Insight into the Role of Conductive Polypyrrole Coated on Rice Husk-Derived Nanosilica-Reduced Graphene Oxide as the Anodes: Electrochemical Improvement in Sustainable Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
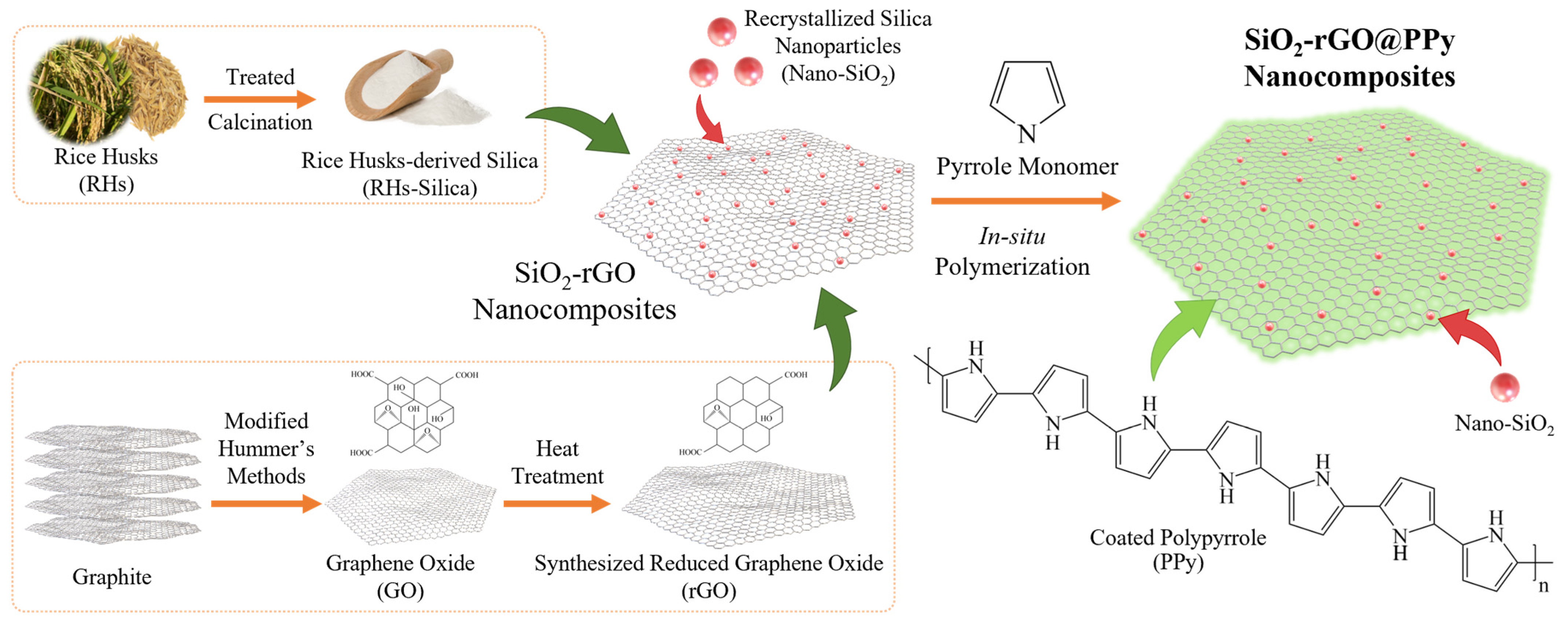
2.2. Synthesis of Rice Husk-Derived Nanosilica-Reduced Graphene Oxide@polypyrrole (SiO2-rGO@PPy)
2.3. Morphological and Structural Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
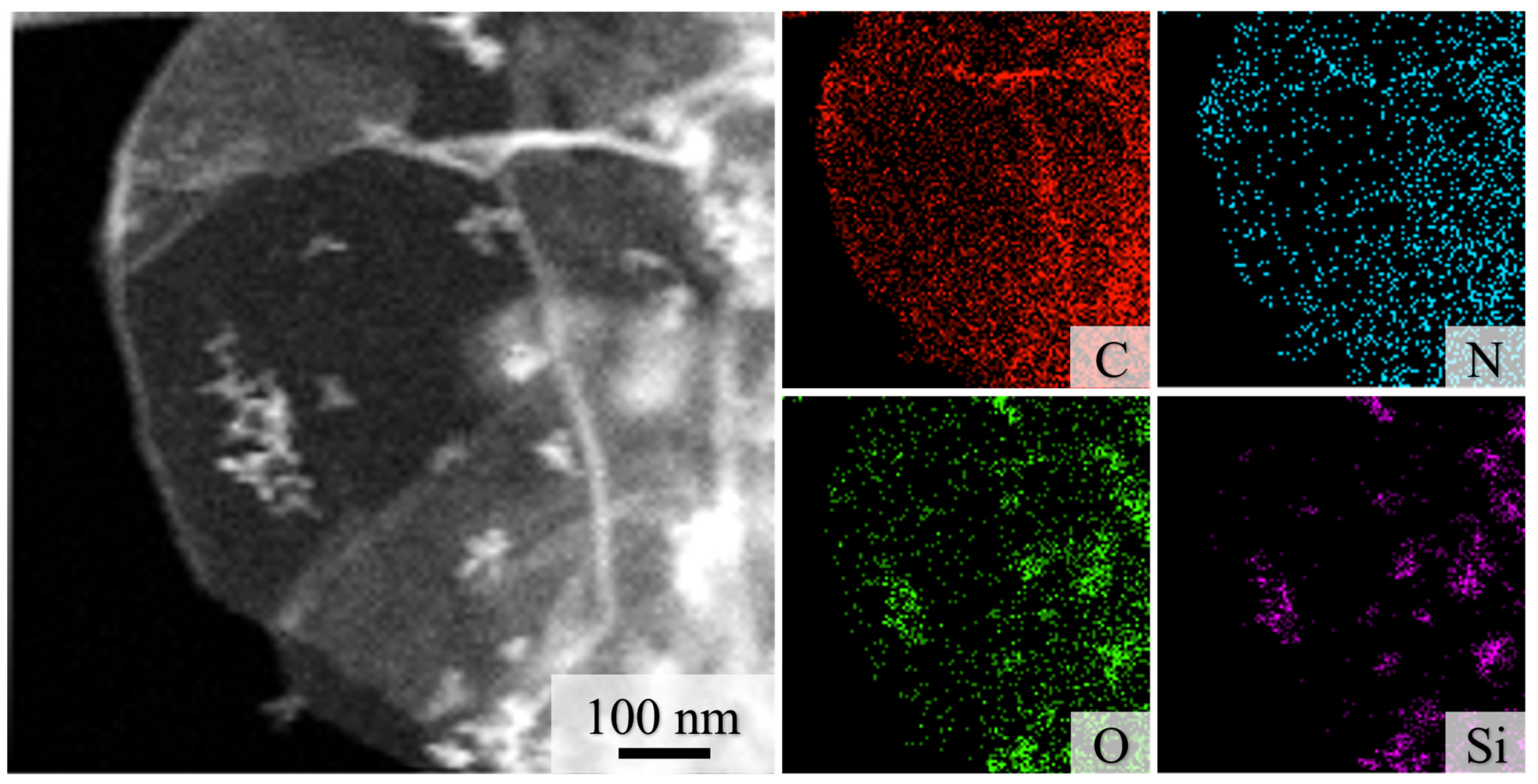
3.1. Characterization of SiO2-rGO@PPy Nanocomposite
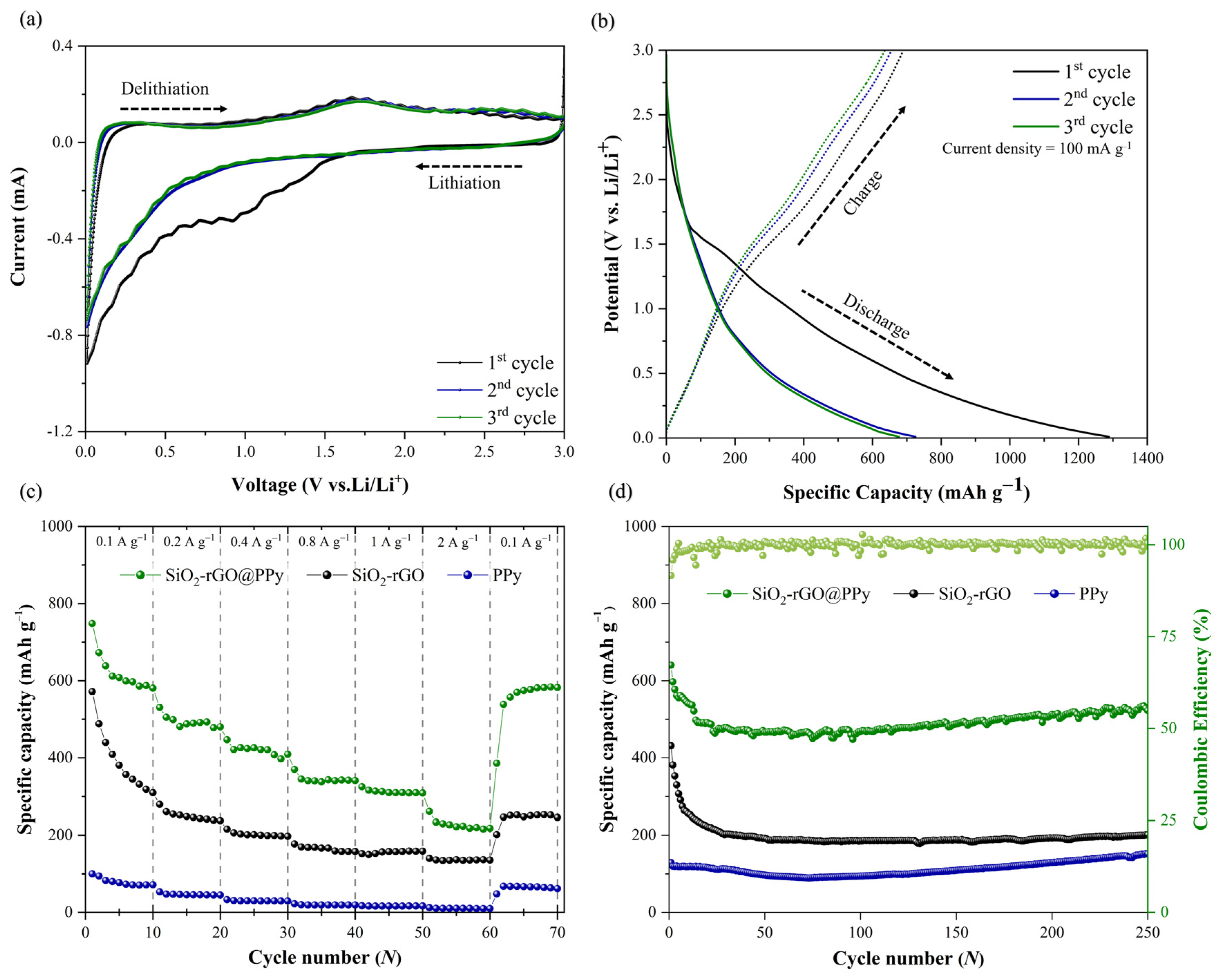
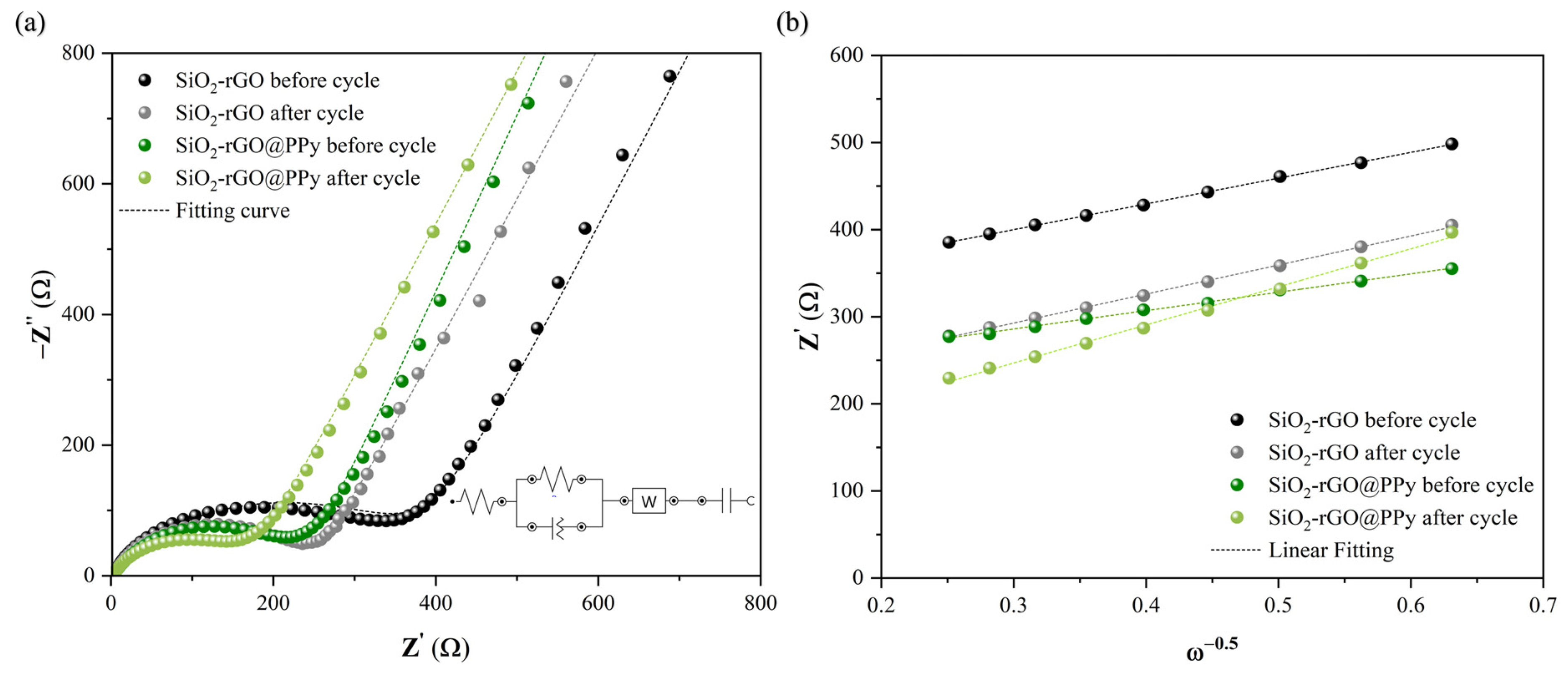
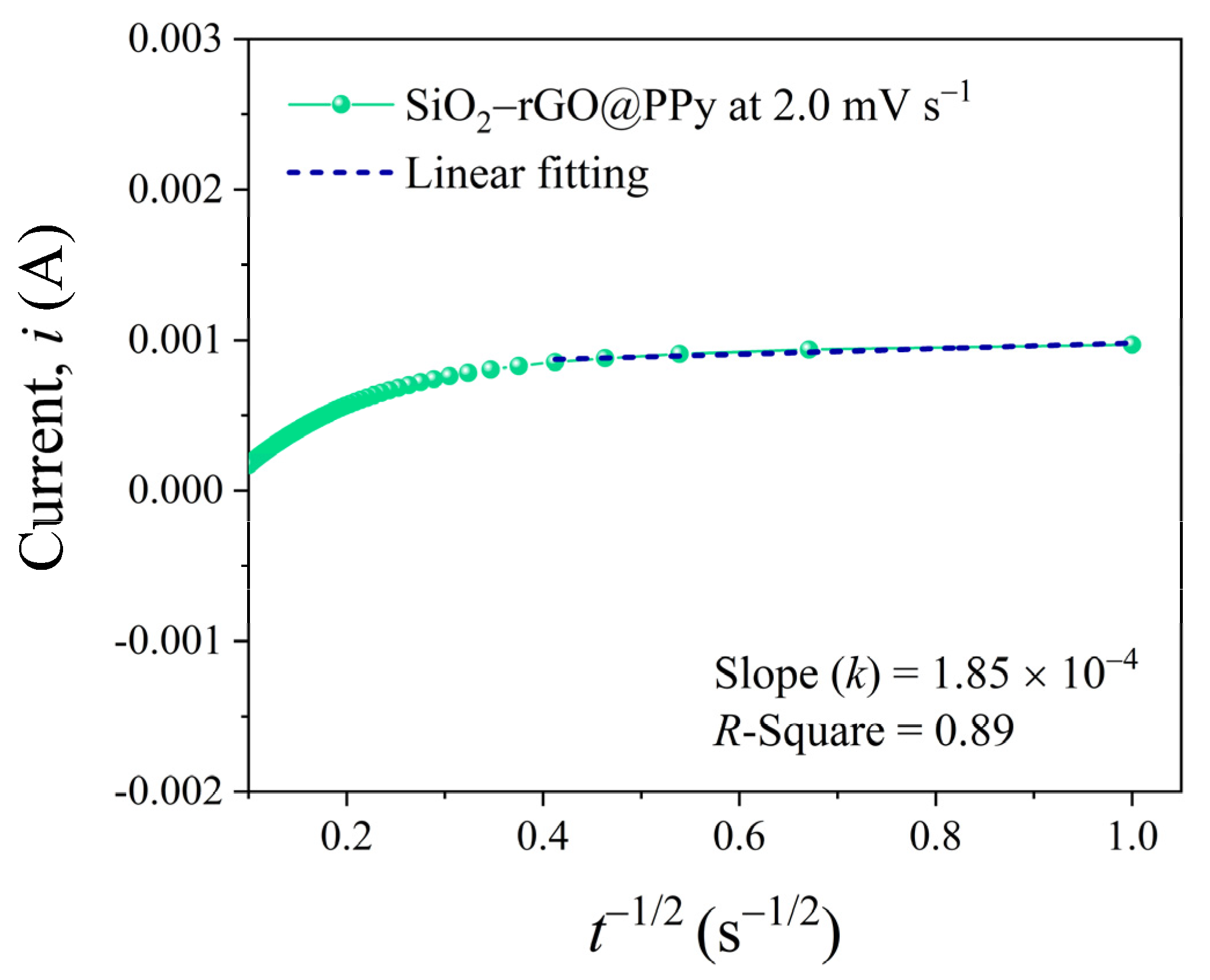
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratsameetammajak, N.; Autthawong, T.; Chairuangsri, T.; Kurata, H.; Yu, A.S.; Sarakonsri, T. Rice Husk-Derived Nano-SiO2 Assembled on Reduced Graphene Oxide Distributed on Conductive Flexible Polyaniline Frameworks towards High-Performance Lithium-Ion Batteries. RSC Adv. 2022, 12, 14621–14630. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon Oxides: A Promising Family of Anode Materials for Lithium-Ion Batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Cui, J.; Cheng, F.; Lin, J.; Yang, J.; Jiang, K.; Wen, Z.; Sun, J. High Surface Area C/SiO2 Composites from Rice Husks as a High-Performance Anode for Lithium Ion Batteries. Powder Technol. 2017, 311, 1–8. [Google Scholar] [CrossRef]
- Autthawong, T.; Yodbunork, C.; Yodying, W.; Boonprachai, R.; Namsar, O.; Yu, A.S.; Chimupala, Y.; Sarakonsri, T. Fast-Charging Anode Materials and Novel Nanocomposite Design of Rice Husk-Derived SiO2 and Sn Nanoparticles Self-Assembled on TiO2(B) Nanorods for Lithium-Ion Storage Applications. ACS Omega 2022, 7, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, B.; Peng, C.; Peng, X.; Fu, J.; Chu, P.K.; Huo, K. Bamboo Leaf Derived Ultrafine Si Nanoparticles and Si/C Nanocomposites for High-Performance Li-Ion Battery Anodes. Nanoscale 2015, 7, 13840–13847. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, Y.; Chu, B.; Sun, B.; Song, B.; Wu, S.; Su, Y.; He, Y. Plant-Derived Fluorescent Silicon Nanoparticles Featuring Excitation Wavelength-Dependent Fluorescence Spectra for Anti-Counterfeiting Applications. Chem. Commun. 2016, 52, 7047–7050. [Google Scholar] [CrossRef]
