Stretchable, Adhesive, and Biocompatible Hydrogel Based on Iron–Dopamine Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
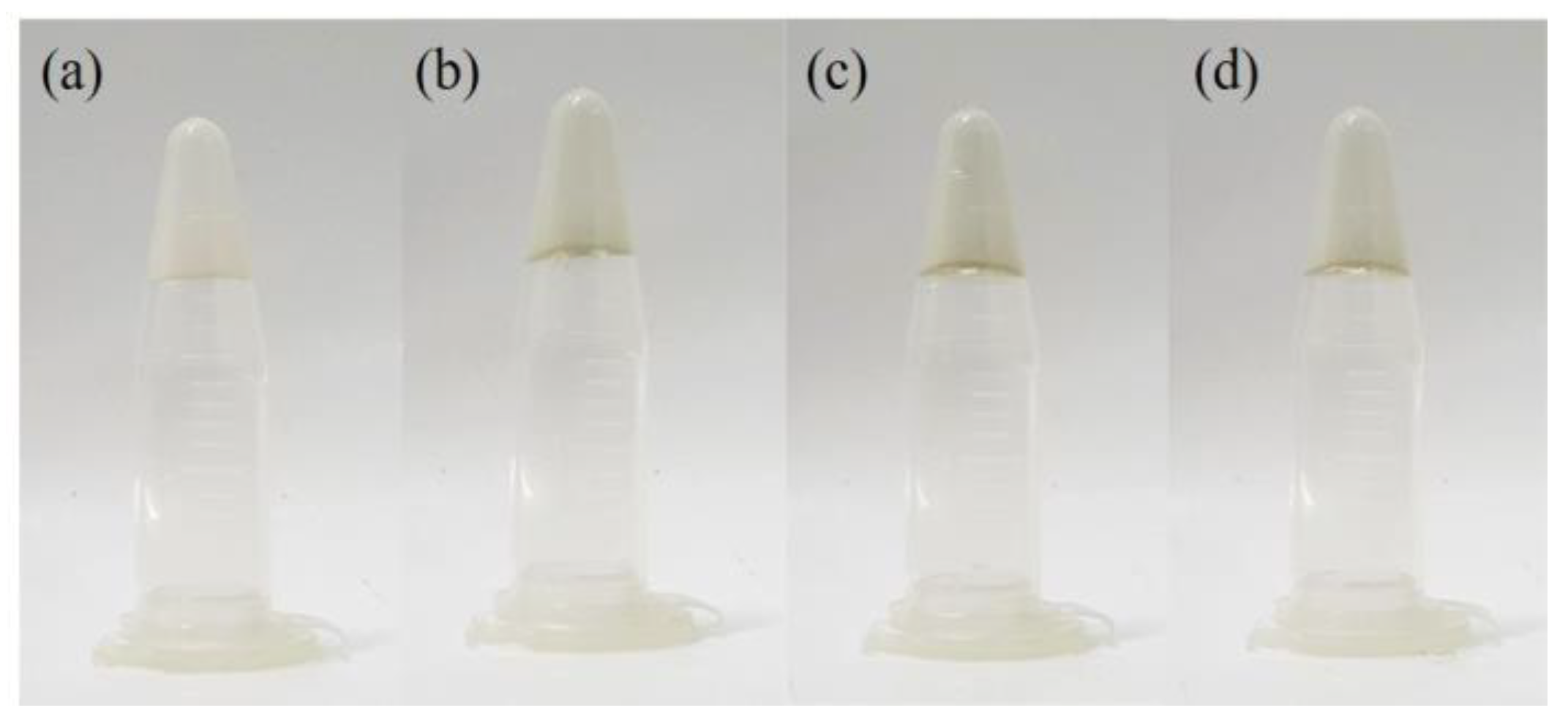
2.2. Preparation of Hydrogels
2.3. Characterizations
2.4. Mechanical Measurements
2.5. Biocompatibility of Hydrogels
3. Results and Discussion
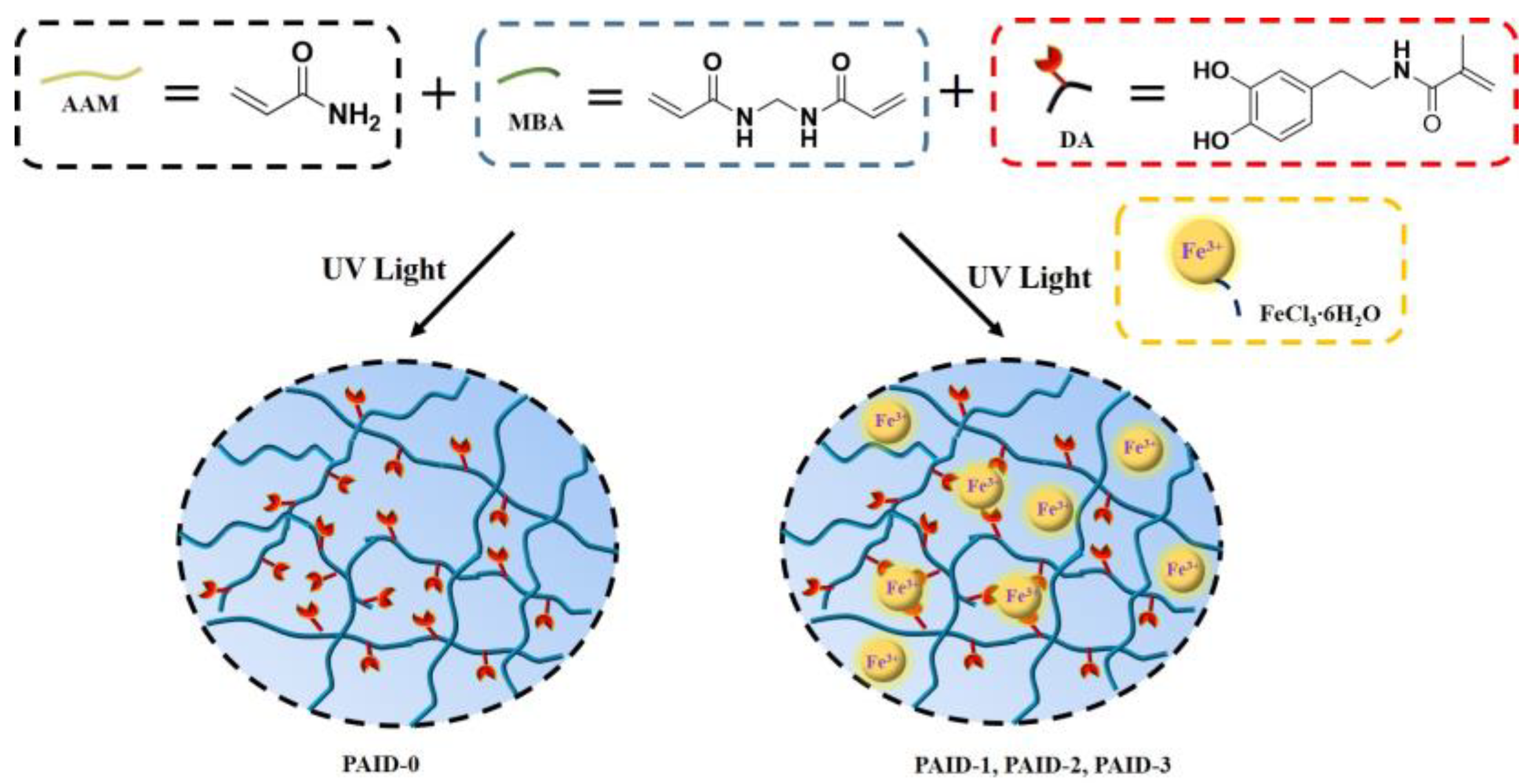
3.1. Preparation of PAID Hydrogels
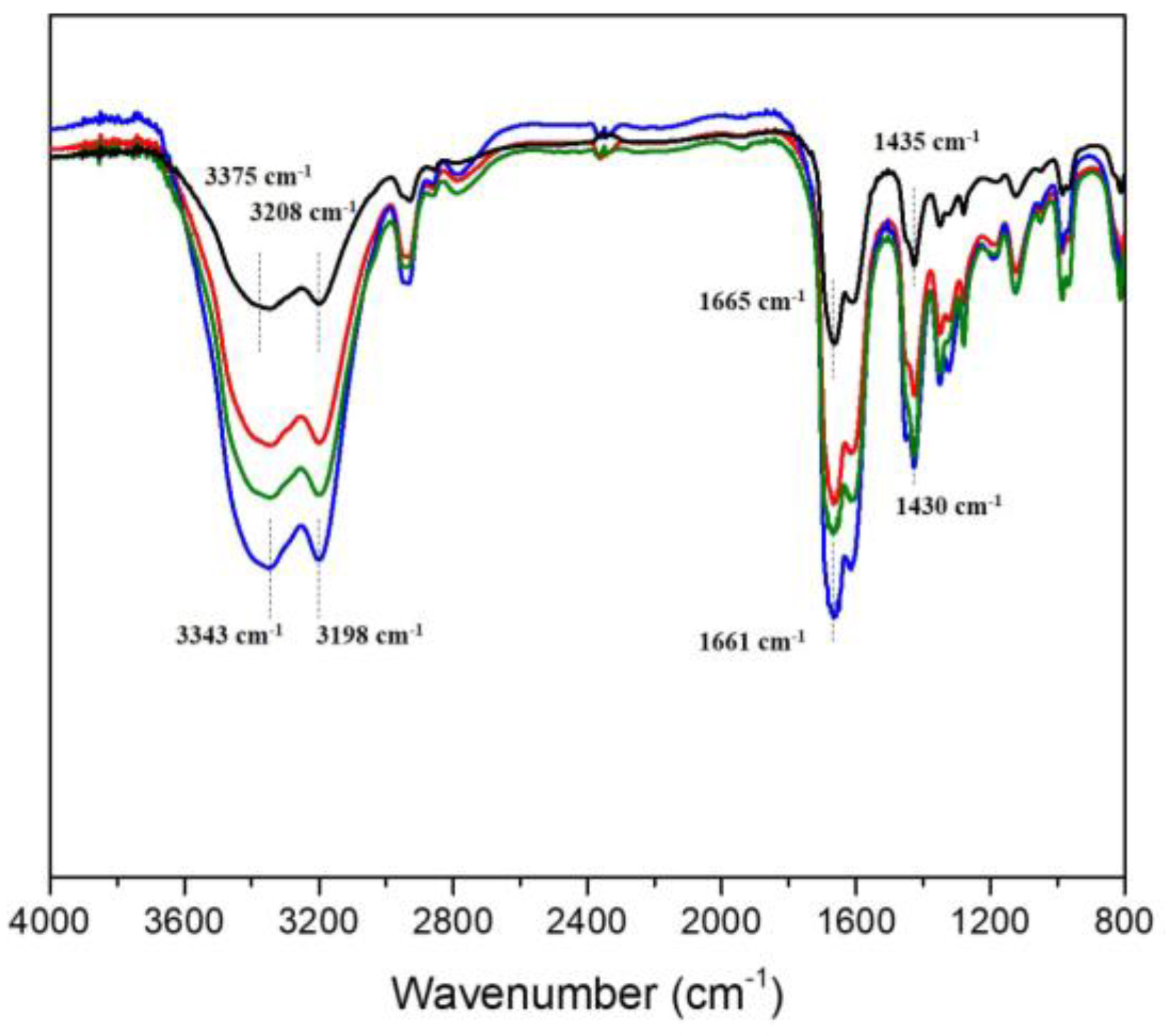
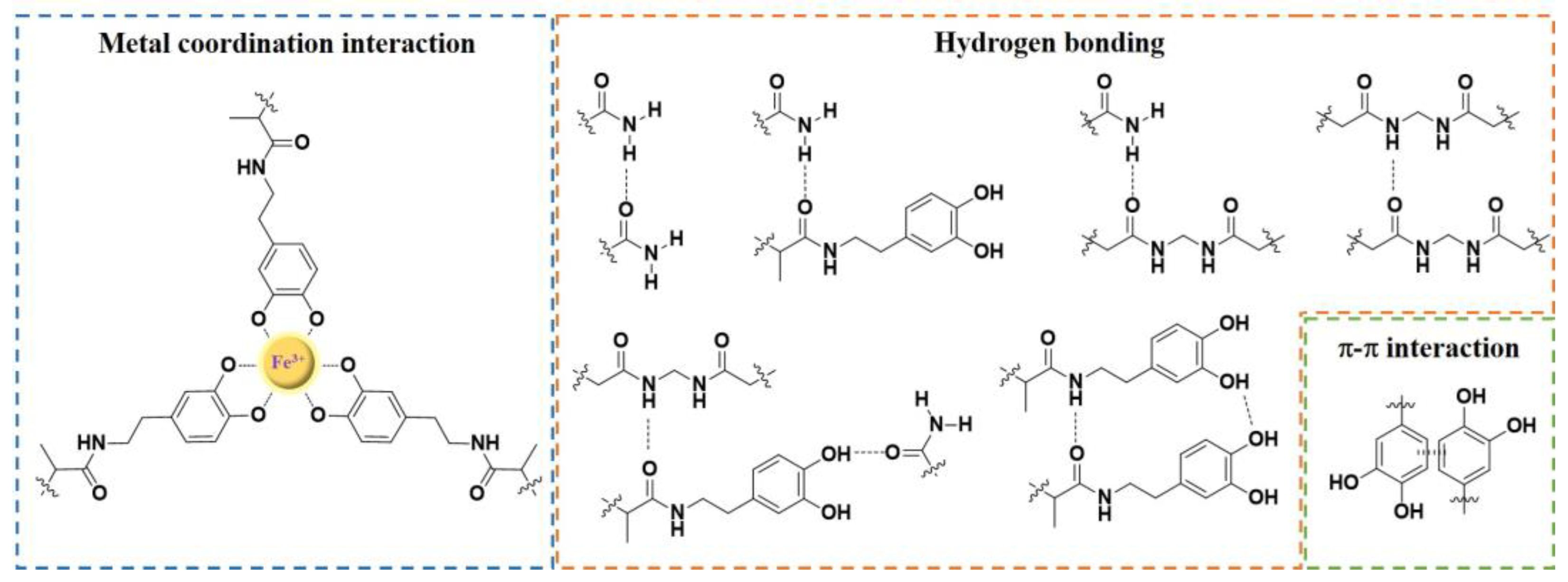
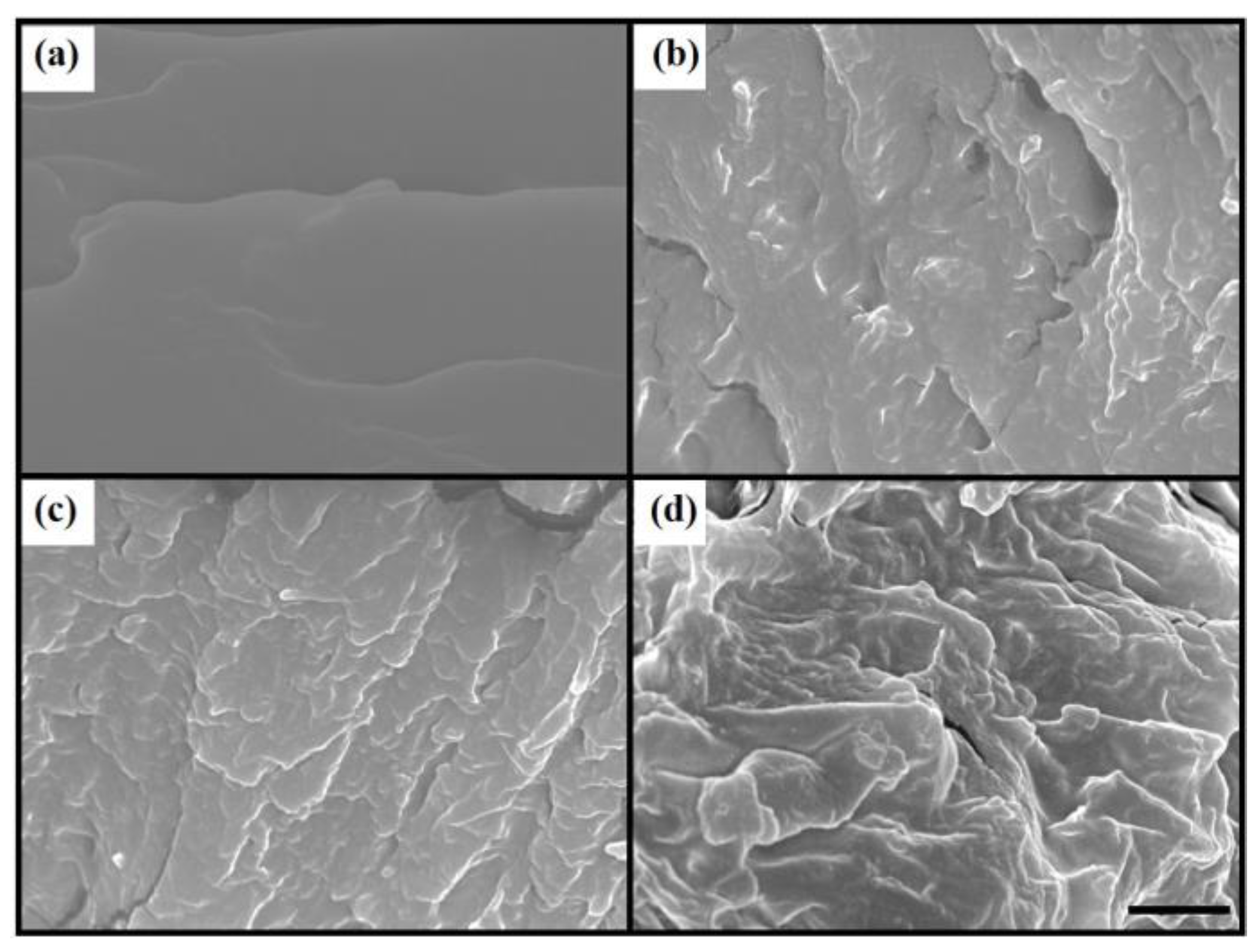
3.2. Spectral Analysis and Morphologies of PAID Hydrogels
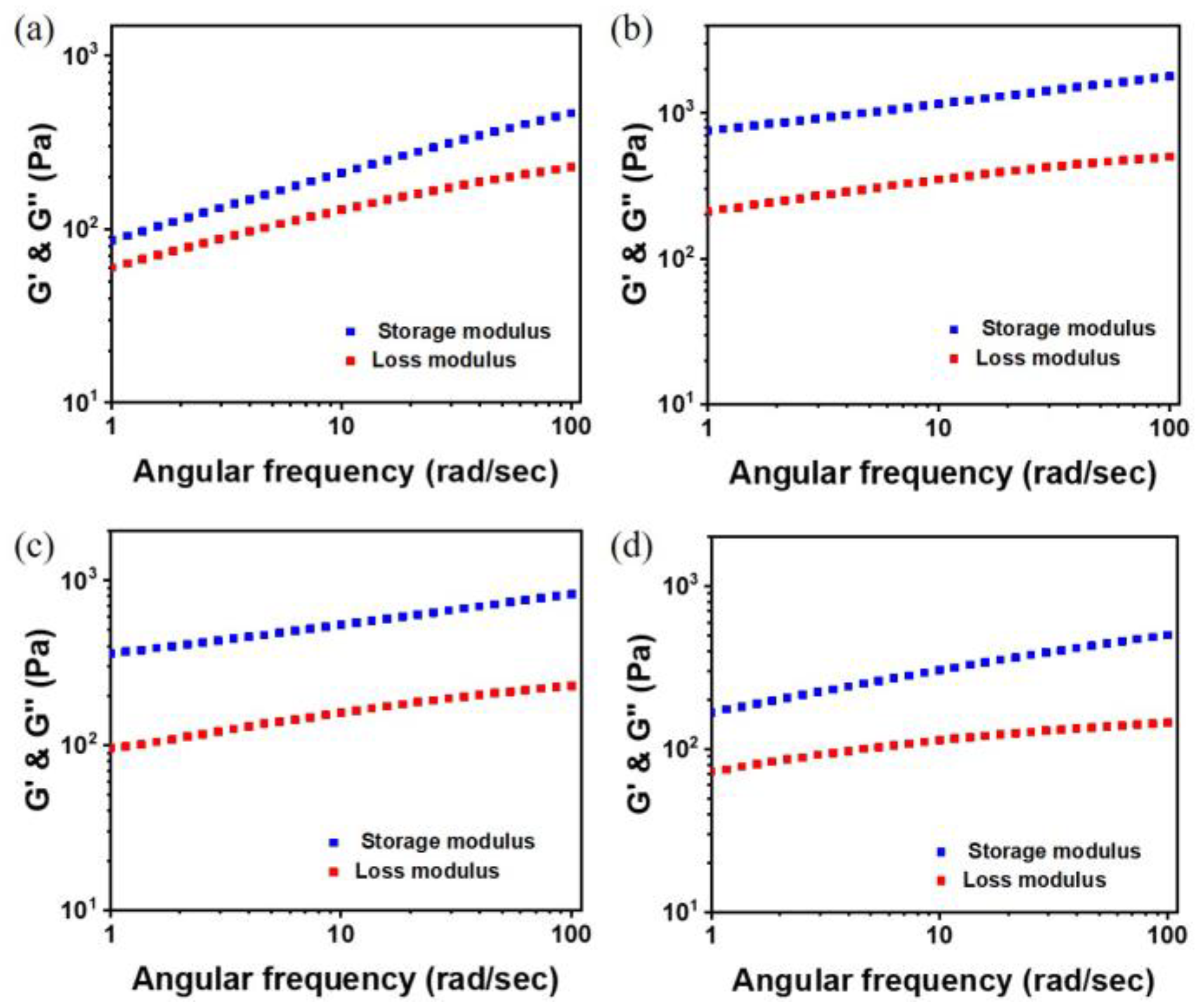
3.3. Mechanical Properties of PAID Hydrogels
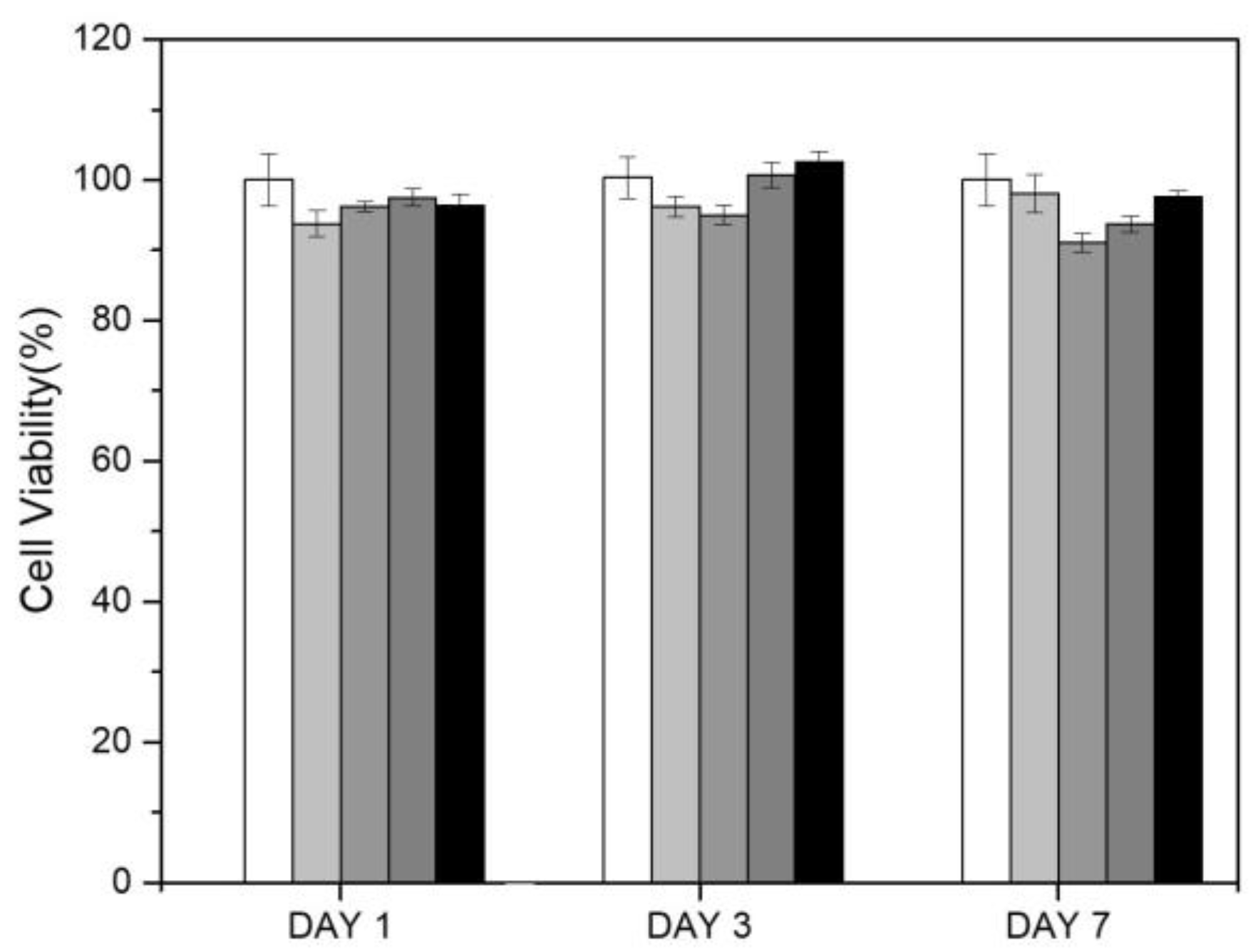
3.4. Biocompatibility of PAID Hydrogels
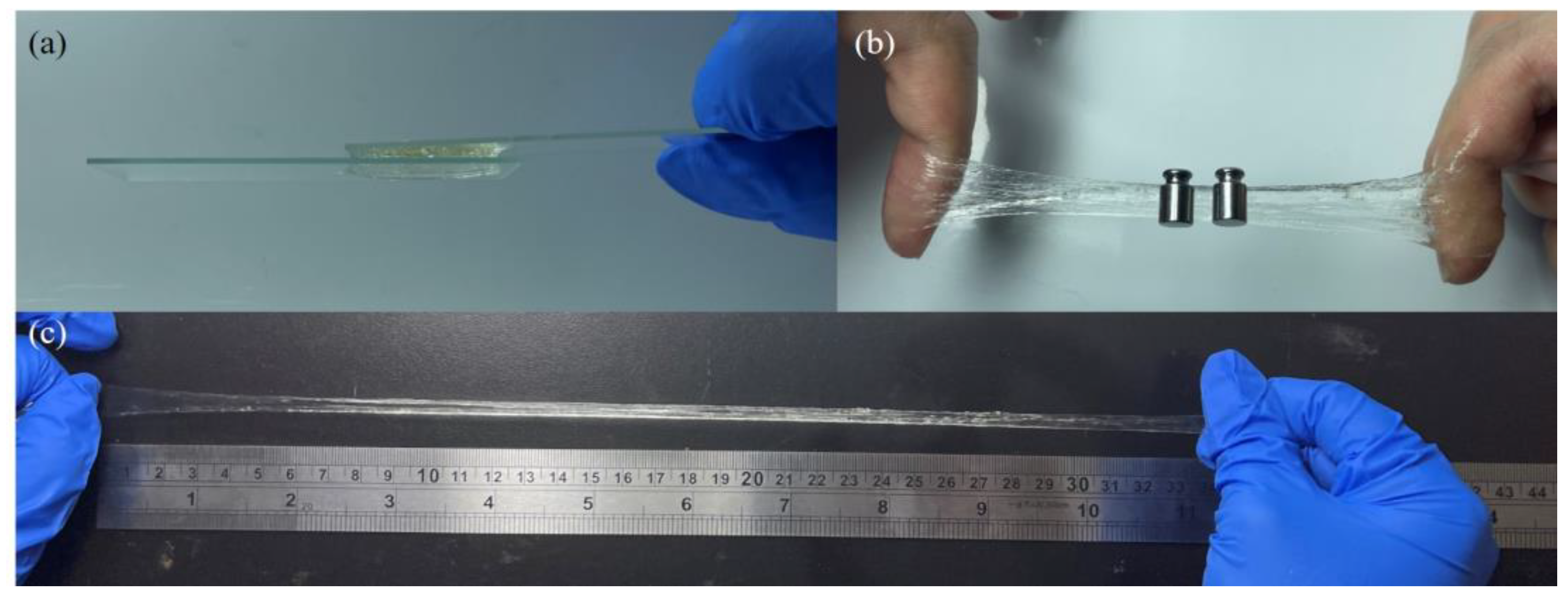
3.5. Adhesive and Stretchable Capacities of PAID Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P. Materials both Tough and Soft. Science 2014, 344, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C 2020, 114, 111023. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; Loh, X.J.; Jones, S.T.; Biedermann, F.; Dreiss, C.A.; Scherman, O.A. Ultrahigh-Water-Content Supramolecular Hydrogels Exhibiting Multistimuli Responsiveness. J. Am. Chem. Soc. 2012, 134, 11767–11773. [Google Scholar] [CrossRef]
- Kuang, X.; Arican, M.O.; Zhou, T.; Zhao, X.; Zhang, Y.S. Functional Tough Hydrogels: Design, Processing, and Biomedical Applications. Acc. Mater. Res. 2023, 4, 101–114. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Ting, M.S.; Travas-Sejdic, J.; Malmström, J. Modulation of hydrogel stiffness by external stimuli: Soft materials for mechanotransduction studies. J. Mater. Chem. B 2021, 9, 7578–7596. [Google Scholar] [CrossRef]
