Study on Press Formability and Properties of UV-Curable Polyurethane Acrylate Coatings with Different Reactive Diluents
Abstract
:1. Introduction
2. Experimental
2.1. Materials
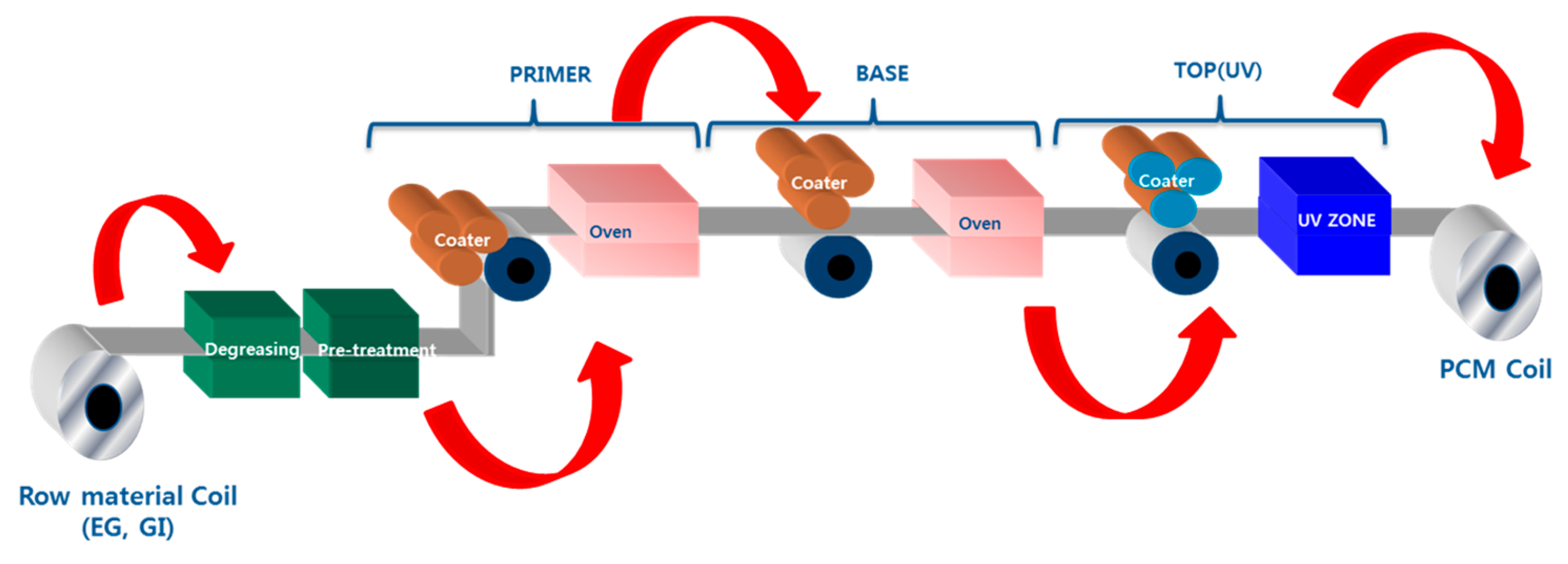
2.2. Method
2.3. Characterizations
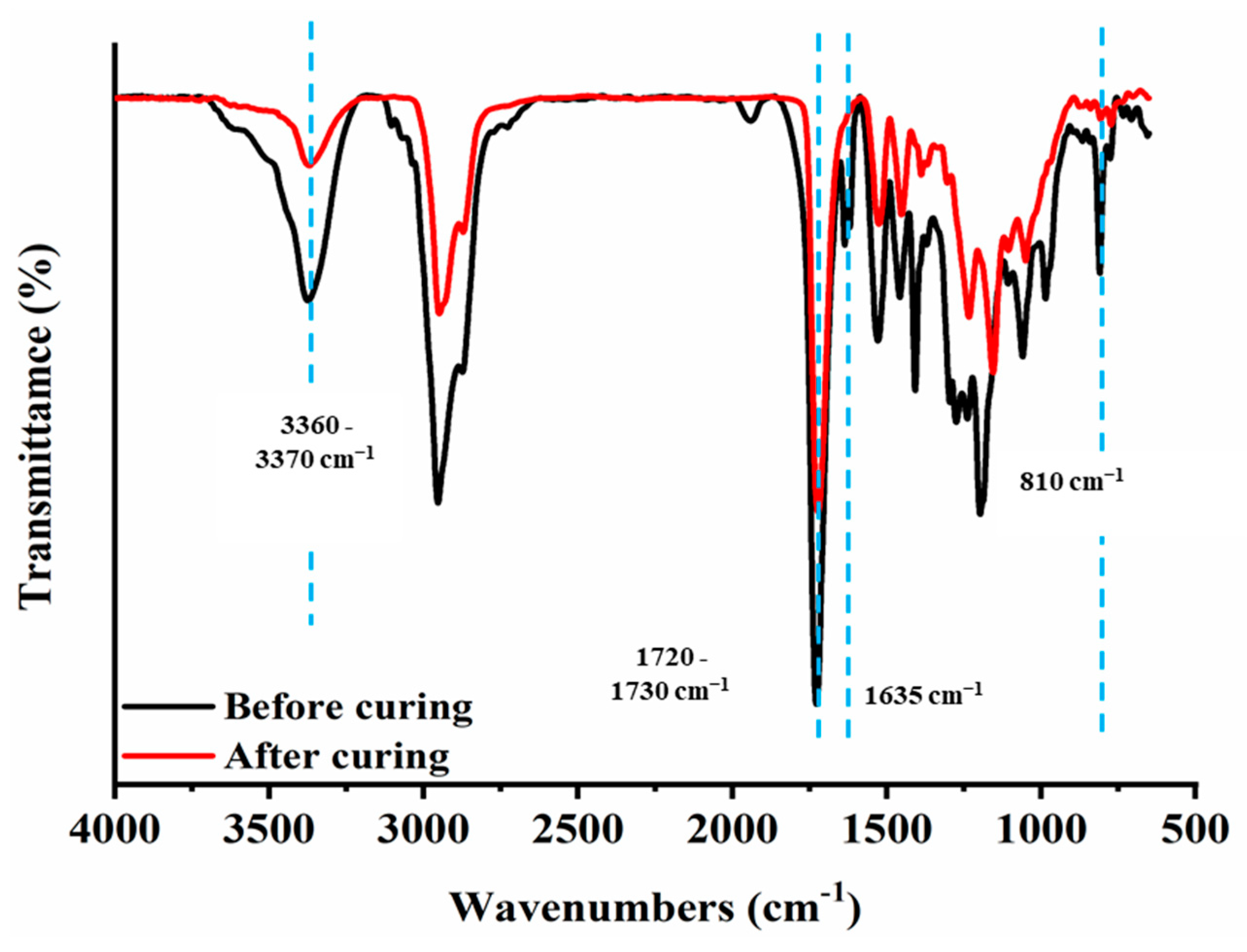
2.3.1. FT-IR
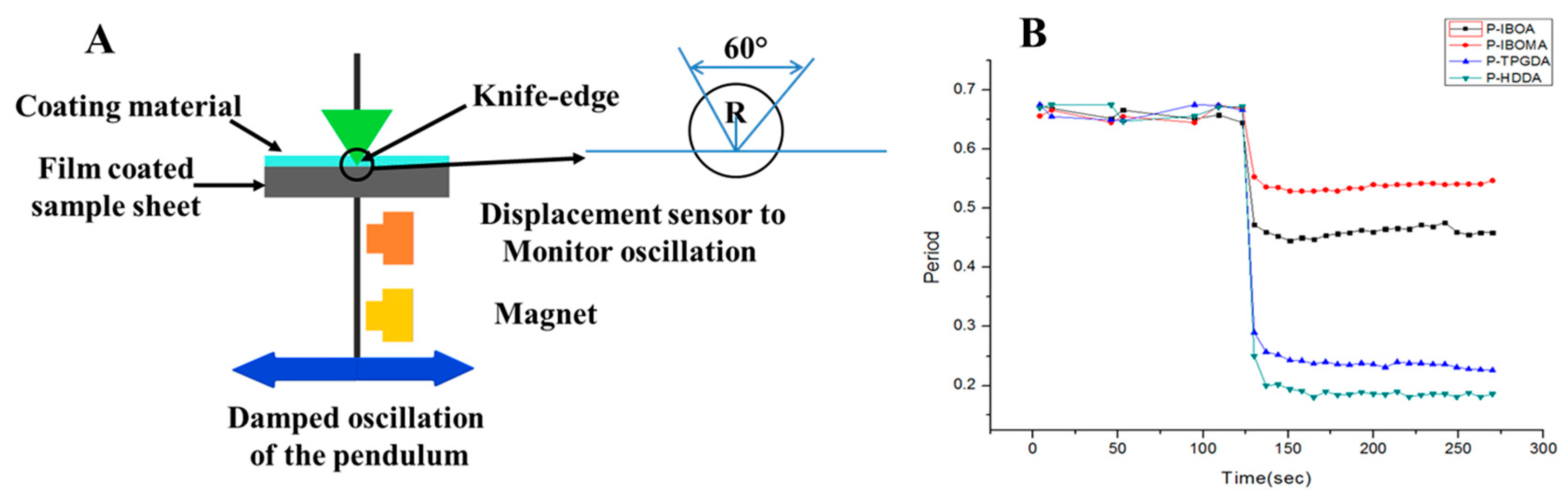
2.3.2. Rigid-Body Pendulum Testing (RPT)
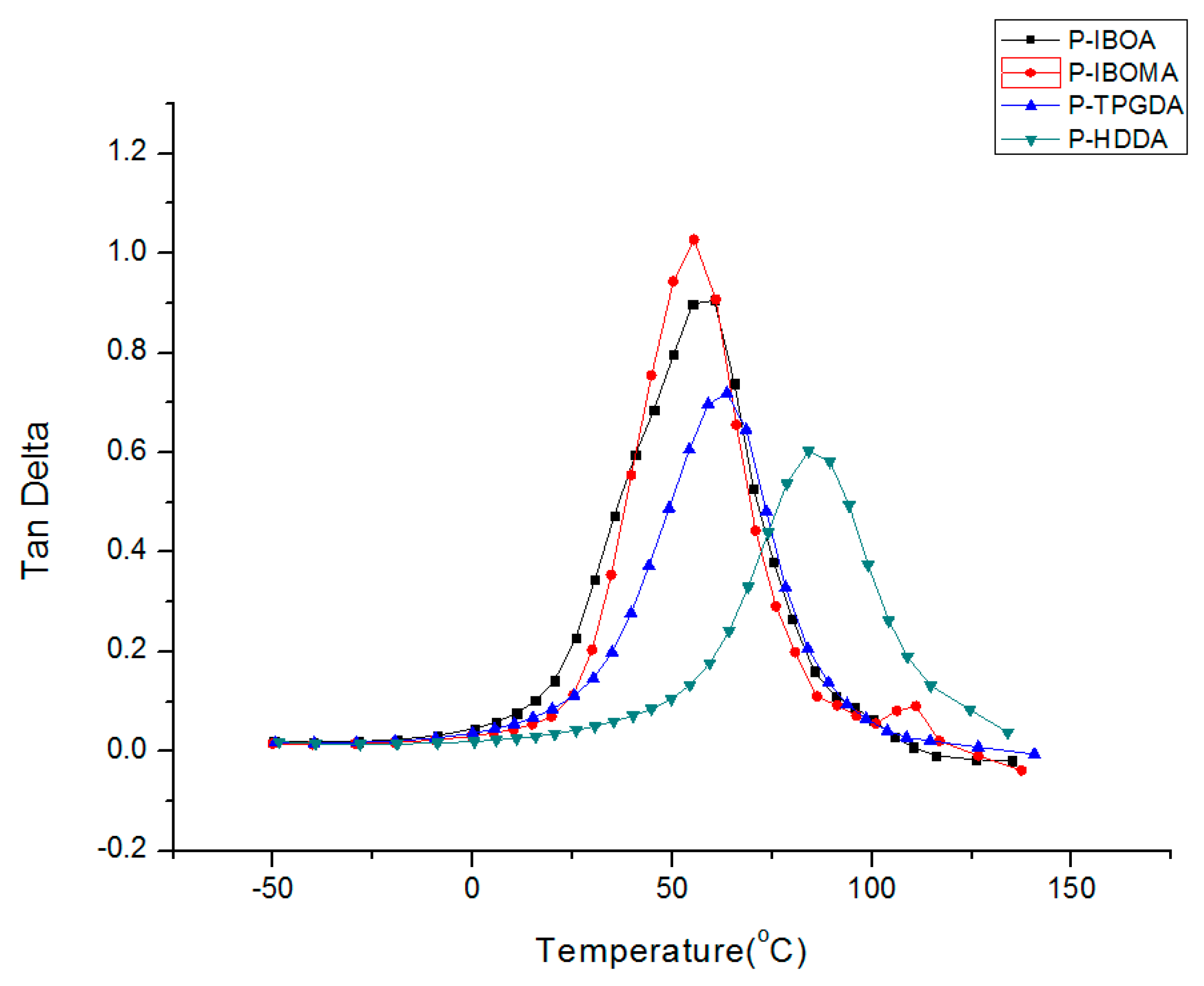
2.3.3. Dynamic Mechanical Analysis (DMA)
2.3.4. Cross Cutter Erichsen Test (CCET)
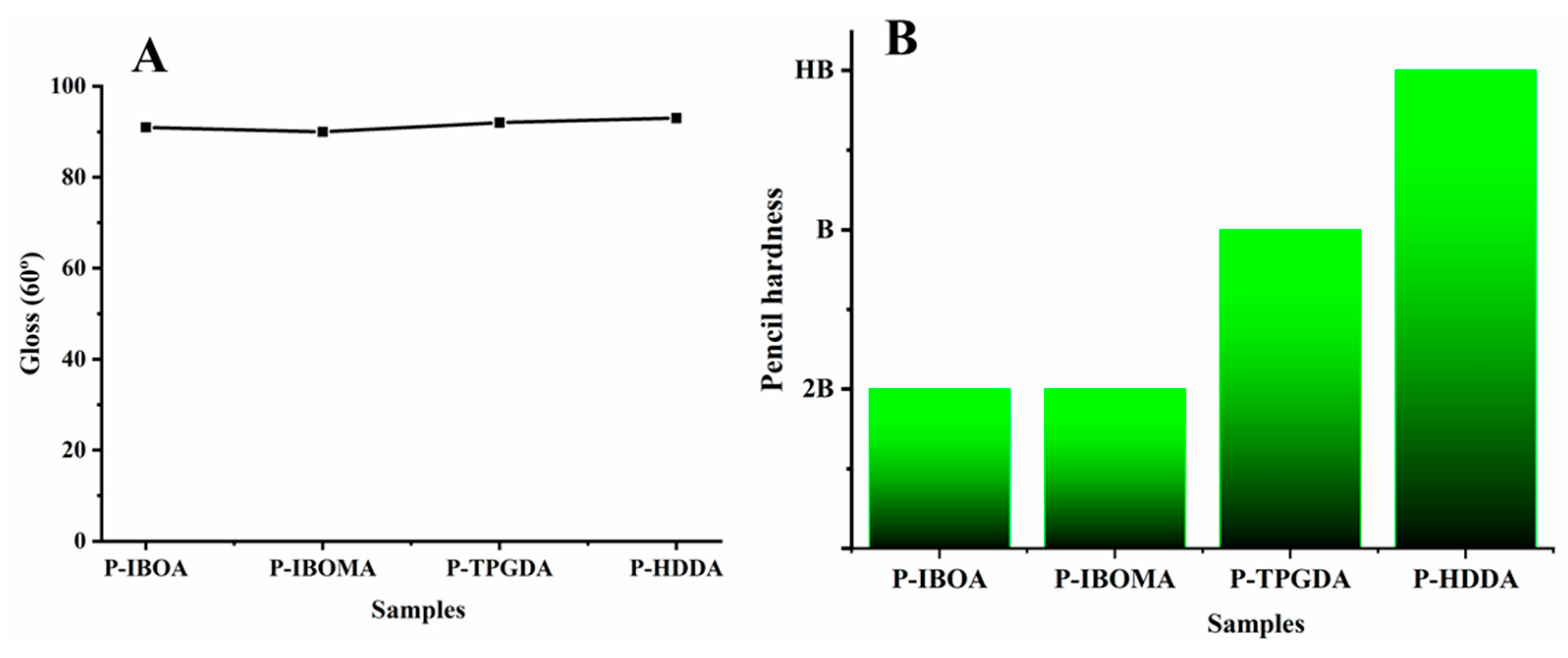
2.3.5. Gloss
2.3.6. Pencil Hardness
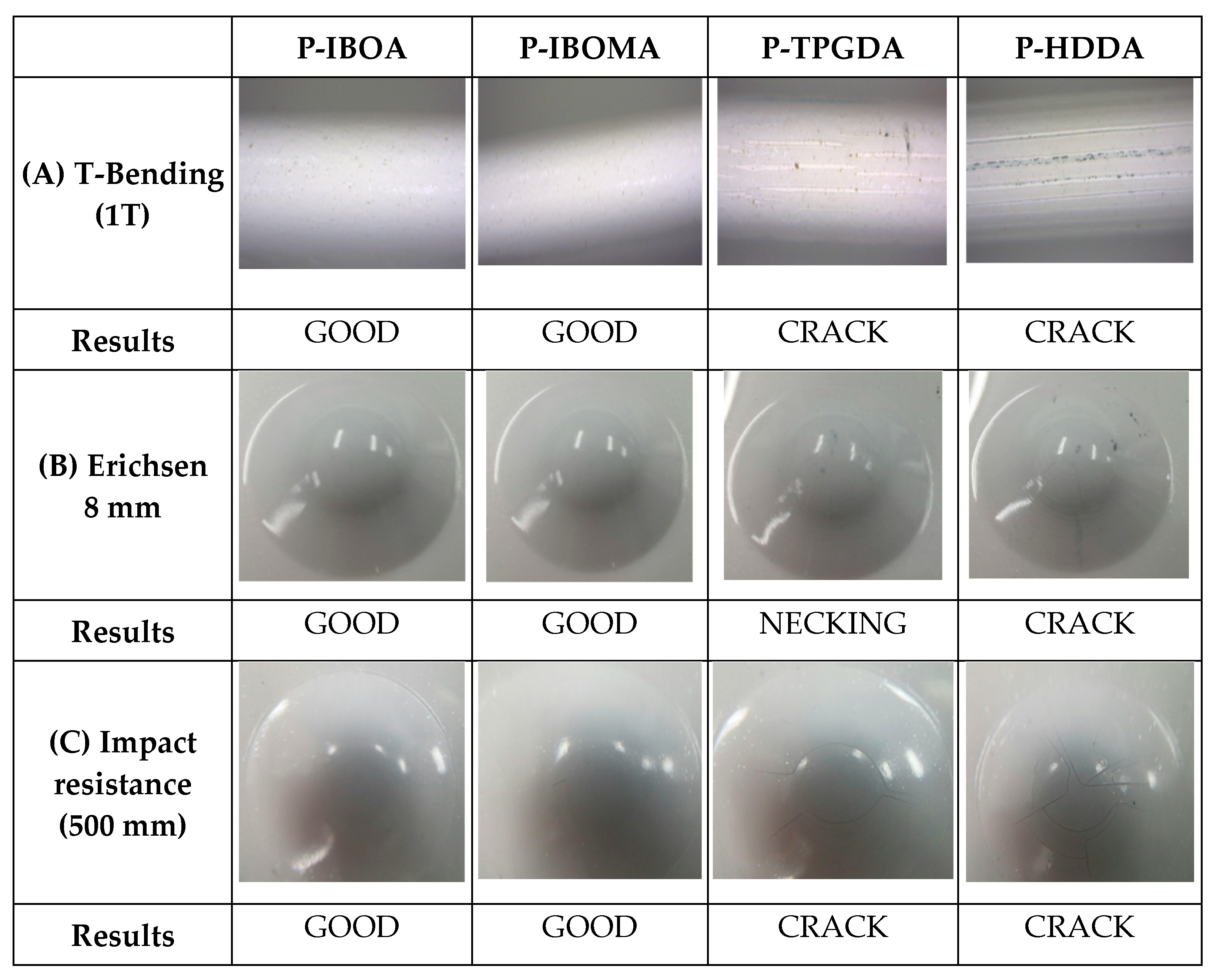
2.3.7. T-Bending Test
2.3.8. Erichsen Test
2.3.9. Impact Resistance
2.3.10. Chemical Resistance
2.3.11. UV Light Resistance
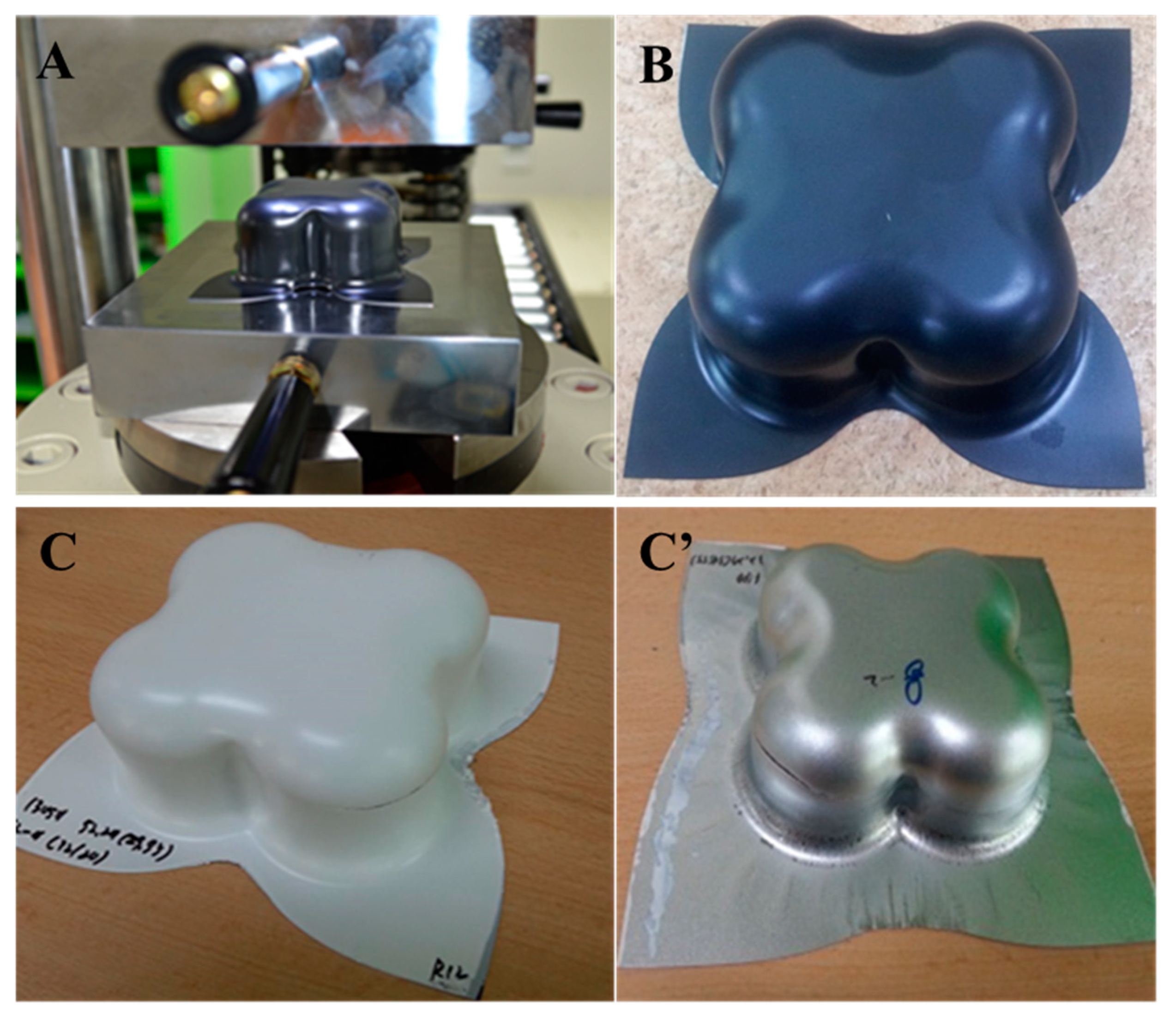
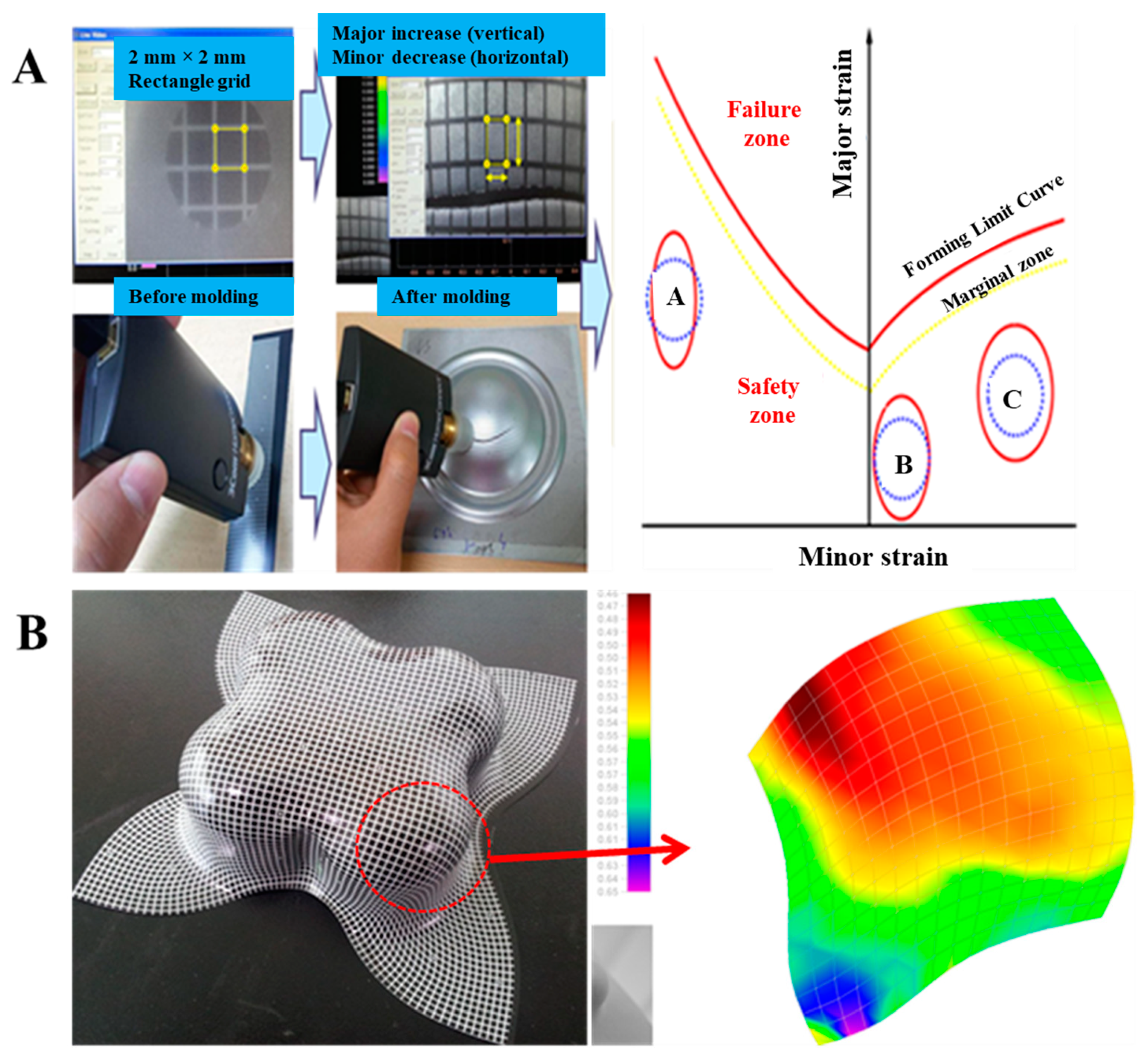
2.3.12. Formability
2.3.13. Gel Content
3. Results and Discussion
Film Properties by Curing Atmosphere
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cheon, J.; Park, S.-Y.; Jeong, B.Y.; Chun, J.H. Preparation and properties of UV-curable polyurethane-acrylate coatings of pre-coated metal (PCM): Effect of polyol type/contents on adhesive property. Mol. Cryst. Liq. Cryst. 2020, 706, 62–71. [Google Scholar] [CrossRef]
