One-Dimensional Nickel Molybdate Nanostructures with Enhanced Supercapacitor Performance
Abstract
:1. Introduction
2. Experiment
2.1. Chemicals
2.2. NiMoO4 Nanofibers’ Synthesis Method
2.3. Characterization
2.4. Electrochemical Measurement Technology
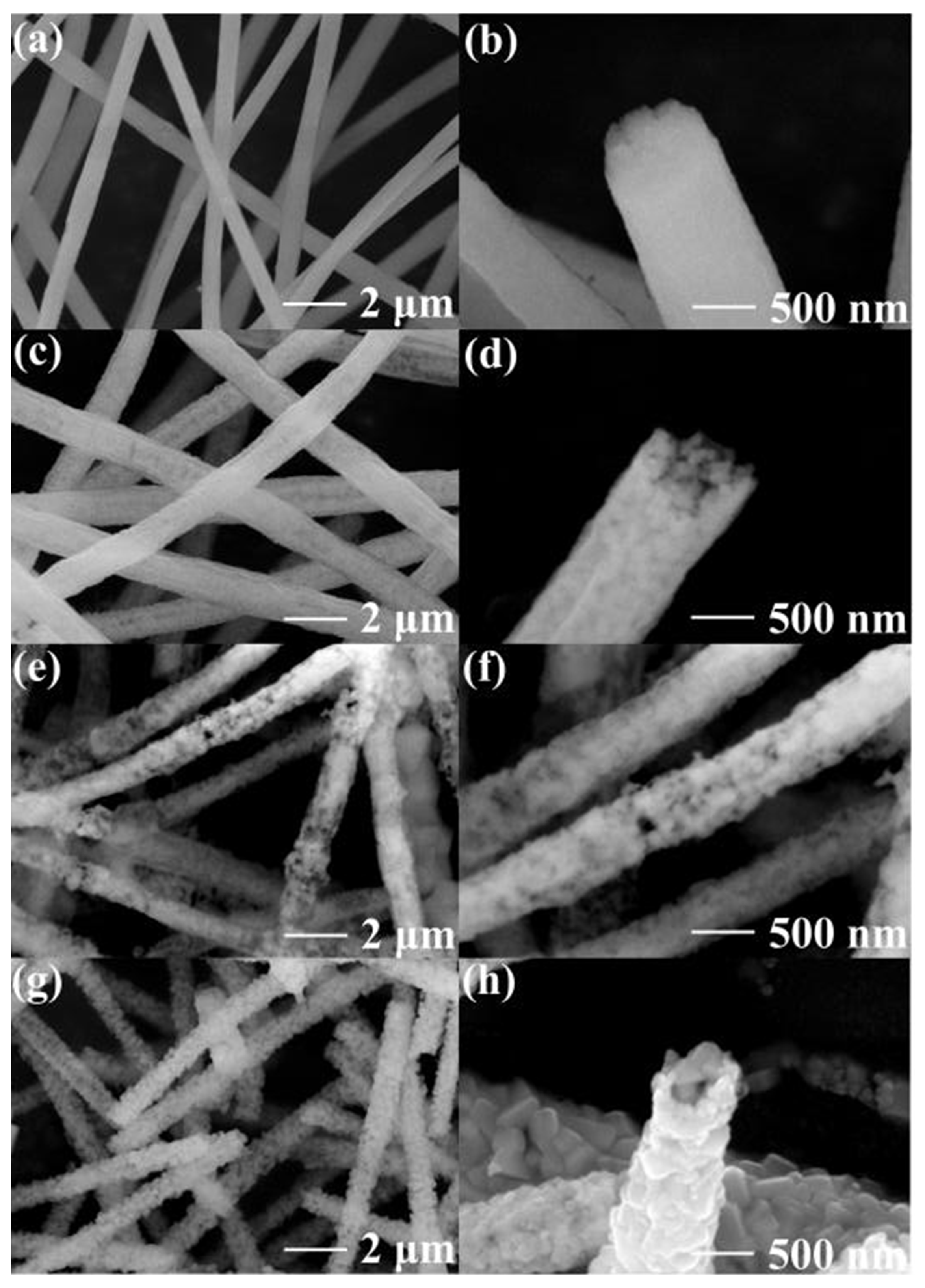
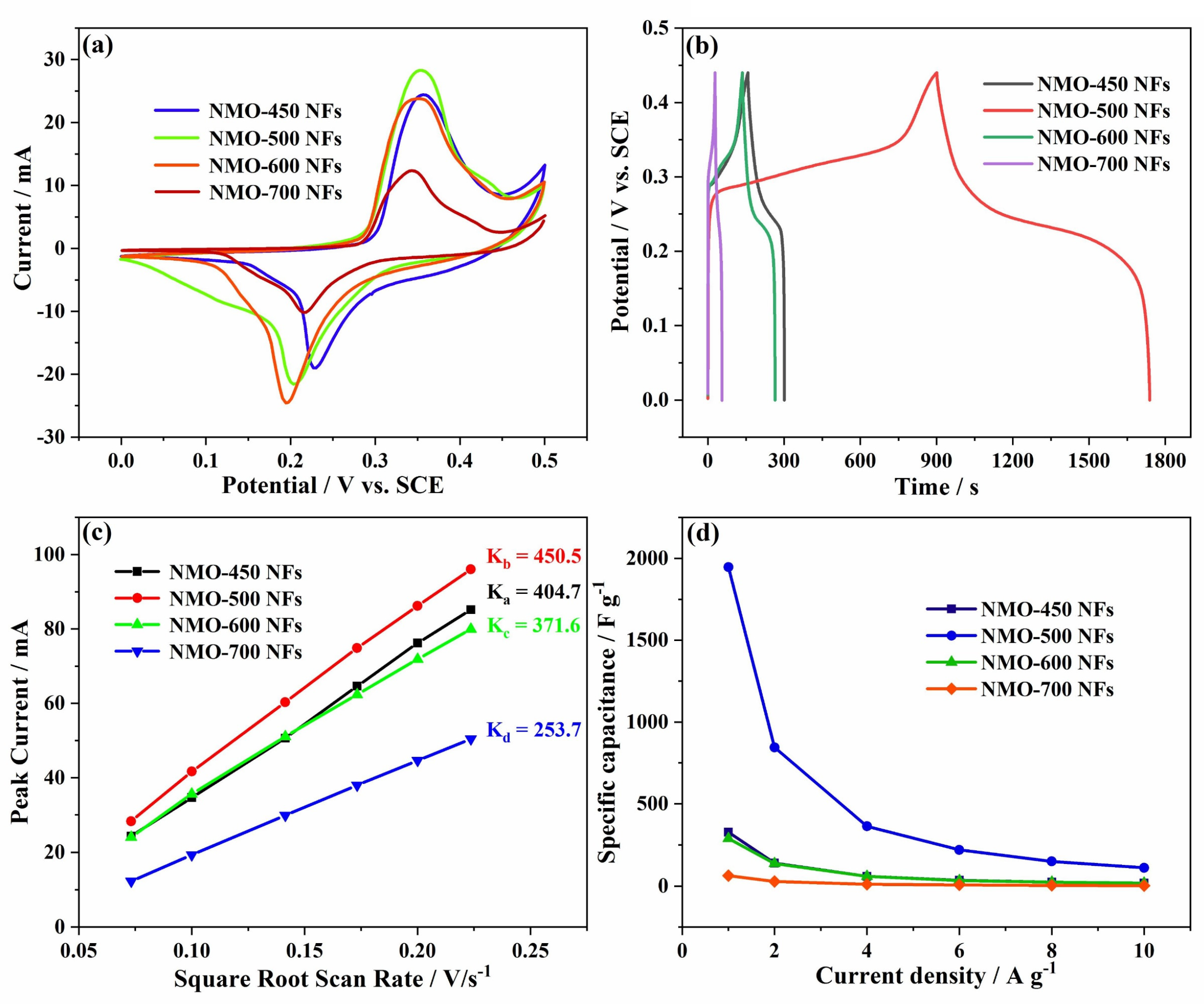
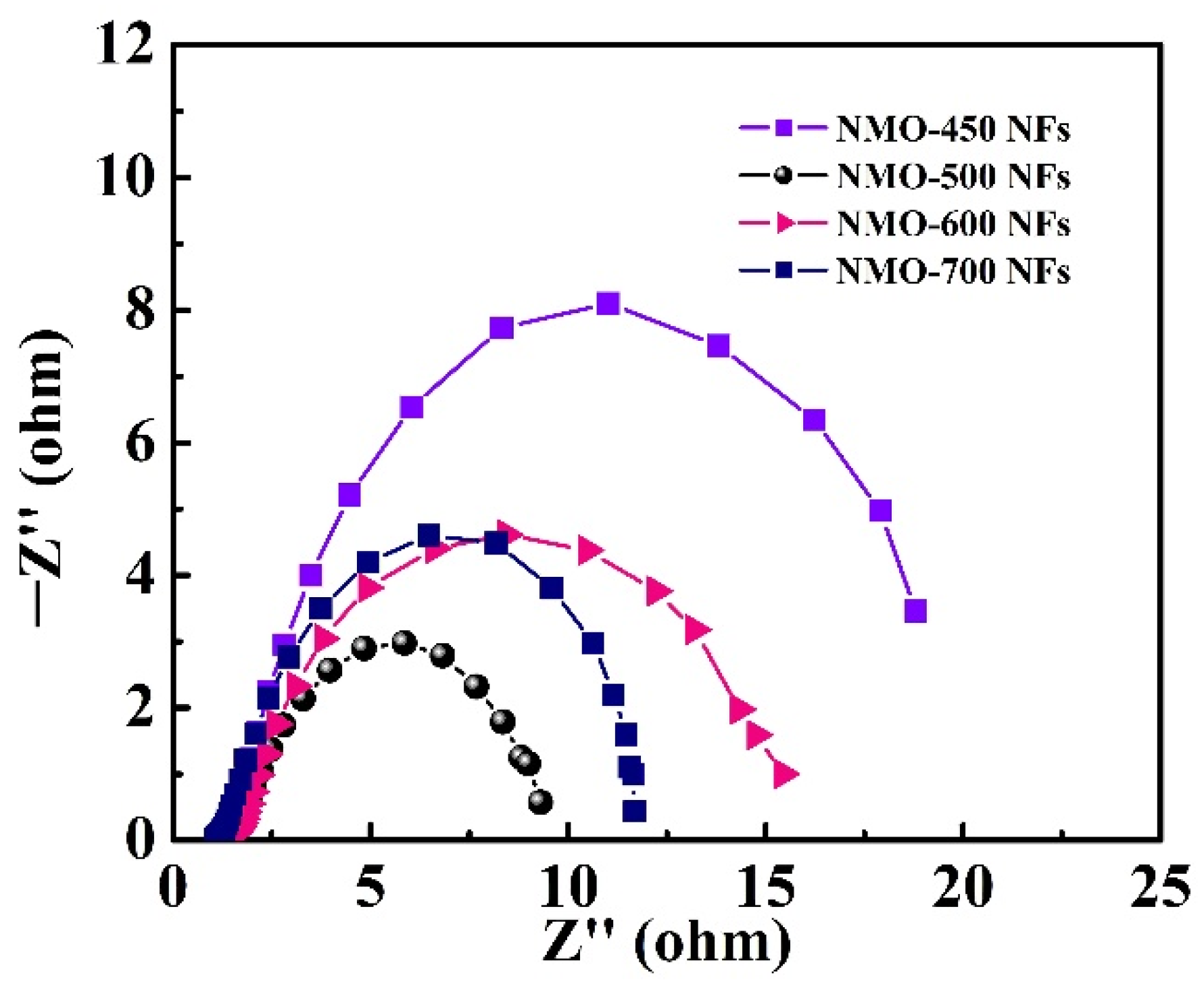
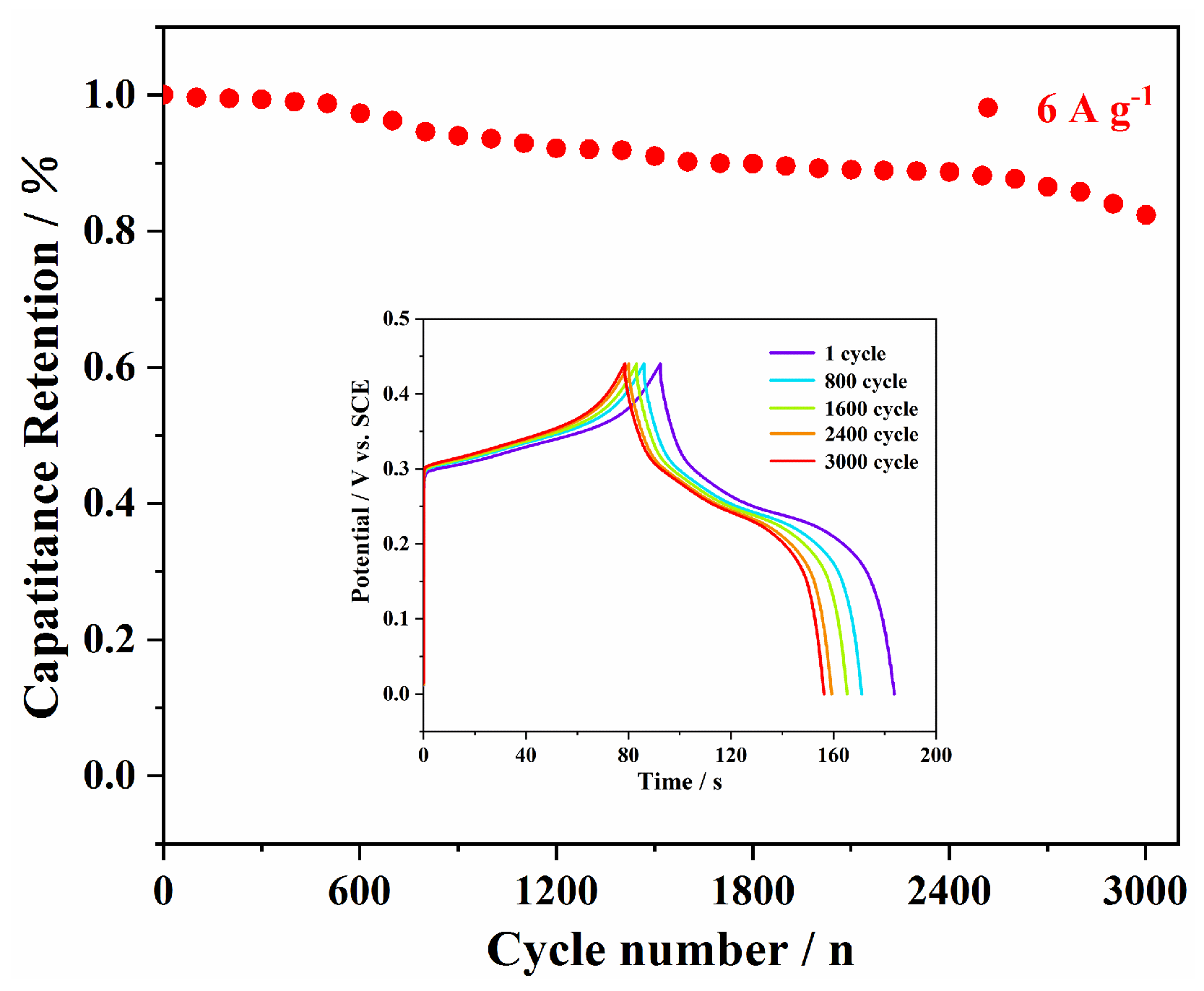
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zardkhoshoui, A.M.; Davarani, S.S.H. Formation of Graphene-wrapped Multi-shelled NiGa2O4 Hollow Spheres and Graphene-wrapped Yolk-shell NiFe2O4 Hollow Spheres Derived from Metal-organic Frameworks for High-performance Hybrid Supercapacitors. Nanoscale 2020, 12, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Zardkhoshoui, A.; Hosseiny Davarani, S.S.; Ashtiani, M.M.; Sarparast, M. High-Performance Energy Storage Device Based on Triple-Shelled Cobalt Gallium Oxide Hollow Spheres and Graphene Wrapped Copper Iron Disulfide Porous Spheres. ACS Sustain. Chem. Eng. 2019, 7, 7908. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards Flexible Solid-state Supercapacitors for Smart and Wearable Electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, K.; Huang, T.; Shen, G. Recent Advances in Fiber Supercapacitors, Materials, Device Configurations, and Applications. Adv. Mater. 2020, 32, 1901806. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Perspectives for Electrochemical Capacitors and Related Devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.M. Biomass-derived Porous Carbon Materials with Different Dimensions for Supercapacitor Electrodes: A Review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.; Huang, Y.; Li, P.; Yan, J.; Liu, K. Mesoporous Hollow Carbon Spheres Boosted, Integrated High Performance Aqueous Zn-Ion Energy Storage. Energy Storage Mater. 2020, 25, 858–865. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Huang, S.S.; Lin, Y.H.; Lin, Y.C.; Shih, B.Y.; Sheu, H.S.; Liao, Y.-F.; Wu, N.L. High-performance Carbon-coated ZnMn2O4 Nanocrystallite Supercapacitors with Tailored Microstructures Enabled by a Novel Solution Combustion Method. J. Power Sources 2018, 378, 90–97. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; Ren, Y.; He, Y. Self-supported 3D Layered Zinc/nickel Metal-organic-framework with Enhanced Performance for Supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 18101–18110. [Google Scholar] [CrossRef]
- Lu, X.; Jia, W.; Chai, H.; Hu, J.; Wang, S.; Cao, Y. Solid-State Chemical Fabrication of One-Dimensional Mesoporous β-Nickel Molybdate Nanorods as Remarkable Electrode Material for Supercapacitors. J. Colloid Interface Sci. 2018, 5, 322–331. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, T.; Huang, Y.; Cheng, S.; Liao, G.; Tang, Z. One-step Synthesis of Porous Carbon Derived from Starch For all-carbon Binder-free High-rate Supercapacitor. Electrochim. Acta 2018, 269, 676–685. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Jones, R.T.; Pramanik, N.C.; Jean-Fulcrand, A.; Garnweitner, G. A Hybrid Electrochemical Energy Storage Device Using Sustainable Electrode Materials. Chemistry Select 2020, 5, 1597–1606. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W. General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wang, D.; Liu, B.; Wang, Y.; Liu, Y.; Wang, L.; Li, H.; Huang, H.; Li, Q.; Wang, T. Comparison of the electrochemicalperformance of NiMoO4 nanorods and hierarchical nanospheres for supercapacitor applications. ACS Appl. Mater. Interfaces 2013, 5, 12905–12910. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Lui, Y.H.; Hu, S. Ni-Mn bimetallic oxide nanosheets as high-performance electrode materials for asymmetric supercapacitors. J. Energy Storage 2019, 25, 100897. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Baur, C.; Chable, J.; Barlow, A.J.; Fichtner, M.; Banerjee, A.; Chakraborty, S.; Ahuja, R. Phase evolution in calcium molybdate nanoparticles as a function of synthesis temperature and its electrochemical effect on energy storage. Nanoscale Adv. 2019, 1, 565–580. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi, M.; Whale, J.; Jean-Fulcrand, A.; Garnweitner, G. Effect of the Anionic Counterpart: Molybdate vs. Tungstate in Energy Storage for Pseudo-Capacitor Applications. Nanomaterials 2021, 11, 580. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn Metal Atom Doping on the Surface Plane of One-Dimesional NiMoO4 Nanorods with Improved Redox Chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Chen, Z.Y.; He, B.; Yan, D.; Yu, X.F.; Li, W.C. Peapod-like MnO@Hollow Carbon Nanofibers Film as Self-standing Electrode for Li-Ion Capacitors with Enhanced Rate Capacity. J. Power Sources 2020, 472, 228501. [Google Scholar] [CrossRef]
- Gao, S.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; He, Y. Facile Synthesis of Cuboid Ni-MoF for High-performance Supercapacitors. J. Mater. Sci. 2018, 53, 6807–6818. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Wang, L.; Yang, X.; Yang, W.; Zheng, J.; Hou, H. Advanced Supercapacitors Based on Porous Hollow Carbon Nanofiber Electrodes with High Specific Capacitance and Large Energy Density. ACS Appl. Mater. Interfaces 2020, 12, 4777–4786. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wang, J.; Liu, C.; Wang, H.; Yao, H.; Zheng, G.; Seh, Z.W.; Cai, Q.; Li, W.; Zhou, G.; et al. Balancing Surface Adsorption and Diffusion of Lithium-polysulfides on Nonconductive Oxides for Lithium-sulfur Battery Design. Nat. Chem. 2016, 7, 11203. [Google Scholar] [CrossRef] [PubMed]