- Du, F.H.; Li, B.; Fu, W.; Xiong, Y.J.; Wang, K.X.; Chen, J.S. Surface Binding of Polypyrrole on Porous Silicon Hollow Nanospheres for Li-Ion Battery Anodes with High Structure Stability. Adv. Mater. 2014, 26, 6145–6150. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Luo, W.; Zhong, C.; Wexler, D.; Chou, S.L.; Liu, H.K.; Shi, Z.; Chen, G.; Ozawa, K.; Wang, J.Z. Novel Germanium/Polypyrrole Composite for High Power Lithium-Ion Batteries. Sci. Rep. 2014, 4, 6095. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Zheng, L.; Duan, X.; Wang, L.; Yang, J.; Qian, Y. Preparation of Polypyrrole-Coated CuFe2O4 and Their Improved Electrochemical Performance as Lithium-Ion Anodes. J. Energy Chem. 2014, 23, 354–357. [Google Scholar] [CrossRef]
- Guo, X.; Xie, K.; Wang, Y.; Kang, Z.; Zhou, W.; Cheng, S. Reduced Graphene Oxide-Coated 3D Interconnected SiO2 Nanoparticles with Enhanced Lithium Storage Performance. Int. J. Electrochem. Sci. 2018, 13, 5645–5653. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Rubinson, J.F.; Kayinamura, Y.P. Charge Transport in Conducting Polymers: Insights from Impedance Spectroscopy. Chem. Soc. Rev. 2009, 38, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Rudge, A.; Davey, J.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. Conducting Polymers as Active Materials in Electrochemical Capacitors. J. Power Sources 1994, 47, 89–107. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a Flexible Electrode Material of Supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Abdelhamid, M.E.; O’Mullane, A.P.; Snook, G.A. Storing Energy in Plastics: A Review on Conducting Polymers & Their Role in Electrochemical Energy Storage. RSC Adv. 2015, 5, 11611–11626. [Google Scholar]
- Levi, M.D.; Gofer, Y.; Aurbach, D. A Synopsis of Recent Attempts toward Construction of Rechargeable Batteries Utilizing Conducting Polymer Cathodes and Anodes. Polym. Adv. Technol. 2002, 13, 697–713. [Google Scholar] [CrossRef]
- Mao, Y.; Kong, Q.; Guo, B.; Fang, X.; Guo, X.; Shen, L.; Armand, M.; Wang, Z.; Chen, L. Polypyrrole-Iron-Oxygen Coordination Complex as High Performance Lithium Storage Material. Energy Environ. Sci. 2011, 4, 3442–3447. [Google Scholar] [CrossRef]
- Wang, J.Z.; Chou, S.L.; Liu, H.; Wang, G.X.; Zhong, C.; Yen Chew, S.; Kun Liu, H. Highly Flexible and Bendable Free-Standing Thin Film Polymer for Battery Application. Mater. Lett. 2009, 63, 2352–2354. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, S.; Qian, J.; Wang, Y.; Cao, Y.; Yang, H.; Ai, X. Li+-Conductive Polymer-Embedded Nano-Si Particles as Anode Material for Advanced Li-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 3508–3512. [Google Scholar] [CrossRef]
- Li, J.; Huang, J. A Nanofibrous Polypyrrole/Silicon Composite Derived from Cellulose Substance as Anode Material for Lithium-Ion Batteries. Chem. Commun. 2015, 20, 14590–14593. [Google Scholar] [CrossRef]