- Choi, W.-C.; Lee, W.-K.; Ha, C.-S. Synthesis and properties of UV-curable polyurethane acrylates based on different polyols for coating of metal sheets. Mol. Cryst. Liq. Cryst. 2018, 660, 104–109. [Google Scholar] [CrossRef]
- Meuthen, B.; Jandel, A.-S. Coil Coating; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Lee, S.; Gavande, V.; Chun, J.H.; Cheon, J.M.; Jin, Y.; Lee, W.-K. Synthesis and properties of UV-curable polyurethane acrylates with reactive silicones. Mol. Cryst. Liq. Cryst. 2020, 706, 86–93. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Liu, R.; Long, L.; Xu, J.; Chen, M.; Qiu, H. Preparation of a Fast Water-Based UV Cured Polyurethane-Acrylate Wood Coating and the Effect of Coating Amount on the Surface Properties of Oak (Quercus alba L.). Polymers 2019, 11, 1414. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Gavande, V.; Lee, W.-K. Synthesis and characteristics of cardanol-based acrylates as reactive diluents in UV-curing coatings. Mol. Cryst. Liq. Cryst. 2023, 1–8. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Saif, M.J. Recent trends in environmentally friendly water-borne polyurethane coatings: A review. Korean J. Chem. Eng. 2016, 33, 388–400. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-step synthesis of novel renewable vegetable oil-based acrylate prepolymers and their application in UV-curable coatings. Polymers 2020, 12, 1165. [Google Scholar] [CrossRef]