- Çıplak, Z.; Yıldız, A.; Yıldız, N. Green Preparation of Ternary Reduced Graphene Oxide-au@polyaniline Nanocomposite for Supercapacitor Application. J. Energy Storage 2020, 32, 101846. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W.; Li, D.; Han, P.; Shi, L.; Luo, Y.; Cong, G.; Li, C.; Yu, J.; Zhu, C.; et al. One-pot Synthesis of Hierarchical Co1-x S/NC@MoS2/C Hollow Nanofibers Based on One-dimensional Metal Coordination Polymers for Enhanced Lithium and Sodium-ion Storage. Sci. Bull. 2020, 65, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, C.R.; Gao, H.L.; Wang, S.W.; Yan, J.; Gao, K.Z.; Jia, X.D.; Luo, H.W.; Fang, H.; Zhang, A.Q.; et al. High-performance supercapacitor electrodes based on NiMoO4 nanorods. J. Mater. Res. 2019, 34, 2435–2444. [Google Scholar] [CrossRef]
- Neeraj, N.S.; Mordina, B.; Srivastava, A.K.; Mukhopadhyay, K.; Prasad, N.E. Impact of Process Conditions on the Electrochemical Performances of NiMoO4 Nanorods and Activated Carbon-Based Asymmetric Supercapacitor. Appl. Surf. Sci. 2019, 473, 807–819. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, C.R.; Jia, X.D.; Huo, Q.Y.; Gao, H.L.; Yan, J.; Zhang, A.Q.; Ru, Y.; Mei, H.X.; Gao, K.Z.; et al. Morphology-Dependent NiMoO4/Carbon Composites for High Performance Supercapacitors. Inorg. Chem. Commun. 2020, 111, 107631. [Google Scholar] [CrossRef]