- Guo, Z.P.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Study of Silicon/Polypyrrole Composite as Anode Materials for Li-Ion Batteries. J. Power Sources 2005, 146, 448–451. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Zhu, X.; Yang, H.; Zhou, K.; Pan, D. Polypyrrole@Silica Composites as High Performance Electrode Materials for Lithium-Ion Batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 6098–6104. [Google Scholar] [CrossRef]
- Cui, L.; Shen, J.; Cheng, F.; Tao, Z.; Chen, J. SnO2 Nanoparticles@polypyrrole Nanowires Composite as Anode Materials for Rechargeable Lithium-Ion Batteries. J. Power Sources 2011, 196, 2195–2201. [Google Scholar] [CrossRef]
- Han, F.; Li, D.; Li, W.C.; Lei, C.; Sun, Q.; Lu, A.H. Nanoengineered Polypyrrole-Coated Fe2O3@C Multifunctional Composites with an Improved Cycle Stability as Lithium-Ion Anodes. Adv. Funct. Mater. 2013, 23, 1692–1700. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.; Wang, H.; Cao, Y.; Han, X.; Zhang, S.; Wang, H.; Zhang, Y.; Chen, A.; Yang, Z.; et al. Core-Shell Structure of a Polypyrrole-Coated Phosphorus/Carbon Nanotube Anode for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4112–4118. [Google Scholar] [CrossRef]
- Thirugnanam, L.; Ganguly, D.; Sundara, R. PPy Coated on SiO2 Encapsulated Porous Carbon Nanofibers as a Potential Anode Material for High Rate Capable and Long-Life Li-Ion Battery. Mater. Lett. 2021, 298, 130029. [Google Scholar] [CrossRef]
- Babaa, M.R.; Moldabayeva, A.; Karim, M.; Zhexembekova, A.; Zhang, Y.; Bakenov, Z.; Molkenova, A.; Taniguchi, I. Development of a Novel SiO2 Based Composite Anode Material for Li-Ion Batteries. Mater. Today Proc. 2017, 4, 4542–4547. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, Y.; Liu, J.; Fang, X. Hydrothermal Synthesis and Electrochemical Performance of Amorphous SiO2 Nanospheres/Graphene Composites. Mater. Sci. Appl. 2017, 8, 959–965. [Google Scholar]
- Meng, J.; Cao, Y.; Suo, Y.; Liu, Y.; Zhang, J.; Zheng, X. Facile Fabrication of 3D SiO2@Graphene Aerogel Composites as Anode Material for Lithium Ion Batteries. Electrochim. Acta 2015, 176, 1001–1009. [Google Scholar] [CrossRef]
- Zhuo, Y.; Prestat, E.; Kinloch, I.A.; Bissett, M.A. Self-Assembled 1T-MoS2/Functionalized Graphene Composite Electrodes for Supercapacitor Devices. ACS Appl. Energy Mater. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Das, G.S.; Hwang, J.Y.; Jang, J.H.; Tripathi, K.M.; Kim, T.Y. Biomass-Based Functionalized Graphene for Self-Rechargeable Zinc-Air Batteries. ACS Appl. Energy Mater. 2022, 5, 6663–6670. [Google Scholar] [CrossRef]
- Kim, H.; Lim, H.D.; Kim, S.W.; Hong, J.; Seo, D.H.; Kim, D.C.; Jeon, S.; Park, S.; Kang, K. Scalable Functionalized Graphene Nano-Platelets as Tunable Cathodes for High-Performance Lithium Rechargeable Batteries. Sci. Rep. 2013, 3, 1506. [Google Scholar] [CrossRef] [PubMed]