- Hu, Y.; Shang, Q.; Bo, C.; Jia, P.; Feng, G.; Zhang, F.; Liu, C.; Zhou, Y. Synthesis and Properties of UV-Curable Polyfunctional Polyurethane Acrylate Resins from Cardanol. ACS Omega 2019, 4, 12505–12511. [Google Scholar] [CrossRef]
- Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Glöckner, P.; Struck, S.; Jung, T.; Studer, K. Radiation Curing: Coatings and Printing Inks; Vincentz Network: Hannover, Germany, 2009. [Google Scholar]
- Choi, W.-C.; Lee, W.-K.; Ha, C.-S. Low-viscosity UV-curable polyurethane acrylates containing dendritic acrylates for coating metal sheets. J. Coat. Technol. Res. 2019, 16, 377–385. [Google Scholar] [CrossRef]
- Seo, J.; Jang, E.-S.; Song, J.-H.; Choi, S.; Khan, S.B.; Han, H. Preparation and properties of poly(urethane acrylate) films for ultraviolet-curable coatings. J. Appl. Polym. Sci. 2010, 118, 2454–2460. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Wróblewska, A.; Markowska-Szczupak, A.; Ossowicz-Rupniewska, P.; Nowak, M.; Kujbida, M.; Kamińska, A.; Czech, Z. UV Curable Coatings Based on Urethane Acrylates Containing Eugenol and Evaluation of Their Antimicrobial Activity. Coatings 2021, 11, 1556. [Google Scholar] [CrossRef]