- Nti, F.; Anang, D.A.; Han, J.I. Facilely Synthesized NiMoO4/CoMoO4 Nanorods as Electrode Material for High Performance Supercapacitor. J. Alloys Compd. 2018, 742, 342–350. [Google Scholar] [CrossRef]
- Arshadi Rastabi, S.; Sarraf Mamoory, R.; Dabir, F.; Blomquist, N.; Phadatare, M.; Olin, H. Synthesis of NiMoO4/3D-rGO Nanocomposite in Alkaline Environments for Supercapacitor Electrodes. Crystals 2019, 9, 31. [Google Scholar] [CrossRef]
- Chu, Y.; Xiong, S.; Li, B.; Qian, Y.; Xi, B. Designed Formation of MnO2@NiO/NiMoO4 Nanowires@Nanosheets Hierarchical Structures with Enhanced Pseudocapacitive Properties. ChemElectroChem 2016, 3, 1347–1353. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Jia, Y.; Zheng, H. Three-dimensional NiCo2O4@NiWO4 core–shell nanowire arrays for high performance supercapacitors. J. Mater. Chem. A 2017, 5, 1028–1034. [Google Scholar] [CrossRef]
- Niu, L.; Li, Z.; Xu, Y.; Sun, J.; Hong, W.; Liu, X.; Wang, J.; Yang, S. Simple synthesis of amorphous NiWO4 nanostructure and its application as a novel cathode material for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 8044–8052. [Google Scholar] [CrossRef] [PubMed]






| Electrode Material | Electrolyte | Specific Capacitance | Cycle Life | Ref. |
|---|---|---|---|---|
| NiMoO4 nanospheres | 3 M KOH | 974.4 F g−1 (at 1 A g−1) | 75% (2000th at 5 A g−1) | [14] |
| NiMoO4 | 2 M NaOH | 392.53 F g−1 | 87.14% (1000th at 5 A g−1) | [17] |
| NiMoO4 nanorods | 3 M KOH | 672 F g−1 (at 4 A g−1) | 72% (1000th at 1 A g−1) | [25] |
