) metal ions. Adsorp. Sci. Technol. 2018, 37, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Hanjabam, M.D.; Kumar, A.; Tejpal, C.S.; Krishnamoorthy, E.; Kishore, P.; Kumar, K.A. Isolation of crude fucoidan from Sargassum wightii using conventional and ultra-sonication extraction methods. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100200. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Montero, M.P.; Gómez-Guillén, M.C. Antioxidant film development from unrefined extracts of brown seaweeds Laminaria Digit. Ascophyllum Nodosum. Food Hydrocoll 2014, 37, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Sandoval-Cárdenas, D.I.; Feregrino-Pérez, A.A.; Favela-Camacho, S.E.; Arredondo-Ochoa, T.; Escamilla-García, M.; Regalado, C.; Amaro-Reyes, A. Potential antioxidant activity of multienzymatically hydrolyzed corncob. Biologia 2022, 77, 803–813. [Google Scholar] [CrossRef]

| Source | DF | Adjusted Sum of Squares | Adjusted Mean Squares | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 9 | 157.166 | 17.4629 | 16.88 | 0.001 |
| Lineal | 3 | 23.246 | 7.7486 | 7.49 | 0.019 |
| Magnetic composite (%) w/v−1 | 1 | 4.629 | 4.6295 | 4.47 | 0.079 |
| Glutaraldehyde (%) w/v−1 | 1 | 19.351 | 19.351 | 18.71 | 0.005 |
| EDTA (g mL−1) | 1 | 19.388 | 19.3879 | 18.74 | 0.005 |
| Square | 3 | 114.931 | 38.3105 | 37.03 | 0 |
| Magnetic composite (%) w/v−1 | 1 | 82.301 | 82.3007 | 79.55 | 0 |
| Glutaraldehyde (%) w/v−1 | 1 | 17.554 | 17.5535 | 16.97 | 0.006 |
| EDTA (g mL−1) | 1 | 17.734 | 17.7336 | 17.14 | 0.006 |
| Interaction | 3 | 21.641 | 7.2137 | 6.97 | 0.022 |
| Glutaraldehyde (%) w/v−1 | 1 | 18.532 | 18.5324 | 17.91 | 0.005 |
| EDTA (g mL−1) | 1 | 18.841 | 18.8414 | 18.21 | 0.005 |
| EDTA (g mL−1) | 1 | 18.826 | 18.8256 | 18.2 | 0.005 |
| Error | 6 | 6.207 | 1.0345 | ||
| Lack of fit | 1 | 0.173 | 0.1727 | 0.14 | 0.721 |
| Pure error | 5 | 6.035 | 1.2069 | ||
| Total | 15 | 163.373 |
| Composite | Solids Yield (%) | Attracted Mass by a Magnet (%) |
|---|---|---|
| Fe3O4@Sargassum | 86.0 ± 7.20 a | 92.1 ± 3.40 a |
| Fe3O4@Sargassum@EDTA | 60.1 ± 17.2 a | 76.0 ± 6.62 b |
| Adsorbents | Initial Hg2+ Concentration (mg L−1) | Final Hg 2+ Concentration (mg L−1) | Amount of Adsorbent Used (g L−1) | Contact Time for Maximum Adsorption (min) | qe = Amount of Hg2+ Adsorbed per Adsorbent (mg/g) | Number of Reuse Cycles | Desorbing Agent | Optimal Temperature (°C) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Sargassum@magnetite composite EDTA-functionalized | 100 | 0 (cycle 1), 23 (cycle 2), 28 (cycle 3), 25 (cycle 4) | 5.0 | 720 | 29.8 | 4 | 0.1 M NaOH, 0.1 M HCl, 2 M EDTA | 25 | Former study |
| Sugarcane bagasse native, NaOH-treated, and HCl-treated | 76 | 5.0, 8.0, and 13 | 5.0 | 5, 45, and 15 | 35.7 | Single use | Not used | 50 | [1] |
| Calcium-alginate from Sargassum sp. | Ni2+ 92–99, Cu2+ 99–108 | 41, 82 | 1.0 | 180, 360 | Not reported | Single use | Not used | 50 | [3] |
| Egyptian mandarin peel dried, NaOH treated, andcarbonized | 100 | Not reported | 5.0 | 60 | 10, 13, and 17 | Single use | Not used | 20 | [4] |
| Activated carbon, sulfur-impregnated activated carbon, and andorganoclay | 0.1 | 0.055, 0.072, and 0.076 | 0.13 | 240 | 0, 0, and 0.4 | Single use | Not used | Not reported | [24] |
| Schiff-base functionalized magnetic Fe3O4 prepared by the one pot subsequent homogeneus method andtraditional heterogeneus method | 401 | Not reported | 1.0 | 180 | 0.57, 0.52 | 5.0 | 5% thiourea-0.5 mol L−1 nitric acid | 35 | [28] |
| Sargassum muticum native and treated for modification of carboxyl groups | 501 | 401, 326 | 2.5 | 2880 | 0.9, 0.85 | Single use | Not used | 25 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-Cárdenas, D.I.; Pool, H.; Favela-Camacho, S.E.; Santos-Cruz, J.; Campos-Guillén, J.; Ramos-López, M.A.; Rodríguez-deLeón, E.; Urbina-Arroyo, J.V.; Amaro-Reyes, A. Sargassum@magnetite Composite EDTA-Functionalized for the Potential Removal of Mercury. Polymers 2023, 15, 1405. https://doi.org/10.3390/polym15061405
Sandoval-Cárdenas DI, Pool H, Favela-Camacho SE, Santos-Cruz J, Campos-Guillén J, Ramos-López MA, Rodríguez-deLeón E, Urbina-Arroyo JV, Amaro-Reyes A. Sargassum@magnetite Composite EDTA-Functionalized for the Potential Removal of Mercury. Polymers. 2023; 15(6):1405. https://doi.org/10.3390/polym15061405
Chicago/Turabian StyleSandoval-Cárdenas, Diana Issell, Hector Pool, Sarai E. Favela-Camacho, José Santos-Cruz, Juan Campos-Guillén, Miguel Angel Ramos-López, Eloy Rodríguez-deLeón, Jessica Viridiana Urbina-Arroyo, and Aldo Amaro-Reyes. 2023. "Sargassum@magnetite Composite EDTA-Functionalized for the Potential Removal of Mercury" Polymers 15, no. 6: 1405. https://doi.org/10.3390/polym15061405