- Gavande, V.; Im, D.; Lee, W.-K. Development of highly transparent UV-curable nylon 6 nanofiber-reinforced polyurethane acrylate nanocomposite coatings for pre-coated metals. J. Appl. Polym. Sci. 2021, 138, 50614. [Google Scholar] [CrossRef]
- Van den Bosch, M.J.; Schreurs, P.J.G.; Geers, M.G.D. On the prediction of delamination during deep-drawing of polymer coated metal sheet. J. Mater. Process. Technol. 2009, 209, 297–302. [Google Scholar] [CrossRef]
- Kim, H.Y.; Hwang, B.C.; Bae, W.B. An experimental study on forming characteristics of pre-coated sheet metals. J. Mater. Process. Technol. 2002, 120, 290–295. [Google Scholar] [CrossRef]
- Vayeda, R.; Wang, J. Adhesion of coatings to sheet metal under plastic deformation. Int. J. Adhes. Adhes. 2007, 27, 480–492. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, S.J.; Park, J.W.; Kim, H.J. Synthesis and properties of flexible polyester with urethane polyol for automotive pre-coated metals. J. Adhes. Sci. Technol. 2016, 30, 1537–1554. [Google Scholar] [CrossRef]
- Phalak, G.; Patil, D.; Vignesh, V.; Mhaske, S. Development of tri-functional biobased reactive diluent from ricinoleic acid for UV curable coating application. Ind. Crops Prod. 2018, 119, 9–21. [Google Scholar] [CrossRef]