- Huang, J.; Frauenlob, M.; Shibata, Y.; Wang, L.; Nakajima, T.; Nonoyama, T.; Tsuda, M.; Tanaka, S.; Kurokawa, T.; Gong, J.P. Chitin-Based Double-Network Hydrogel as Potential Superficial Soft-Tissue-Repairing Materials. Biomacromolecules 2020, 21, 4220–4230. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Song, W.J.; Sun, J.-Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Ouyang, Y.; Huang, G.; Cui, J.; Zhu, H.; Yan, G.; Mei, Y. Advances and Challenges of Hydrogel Materials for Robotic and Sensing Applications. Chem. Mater. 2022, 34, 9307–9328. [Google Scholar] [CrossRef]
- Jing, L.; Hsiao, L.-Y.; Li, S.; Yang, H.; Ng, P.L.P.; Ding, M.; Truong, T.V.; Ga, S.-P.; Li, K.; Guo, Y.-X.; et al. 2D-Material-integrated hydrogels as multifunctional protective skins for soft robots. Mater. Horiz. 2021, 8, 2065–2078. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xu, S.; Ma, H.; Li, C.; Huang, Z. Photoresponsive hydrogel-based soft robot: A review. Mater. Today Bio 2023, 20, 100657. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Luo, X.; Zhao, H.; Qiao, C.; Li, J.; Yi, J.; Yang, L.; Oropeza, F.J.; Hu, T.S.; Xu, Q.; et al. Recent advances in biomimetic soft robotics: Fabrication approaches, driven strategies and applications. Soft. Matter. 2022, 18, 7699–7734. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Cao, W.-T.; Ma, M.-G.; Wan, P. Ultrasensitive Wearable Soft Strain Sensors of Conductive, Self-healing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, C.; Su, J.; Zhang, C.; Wei, S.; Tian, W.; Liu, X.; Cheng, W.; Li, X.; Li, X.; et al. A dual-crosslinked self-healing and antibacterial nanocellulose hydrogel for monitoring of human motions. Mater. Des. 2022, 215, 110464. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A. A review on the features, performance and potential applications of hydrogel-based wearable strain/pressure sensors. Adv. Colloid Interface Sci. 2021, 298, 102553. [Google Scholar] [CrossRef] [PubMed]
- Bashari, A.; Shirvan, A.R.; Shakeri, M. Cellulose-based hydrogels for personal care products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Xue, K.; Liow, S.S.; Karim, A.A.; Li, Z.; Loh, X.J. A Recent Perspective on Noncovalently Formed Polymeric Hydrogels. Chem. Rec. 2018, 18, 1517–1529. [Google Scholar] [CrossRef]