| NiMoO4 | 6 M KOH | 594 F g−1 (at 1 A g−1) | 56% (1000th at 1 A g−1) | [26] |
| NiMoO4/MWCNTs | 3 M KOH | 805 F g−1 (at 1 A g−1) | 66.7% (1000th at 1 A g−1) | [27] |
| NiMoO4/CoMoO4 nanorods | 1 M KOH | 1445 F g−1 (at 1 A g−1) | 78.8% (3000th at 10 A g−1) | [28] |
| NiMoO4/3D-rGO (II) | 3 M KOH | 932 F g−1 (at 1 A g−1) | 76% (500th at 1 A g−1) | [29] |
| MnO2/NiMoO4 nanostructure | 5 M KOH | 918 F g−1 (at 1 A g−1) | 80% (10000th at 5 A g−1) | [30] |
| NiCo2O4@NiWO4 core–shell nanowire | 6 M KOH | 1384 F g−1 (at 1 A g−1) | 87.6% (6000th at 5 A g−1) | [31] |
| Amorphous NiWO4 | 2 M KOH | 586.2 F g−1 (at 0.5 A g−1) | 91.4% (5000th at 2A g−1) | [32] |
| NiMoO4 NFs | 1 M KOH | 1947 F g−1 (at 1 A g−1) | 82.35% (3000th at 6 A g−1) | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Wang, S.; Zhang, M. One-Dimensional Nickel Molybdate Nanostructures with Enhanced Supercapacitor Performance. Polymers 2023, 15, 4538. https://doi.org/10.3390/polym15234538
Sun B, Wang S, Zhang M. One-Dimensional Nickel Molybdate Nanostructures with Enhanced Supercapacitor Performance. Polymers. 2023; 15(23):4538. https://doi.org/10.3390/polym15234538
Chicago/Turabian StyleSun, Baodong, Shaomin Wang, and Mingyi Zhang. 2023. "One-Dimensional Nickel Molybdate Nanostructures with Enhanced Supercapacitor Performance" Polymers 15, no. 23: 4538. https://doi.org/10.3390/polym15234538