- Wang, X.; Soucek, M.D. Investigation of non-isocyanate urethane dimethacrylate reactive diluents for UV-curable polyurethane coatings. Prog. Org. Coat. 2013, 76, 1057–1067. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhaske, S.T. Design and synthesis of bio-based UV curable PU acrylate resin from itaconic acid for coating applications. Des. Monomers Polym. 2017, 20, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Cheong, Z.; Sorce, F.S.; Ngo, S.; Lowe, C.; Taylor, A.C. The effect of substrate material properties on the failure behaviour of coatings in the Erichsen cupping test. Prog. Org. Coat. 2021, 151, 106087. [Google Scholar] [CrossRef]
- Sorce, F.S.; Ngo, S.; Lowe, C.; Taylor, A.C. Quantification and analysis of coating surface strains in T-bend tests. Int. J. Adv. Manuf. Technol. 2021, 113, 1125–1142. [Google Scholar] [CrossRef]
- Merklein, M.; Johannes, M.; Lechner, M.; Kuppert, A. A review on tailored blanks—Production, applications and evaluation. J. Mater. Process. Technol. 2014, 214, 151–164. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Su, W.; Zhu, L.; Cheng, X.; Wu, J.; Zhao, S.; Zhou, C. Construction of a durable superhydrophobic surface based on the oxygen inhibition layer of organosilicon resins. Thin Solid Film. 2021, 717, 138467. [Google Scholar] [CrossRef]