- Xu, K.; Shen, K.; Yu, J.; Yang, Y.; Wei, Y.; Lin, P.; Zhang, Q.; Xiao, C.; Zhang, Y.; Cheng, Y. Ultradurable Noncovalent Cross-Linked Hydrogels with Low Hysteresis and Robust Elasticity for Flexible Electronics. Chem. Mater. 2022, 34, 3311–3322. [Google Scholar] [CrossRef]
- Panzer, M.J. Holding it together: Noncovalent cross-linking strategies for ionogels and eutectogels. Mater. Adv. 2022, 3, 7709–7725. [Google Scholar] [CrossRef]
- Panja, S.; Seddon, A.; Adams, D.J. Controlling hydrogel properties by tuning non-covalent interactions in a charge complementary multicomponent system. Chem. Sci. 2021, 12, 11197–11203. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, J.; Shen, K.; Wang, R.; Du, J.; Zhao, X.; Yang, Y.; Xu, K.; Zhang, Q.; Zhang, Y.; et al. Highly Stretchable Nanocomposite Hydrogels with Outstanding Antifatigue Fracture Based on Robust Noncovalent Interactions for Wound Healing. Chem. Mater. 2021, 33, 6453–6463. [Google Scholar] [CrossRef]
- Yu, A.C.; Lian, H.; Kong, X.; Hernandez, H.L.; Qin, J.; Appel, E.A. Physical networks from entropy-driven non-covalent interactions. Nat. Commun. 2021, 12, 746. [Google Scholar] [CrossRef] [PubMed]
- Pinnataip, R.; Lee, B.P. Oxidation Chemistry of Catechol Utilized in Designing Stimuli-Responsive Adhesives and Antipathogenic Biomaterials. ACS Omega 2021, 6, 5113–5118. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, J.; Wang, J.; Zeng, H.; Yu, J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, S.; Oh, D.X.; Masic, A.; Cha, H.J.; Hwang, D.S. Mussel-inspired adhesive protein-based electrospun nanofibers reinforced by Fe(iii)–DOPA complexation. J. Mater. Chem. B 2015, 3, 112–118. [Google Scholar] [CrossRef]
- Li, L.; Smitthipong, W.; Zeng, H. Mussel-inspired hydrogels for biomedical and environmental applications. Polym. Chem. 2015, 6, 353–358. [Google Scholar] [CrossRef]