- Bakandritsos, A.; Jakubec, P.; Pykal, M.; Otyepka, M. Covalently Functionalized Graphene as a Supercapacitor Electrode Material. FlatChem 2019, 13, 25–33. [Google Scholar] [CrossRef]
- Li, J.; Yan, L. Synthesis of Fe2O3/RGO Based Composites as Anode Material for Lithium Ion Batteries Using Sewage Sludge as Source of Iron (III) Oxide. Int. J. Electrochem. Sci. 2022, 17, 221173. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, R.; Yadav, A.; Kumari, R. Recent Advancement Made in the Field of Reduced Graphene Oxide-Based Nanocomposites Used in the Energy Storage Devices: A Review. J. Energy Storage 2021, 33, 102032. [Google Scholar] [CrossRef]
- Ma, C.; Yang, K.; Wang, L.; Wang, X. Facile Synthesis of Reduced Graphene Oxide/Fe3O4 Nanocomposite Film. J. Appl. Biomater. Funct. Mater. 2017, 15, S1–S6. [Google Scholar] [CrossRef]
- Jia, P.Y.; Liu, X.M.; Li, G.Z.; Yu, M.; Fang, J.; Lin, J. Sol-Gel Synthesis and Characterization of SiO2@CaWO4, SiO2@CaWO4:Eu3+/Tb3+ Core-Shell Structured Spherical Particles. Nanotechnology 2006, 17, 734–742. [Google Scholar] [CrossRef]
- Huang, F.; Huang, T.; Wu, X.; Pang, W. Synthesis and Characterization of ZnSe: Ag/SiO2 Nanoparticles. E3S Web Conf. 2021, 261, 02063. [Google Scholar] [CrossRef]
- Liu, G.; Shi, Y.; Wang, L.; Song, Y.; Gao, S.; Liu, D.; Fan, L. Reduced Graphene Oxide/Polypyrrole Composite: An Advanced Electrode for High-Performance Symmetric/Asymmetric Supercapacitor. Carbon Lett. 2020, 30, 389–397. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Du, X.; Wang, J.; Ma, H.; Jing, X. Polypyrrole Composites with Carbon Materials for Supercapacitors. Chem. Pap. 2017, 71, 293–316. [Google Scholar] [CrossRef]
- Jumari, A.; Yudha, C.S.; Widiyandari, H.; Lestari, A.P.; Rosada, R.A.; Santosa, S.P.; Purwanto, A. SiO2/C Composite as a High Capacity Anode Material of Lini0.8Co0.15Al0.05O2 Battery Derived from Coal Combustion Fly Ash. Appl. Sci. 2020, 10, 8428. [Google Scholar] [CrossRef]
- Yi, G.; Xing, B.; Zeng, H.; Wang, X.; Zhang, C.; Cao, J.; Chen, L. One-Step Synthesis of Hierarchical Micro-Mesoporous SiO2/Reduced Graphene Oxide Nanocomposites for Adsorption of Aqueous Cr(VI). J. Nanomater. 2017, 7, 27224–27234. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of Graphene Microstructures on Electrochemical Performance for Supercapacitors. Prog. Nat. Sci. Mater. 2015, 25, 379–385. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and Characterization of Sulfophenyl-Functionalized Reduced Graphene Oxide Sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef]
- Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (RGO) for Gas Sensing Application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Babaei, F.; Fakhri, P.; Jaleh, B. Synthesis, Characterization, Structural, Optical Properties and Catalytic Activity of Reduced Graphene Oxide/Copper Nanocomposites. RSC Adv. 2015, 5, 10782–10789. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Li, M.; Yin, H.; Lin, H.; Zhou, J.; Zhuo, S. Three-Dimensional ZnS/Reduced Graphene Oxide/Polypyrrole Composite for High-Performance Supercapacitors and Lithium-Ion Battery Electrode Material. J. Solid State Electrochem. 2019, 23, 3419–3428. [Google Scholar] [CrossRef]
- Ali Shah, A.u.H.; Ullah, S.; Bilal, S.; Rahman, G.; Seema, H. Reduced Graphene Oxide/Poly(Pyrrole-co-Thiophene) Hybrid Compositematerials: Synthesis, Characterization, and Supercapacitive Properties. Polymers 2020, 12, 1110. [Google Scholar] [CrossRef]
- Wu, T.M.; Lin, S.H. Synthesis, Characterization, and Electrical Properties of Polypyrrole/Multiwalled Carbon Nanotube Composites. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6449–6457. [Google Scholar] [CrossRef]
- Ruhi, G.; Dhawan, S.K. Conducting Polymer Nano Composite Epoxy Coatings for Anticorrosive Applications. In Modern Electrochemical Methods in Nano, Surface and Corrosion Science; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Wang, Y.; Pan, X.; Chen, Y.; Wen, Q.; Lin, C.; Zheng, J.; Li, W.; Xu, H.; Qi, L. A 3D Porous Nitrogen-Doped Carbon Nanotube Sponge Anode Modified with Polypyrrole and Carboxymethyl Cellulose for High-Performance Microbial Fuel Cells. J. Appl. Electrochem. 2020, 50, 1281–1290. [Google Scholar] [CrossRef]
- Hsu, F.H.; Wu, T.M. In Situ Synthesis and Characterization of Conductive Polypyrrole/Graphene Composites with Improved Solubility and Conductivity. Synth. Met. 2012, 162, 682–687. [Google Scholar] [CrossRef]
- Prasankumar, T.; Karazhanov, S.; Jose, S.P. Three-Dimensional Architecture of Tin Dioxide Doped Polypyrrole/Reduced Graphene Oxide as Potential Electrode for Flexible Supercapacitors. Mater. Lett. 2018, 221, 179–182. [Google Scholar] [CrossRef]
- Xie, D.; Wang, D.H.; Tang, W.J.; Xia, X.H.; Zhang, Y.J.; Wang, X.L.; Gu, C.D.; Tu, J.P. Binder-Free Network-Enabled MoS2-PPY-RGO Ternary Electrode for High Capacity and Excellent Stability of Lithium Storage. J. Power Sources 2016, 307, 510–518. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, X.; Mu, J.; Liu, P.; Yuan, W.; Li, S.; Wang, X.; Fang, H.; Liu, H.; Xing, T.; et al. Robust and Fast Lithium Storage Enabled by Polypyrrole-Coated Nitrogen and Phosphorus Co-Doped Hollow Carbon Nanospheres for Lithium-Ion Capacitors. Front. Chem. 2021, 9, 760473. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Y.; Chen, Q. High Lithium Anodic Performance of Highly Nitrogen-Doped Porous Carbon Prepared from a Metal-Organic Framework. Nat. Commun. 2014, 5, 5261. [Google Scholar] [CrossRef]