- Hermann, A.; Burr, D.; Landry, V. Comparative study of the impact of additives against oxygen inhibition on pendulum hardness and abrasion resistance for UV-curable wood finishes. Prog. Org. Coat. 2020, 148, 105879. [Google Scholar] [CrossRef]
- Lang, M.; Hirner, S.; Wiesbrock, F.; Fuchs, P. A review on modeling cure kinetics and mechanisms of photopolymerization. Polymers 2022, 14, 2074. [Google Scholar] [CrossRef]
- Ariffin, M.M.; Aung, M.M.; Abdullah, L.C.; Salleh, M.Z. Assessment of corrosion protection and performance of bio-based polyurethane acrylate incorporated with nano zinc oxide coating. Polym. Test. 2020, 87, 106526. [Google Scholar] [CrossRef]
- Fu, J.; Wang, L.; Yu, H.; Haroon, M.; Haq, F.; Shi, W.; Wu, B.; Wang, L. Research progress of UV-curable polyurethane acrylate-based hardening coatings. Prog. Org. Coat. 2019, 131, 82–99. [Google Scholar] [CrossRef]
- Studer, K.; Decker, C.; Beck, E.; Schwalm, R. Overcoming oxygen inhibition in UV-curing of acrylate coatings by carbon dioxide inerting, Part I. Prog. Org. Coat. 2003, 48, 92–100. [Google Scholar] [CrossRef]
- Lovestead, T.M.; O’Brien, A.K.; Bowman, C.N. Models of multivinyl free radical photopolymerization kinetics. J. Photochem. Photobiol. A Chem. 2003, 159, 135–143. [Google Scholar] [CrossRef]