- Li, X.; Qiu, H.; Gao, P.; Yang, Y.; Yang, Z.; Huang, N. Synergetic coordination and catecholamine chemistry for catalytic generation of nitric oxide on vascular stents. NPG Asia Mater. 2018, 10, 482–496. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y. The molecular mechanisms underlying mussel adhesion. Nanoscale Adv. 2019, 1, 4246–4257. [Google Scholar] [CrossRef]
- Dutta, A.; Ghosal, K.; Sarkar, K.; Pradhan, D.; Das, R.K. From ultrastiff to soft materials: Exploiting dynamic metal–ligand cross-links to access polymer hydrogels combining customized mechanical performance and tailorable functions by controlling hydrogel mechanics. Chem. Eng. J. 2021, 419, 129528. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Z.; Liu, Y.; Huang, Z.; Chai, C.; Hao, J. Multiple Cross-Linking-Dominated Metal–Ligand Coordinated Hydrogels with Tunable Strength and Thermosensitivity. ACS Appl. Polym. Mater. 2019, 1, 2370–2378. [Google Scholar] [CrossRef]
- Gan, F.; Wang, J.; Wen, J.; Zhou, J.; Mo, J.; Han, S.; Wu, Y.; Yi, N. Preparation of polyimide-metal complexes with tunable Sol-Gel transition by Introduction of Zn(II)-Coordination. Polym. Test. 2022, 111, 107615. [Google Scholar] [CrossRef]
- Sun, W.; Xue, B.; Fan, Q.; Tao, R.; Wang, C.; Wang, X.; Li, Y.; Qin, M.; Wang, W.; Chen, B.; et al. Molecular engineering of metal coordination interactions for strong, tough, and fast-recovery hydrogels. Sci. Adv. 2020, 6, 9531. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Ding, H.; Qian, J.; Yin, J.; Wu, Z.L.; Song, Y.; Zheng, Q. Metal-Coordination Complexes Mediated Physical Hydrogels with High Toughness, Stick–Slip Tearing Behavior, and Good Processability. Macromolecules 2016, 49, 9637–9646. [Google Scholar] [CrossRef]
- Ju, H.; Zhu, Q.L.; Zuo, M.; Liang, S.; Du, M.; Zheng, Q.; Wu, Z.L. Toughening Hydrogels by Forming Robust Hydrazide-Transition Metal Coordination Complexes. Chem. Eur. J. 2023, 29, e202300969. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [PubMed]