- Wasalathilake, K.C.; Hapuarachchi, S.N.S.; Zhao, Y.; Fernando, J.F.S.; Chen, H.; Nerkar, J.Y.; Golberg, D.; Zhang, S.; Yan, C. Unveiling the Working Mechanism of Graphene Bubble Film/Silicon Composite Anodes in Li-Ion Batteries: From Experiment to Modeling. ACS Appl. Energy Mater. 2020, 3, 521–531. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, K.; He, W.; Liu, Y.; Guo, S. SiO2@graphite Composite Generated from Sewage Sludge as Anode Material for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2017, 12, 10221–10229. [Google Scholar] [CrossRef]
- Jayabalan, A.D.; Din, M.M.U.; Indu, M.S.; Karthik, K.; Ragupathi, V.; Nagarajan, G.S.; Panigrahi, P.; Murugan, R. Electrospun 3D CNF–SiO2 Fabricated Using Non-Biodegradable Silica Gel as Prospective Anode for Lithium–Ion Batteries. Ionics 2019, 25, 5305–5313. [Google Scholar] [CrossRef]
- Lener, G.; Otero, M.; Barraco, D.E.; Leiva, E.P.M. Energetics of Silica Lithiation and Its Applications to Lithium Ion Batteries. Electrochim. Acta 2018, 259, 1053–1058. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material-Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.; Lin, S.; Khan, S.; Huang, J.; Liu, S.; Chen, Z.; Wu, D.; Fu, R. Synthesis of SiOx/C Composite Nanosheets As High-Rate and Stable Anode Materials for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 3562–3568. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Zhang, W.; Qiao, Y.; Cao, X.; Xie, X.; Zhou, H.; Pan, A.; Liang, S. Tuning Interface Bridging Between MoSe2 and Three-Dimensional Carbon Framework by Incorporation of MoC Intermediate to Boost Lithium Storage Capability. Nanomicro. Lett. 2020, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Huang, J.; Lin, Z.; Yu, X.; Wu, X.; Zhang, B.; Zhan, Y.; Xie, F.; Zhang, W.; Chen, J.; et al. Amorphous SiO2/C Composite as Anode Material for Lithium-Ion Batteries. J. Mater. Res. 2018, 33, 1219–1225. [Google Scholar] [CrossRef]
- Jia, M.; Qi, T.; Yuan, Q.; Zhao, P.; Jia, M. Polypyrrole Modified MoS2 Nanorod Composites as Durable Pseudocapacitive Anode Materials for Sodium-Ion Batteries. Nanomaterials 2022, 12, 2006. [Google Scholar] [CrossRef] [PubMed]