- Pirman, T.; Ocepek, M.; Likozar, B. Radical Polymerization of Acrylates, Methacrylates, and Styrene: Biobased Approaches, Mechanism, Kinetics, Secondary Reactions, and Modeling. Ind. Eng. Chem. Res. 2021, 60, 9347–9367. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; Ramis, X.; Serra, À. State of the Art in Dual-Curing Acrylate Systems. Polymers 2018, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sabat, G.; Granda, L.A.; Medel, S. Synthesis of UV-curable polyurethane-acrylate hybrids with tuneable hardness and viscoelastic properties on-demand. Mater. Adv. 2022, 3, 5118–5130. [Google Scholar] [CrossRef]
- Geng, Z.; Shin, J.J.; Xi, Y.; Hawker, C.J. Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci. 2021, 59, 963–1042. [Google Scholar] [CrossRef]



| Oligomer | Viscosity (mPa·s) | Density (g/cm3) | Molecular Weight | Functionality | Solid |
|---|---|---|---|---|---|
| UA9359 a | 20,000 (25 °C) | 1.12 | 1000 | 2 | 100% |
| Ebecryl 1290 b | 2000 (60 °C) | 1.19 | 1000 | 6 | 100% |
| Components | P-IBOA | P-IBOMA | P-TPGDA | P-HDDA | |
|---|---|---|---|---|---|
| Oligomer | UA9359 a | 55 | 55 | 55 | 55 |
| Ebecryl 1290 b | 5 | 5 | 5 | 5 | |
| Diluent | IBOA c | 34 | - | - | - |
| IBOMA d | - | 34 | - | - | |
| TPGDA e | - | - | 34 | - | |
| HDDA f | - | - | - | 34 | |
| Photoinitiator | IC-184 g | 5 | 5 | 5 | 5 |