- Vehlow, D.; Wong, J.P.H.; Urban, B.; Weisspflog, J.; Gebert, A.; Schumacher, M.; Gelinsky, M.; Stamm, M.; Muller, M. Catechol Containing Polyelectrolyte Complex Nanoparticles as Local Drug Delivery System for Bortezomib at Bone Substitute Materials. Pharmaceutics 2020, 12, 799. [Google Scholar] [CrossRef]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol polymers for pH-responsive, targeted drug delivery to cancer cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef]
- Chandra Joshi, D.; Ashokan, A.; Jayakannan, M. l-Amino Acid Based Phenol- and Catechol-Functionalized Poly(ester-urethane)s for Aromatic pi-Interaction Driven Drug Stabilization and Their Enzyme-Responsive Delivery in Cancer Cells. ACS Appl. Bio Mater. 2022, 5, 5432–5444. [Google Scholar] [CrossRef]
- Glass, P.; Chung, H.; Washburn, N.R.; Sitti, M. Enhanced Reversible Adhesion of Dopamine Methacrylamide-Coated Elastomer Microfibrillar Structures under Wet Conditions. Langmuir 2009, 25, 6607–6612. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.-N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Double Reversible Networks: Improvement of Self-Healing in Hybrid Materials via Combination of Diels–Alder Cross-Linking and Hydrogen Bonds. Macromolecules 2018, 51, 6099–6110. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Lin, Y.-C.; Chang, J.-W.; Liu, Y.-H.; Lin, H.-C. Intramolecular Interactions of a Phenyl/Perfluorophenyl Pair in the Formation of Supramolecular Nanofibers and Hydrogels. Angew. Chem. Int. Ed. 2014, 53, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-W.; Ji, D.-H.; Zhang, Y.-S.; Lee, C.; Yeh, M.-Y. Electroactive and Stretchable Hydrogels of 3,4-Ethylenedioxythiophene/thiophene Copolymers. ACS Omega 2023, 8, 6753–6761. [Google Scholar] [CrossRef]