- Nita, C.; Fullenwarth, J.; Monconduit, L.; Le Meins, J.M.; Fioux, P.; Parmentier, J.; Matei Ghimbeu, C. Eco-Friendly Synthesis of SiO2 Nanoparticles Confined in Hard Carbon: A Promising Material with Unexpected Mechanism for Li-Ion Batteries. Carbon 2019, 143, 598–609. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Z.Q.; Wang, C.Y.; Jin, J. Nanostructured SiO2/C Composites Prepared via Electrospinning and Their Electrochemical Properties for Lithium Ion Batteries. J. Electroanal. Chem. 2015, 746, 62–67. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, T.; Zhang, A.M.; Li, S.L.; Shen, F.C.; Dong, L.Z.; Li, D.S.; Lan, Y.Q. Polyoxomolybdate-Polypyrrole/Reduced Graphene Oxide Nanocomposite as High-Capacity Electrodes for Lithium Storage. ACS Omega 2017, 2, 5684–5690. [Google Scholar] [CrossRef]
- Mo, L.; Zheng, H. Solid Coated Li4Ti5O12 (LTO) Using Polyaniline (PANI) as Anode Materials for Improving Thermal Safety for Lithium Ion Battery. Energy Rep. 2020, 6, 2913–2918. [Google Scholar] [CrossRef]
- Wang, B.; Gu, L.; Zhang, D.; Wang, W. High-Throughput Production of Zr-Doped Li4Ti5O12 Modified by Mesoporous Libaf3 Nanoparticles for Superior Lithium and Potassium Storage. Asian J. Chem. 2019, 14, 3181–3187. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Liu, H.; Zhao, X.; Pang, L.; Deng, L.; Li, M. Composite with TiO2 and Extension of Discharge Voltage Range for Capacity Enhancement of a Li4Ti5O12 Battery. RSC Adv. 2017, 7, 43894–43904. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Villareal, J.; Alcoutlabi, M. Composite Nanofibers as Advanced Materials for Li-Ion, Li-O2 and Li-S Batteries. Electrochim. Acta 2016, 192, 529–550. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, G.; Qu, Y.; Zheng, Z.; Wang, J.; Zhu, M.; Duan, L. Capacity Contribution Mechanism of RGO for SnO2/RGO Composite as Anode of Lithium-Ion Batteries. Chin. J. Mech. Eng. 2022, 35, 63. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, X.; Deng, Y.; Wang, L.; Hong, H.; Ju, Z. SiO2/N-Doped Graphene Aerogel Composite Anode for Lithium-Ion Batteries. J. Mater. Sci. 2020, 55, 13023–13035. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, R.; Liu, B.; Zhang, Y.; Zhu, K.; Yan, J.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. MXene-Derived TiO2/Reduced Graphene Oxide Composite with an Enhanced Capacitive Capacity for Li-Ion and K-Ion Batteries. J. Mater. Chem. A 2019, 7, 5363–5372. [Google Scholar] [CrossRef]
- Liu, D.; Huang, X.; Wei, Z.; Xia, L.; Pan, H.; Zhang, T.; Wang, H.; Duan, X.; Jia, D.; Zhou, Y.; et al. Orderly Stacked Graphene Sheets Supporting SnO2 Nanoparticles as an Anode Material for Lithium-Ion Batteries with Incremental Capacity. Appl. Surf. Sci. 2021, 564, 150265. [Google Scholar] [CrossRef]