| Additive | E-3035 h | 1 | 1 | 1 | 1 |
| No | Sample | Coating System | LPH (mm) | Reduction Thickness (mm) | ||
|---|---|---|---|---|---|---|
| Reactive Diluent Functionality | Thickness | Condition | ||||
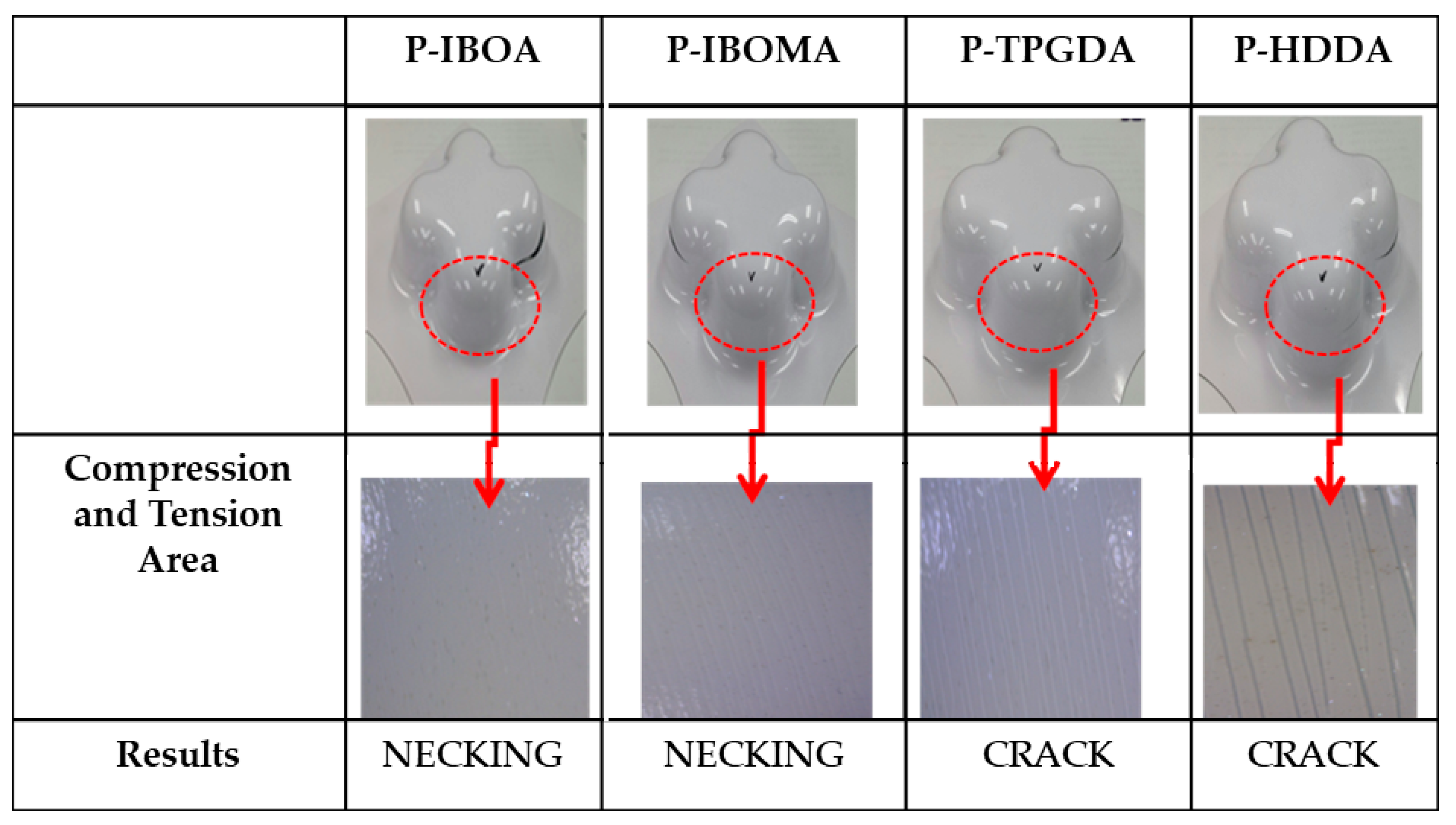
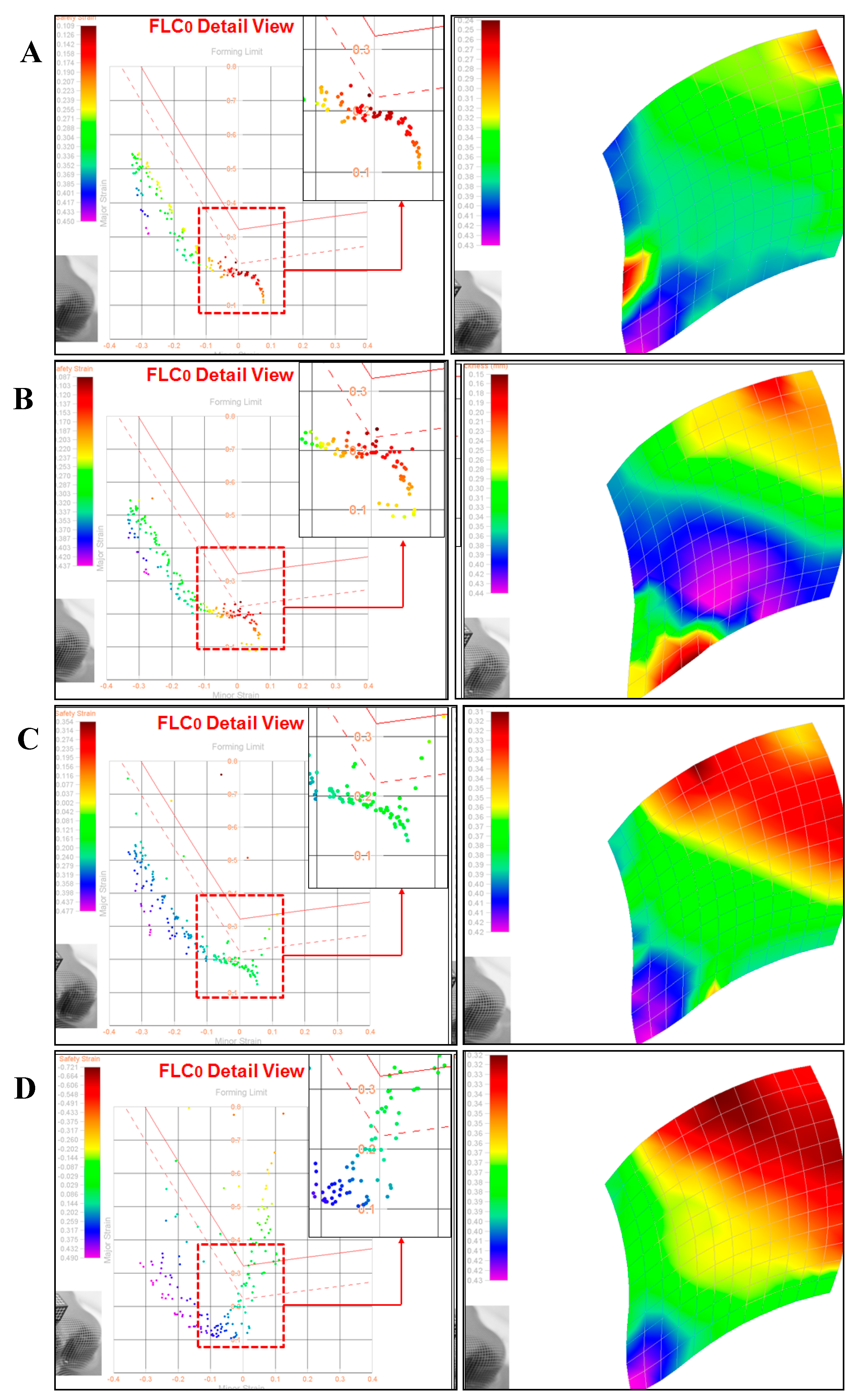
| 1 | P-IBOA | 1 | 30 μm | O2 | 44.8 | 0.53~0.67 |
| 2 | P-IBOMA | 1 | 30 μm | O2 | 45.2 | 0.51~0.64 |
| 3 | P-TPGDA | 2 | 30 μm | O2 | 41.1 | 0.51~0.68 |
| 4 | P-HDDA | 2 | 30 μm | O2 | 37.5 | 0.45~0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.-C.; Gavande, V.; Kim, D.-Y.; Lee, W.-K. Study on Press Formability and Properties of UV-Curable Polyurethane Acrylate Coatings with Different Reactive Diluents. Polymers 2023, 15, 880. https://doi.org/10.3390/polym15040880
Choi W-C, Gavande V, Kim D-Y, Lee W-K. Study on Press Formability and Properties of UV-Curable Polyurethane Acrylate Coatings with Different Reactive Diluents. Polymers. 2023; 15(4):880. https://doi.org/10.3390/polym15040880
Chicago/Turabian StyleChoi, Woo-Chan, Vishal Gavande, Dong-Yun Kim, and Won-Ki Lee. 2023. "Study on Press Formability and Properties of UV-Curable Polyurethane Acrylate Coatings with Different Reactive Diluents" Polymers 15, no. 4: 880. https://doi.org/10.3390/polym15040880