- Yildiz, G.; Çatalgil-Giz, H.; Kadirgan, F. Electrochemically prepared acrylamide/N,N′-methylene bisacrylamide gels. J. Appl. Electrochem. 2000, 30, 71–75. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, H.; Gao, B.; Zhang, S.; Zhao, C.; Zhou, Y.; Xu, B. Fabricating an anionic polyacrylamide (APAM) with an anionic block structure for high turbidity water separation and purification. RSC Adv. 2017, 7, 28918–28930. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Mohamed, A.; Ji, Q.; Jia, H. High strength and flexible aramid nanofiber conductive hydrogels for wearable strain sensors. J. Mater. Chem. C 2021, 9, 575–583. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, J.; Yang, X.; Fan, D.; Cao, J.; Luo, Y.; Zhang, X. Naturally Derived Wearable Strain Sensors with Enhanced Mechanical Properties and High Sensitivity. ACS Appl. Mater. Interfaces 2020, 12, 22163–22169. [Google Scholar] [CrossRef]
- Ramli, H.; Zainal, N.F.A.; Hess, M.; Chan, C.H. Basic principle and good practices of rheology for polymers for teachers and beginners. Chem. Teach. Int. 2022, 4, 307–326. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef]
- Pilipenko, I.; Murzova, A.; Savin, A.; Mohamed, A.A.; Vladimirova, E.; Koshel, E.; Shamova, O.; Kumacheva, E. Dual-Function Hydrogel Dressings with a Dynamic Exchange of Iron Ions and an Antibiotic Drug for Treatment of Infected Wounds. ACS Appl. Bio Mater. 2023, 6, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, K.; Wang, Z.; Xiao, J.; Tang, Z.; Li, H.; Yi, W.; Li, Z.; Luo, Y.; Li, J.; et al. Rapid In Situ Deposition of Iron-Chelated Polydopamine Coating on the Polyacrylamide Hydrogel Dressings for Combined Photothermal and Chemodynamic Therapy of Skin Wound Infection. ACS Appl. Bio Mater. 2022, 5, 4541–4553. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, H.; Wang, S.; Zhao, J.; Hu, L. Preparation and Characterization of Biocompatible Iron/Zirconium/Polydopamine/Carboxymethyl Chitosan Hydrogel with Fenton Catalytic Properties and Photothermal Efficacy. Gels 2023, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ryu, S.B.; Park, K.D. Preparation and characterization of dual-crosslinked gelatin hydrogel via Dopa-Fe3+ complexation and fenton reaction. J. Ind. Eng. Chem. 2018, 58, 105–112. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Owen, T.C.; Cory, A.H.; Gory, J.G. 5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans As cell-viability indicators. Bioorganic Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar]