- Wu, N.; Miao, D.; Zhou, X.; Zhang, L.; Liu, G.; Guo, D.; Liu, X. V3S4 Nanosheets Anchored on N, S Co-Doped Graphene with Pseudocapacitive Effect for Fast and Durable Lithium Storage. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Deng, X.; Wei, Z.; Cui, C.; Liu, Q.; Wang, C.; Ma, J. Oxygen-Deficient Anatase TiO2@C Nanospindles with Pseudocapacitive Contribution for Enhancing Lithium Storage. J. Mater. Chem. A 2018, 6, 4013–4022. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, Y.; Li, D.; Liu, Y.; Chen, W.; Feng, X.; Zhang, J. Construction of 3D Architectures with Ni(HCO3)2 Nanocubes Wrapped by Reduced Graphene Oxide for LIBs: Ultrahigh Capacity, Ultrafast Rate Capability and Ultralong Cycle Stability. Chem. Sci. 2018, 9, 8682–8691. [Google Scholar] [CrossRef]
- Hou, L.; Deng, S.; Jiang, Y.; Cui, R.; Zhou, Y.; Guo, Y.; Gao, F. A Unique Corn-like Architecture Composed of Se-Doped Carbon Load Fe3O4 Particles as High-Performance Lithium-Ion Battery Anodes. Ionics 2021, 27, 2825–2833. [Google Scholar] [CrossRef]



| Electrodes | SiO2 Content in the Composite | Specific Capacity (mAh g−1) | Cycle | Current Density (mA g−1) | Ref. |
|---|---|---|---|---|---|
| C/SiO2 | 28% | 203 | 250 | C/5 (~164) | [68] |
| SiO2/C | 15% | 658 | 100 | 50 | [69] |
| SiO2/MWCNT/G composite | 50% | 260 | 30 | 50 | [27] |
| SiO2-rGO@PPy nanocomposite | 30% | 523 | 250 | 100 | This work |
| Prepared Electrodes | Rs (Ω) | Rct (Ω) | σW (Ω/s1/2) | DLi+ (cm2/s) |
|---|---|---|---|---|
| SiO2-rGO@PPy before cycle | 2.69 | 193 | 211 | 3.02 × 10−15 |
| SiO2-rGO@PPy after cycle | 2.60 | 189 | 437 | 7.04 × 10−16 |
| SiO2-rGO before cycle | 0.85 | 399 | 296 | 1.54 × 10−15 |
| SiO2-rGO after cycle | 2.06 | 212 | 333 | 1.21 × 10−15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratsameetammajak, N.; Autthawong, T.; Khunpakdee, K.; Haruta, M.; Chairuangsri, T.; Sarakonsri, T. Insight into the Role of Conductive Polypyrrole Coated on Rice Husk-Derived Nanosilica-Reduced Graphene Oxide as the Anodes: Electrochemical Improvement in Sustainable Lithium-Ion Batteries. Polymers 2023, 15, 4638. https://doi.org/10.3390/polym15244638
Ratsameetammajak N, Autthawong T, Khunpakdee K, Haruta M, Chairuangsri T, Sarakonsri T. Insight into the Role of Conductive Polypyrrole Coated on Rice Husk-Derived Nanosilica-Reduced Graphene Oxide as the Anodes: Electrochemical Improvement in Sustainable Lithium-Ion Batteries. Polymers. 2023; 15(24):4638. https://doi.org/10.3390/polym15244638
Chicago/Turabian StyleRatsameetammajak, Natthakan, Thanapat Autthawong, Kittiched Khunpakdee, Mitsutaka Haruta, Torranin Chairuangsri, and Thapanee Sarakonsri. 2023. "Insight into the Role of Conductive Polypyrrole Coated on Rice Husk-Derived Nanosilica-Reduced Graphene Oxide as the Anodes: Electrochemical Improvement in Sustainable Lithium-Ion Batteries" Polymers 15, no. 24: 4638. https://doi.org/10.3390/polym15244638