- Yeh, M.-Y.; Zhao, J.-Y.; Hsieh, Y.-R.; Lin, J.-H.; Chen, F.-Y.; Chakravarthy, R.D.; Chung, P.-C.; Lin, H.-C.; Hung, S.-C. Reverse thermo-responsive hydrogels prepared from Pluronic F127 and gelatin composite materials. RSC Adv. 2017, 7, 21252–21257. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tidditt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Guo, M.; Li, G.; Cai, M.; Hou, X.; Huang, K.; Tang, J.; Guo, C.F. A Tough Hydrogel Adhesive for the Repair of Archeological Pottery. Nano Lett. 2023, 23, 1371–1378. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Fang, Y.; Li, P.; Zhou, W.; Wang, Z.; Chen, M.; Liu, H. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater. Sci. Eng. C 2020, 109, 110649. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Lu, L.; Du, R.; Ma, W.; Cai, Y.; An, X.; Wu, H.; Luo, Q.; Xu, Q.; et al. A Highly-Adhesive and Self-Healing Elastomer for Bio-Interfacial Electrode. Adv. Funct. Mater. 2021, 31, 2006432. [Google Scholar] [CrossRef]
- Ivarsson, M.; Prenkert, M.; Cheema, A.; Wretenberg, P.; Andjelkov, N. Mussel Adhesive Protein as a Promising Alternative to Fibrin for Scaffold Fixation during Cartilage Repair Surgery. Cartilage 2021, 13, 663S–671S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, C.; Hou, R.; Li, J.; Cao, Y.; Dong, S.; Lin, C.; Pan, J. Investigation and application of mussel adhesive protein nanocomposite film-forming inhibitor for reinforced concrete engineering. Corros. Sci. 2019, 153, 333–340. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Wang, J.; Zeng, H. Anti-biofouling materials and surfaces based on mussel-inspired chemistry. Mater. Adv. 2021, 2, 2216–2230. [Google Scholar] [CrossRef]
- Guo, G.; Sun, J.; Wu, Y.; Wang, J.; Zou, L.Y.; Huang, J.J.; Ren, K.F.; Liu, C.M.; Wu, Z.L.; Zheng, Q.; et al. Tough complex hydrogels transformed from highly swollen polyelectrolyte hydrogels based on Cu2+ coordination with anti-bacterial properties. J. Mater. Chem. B 2022, 10, 6414–6424. [Google Scholar] [CrossRef]
- Dou, X.; Wang, H.; Yang, F.; Shen, H.; Wang, X.; Wu, D. One-Step Soaking Strategy toward Anti-Swelling Hydrogels with a Stiff “Armor”. Adv. Sci. 2023, 10, e2206242. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Lu, W.; Li, W.; Chen, S.; Chen, T. Mechanical tough and multicolor aggregation-induced emissive polymeric hydrogels for fluorescent patterning. Nanoscale Adv. 2023, 5, 725–732. [Google Scholar] [CrossRef]
- Zhang, H.; He, J.; Qu, J. High-strength, tough, and anti-swelling Schiff base hydrogels with fluorescent encryption writing, solvent response and double shape memory functions. Eur. Polym. J. 2022, 178, 111487. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Cai, C.; Zhang, Y.; Guo, S.; Fu, Y.; Tan, S.C. Dual-Network Liquid Metal Hydrogel with Integrated Solar-Driven Evaporation, Multi-Sensory Applications, and Electricity Generation via Enhanced Light Absorption and Bénard–Marangoni Effect. Adv. Funct. Mater. 2022, 32, 2206287. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Duan, L.; Gao, G. Ultra-stretchable wearable strain sensors based on skin-inspired adhesive, tough and conductive hydrogels. Chem. Eng. J. 2019, 365, 10–19. [Google Scholar] [CrossRef]
- Dong, L.; Wang, M.; Wu, J.; Zhu, C.; Shi, J.; Morikawa, H. Stretchable, Adhesive, Self-Healable, and Conductive Hydrogel-Based Deformable Triboelectric Nanogenerator for Energy Harvesting and Human Motion Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9126–9137. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, Healable, and Adhesive Epidermal Sensors Assembled from Mussel-Inspired Conductive Hybrid Hydrogel Framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W.; Yang, W.; Chen, J.; Peng, Q.; Wang, T.; Yang, D.; Wang, J.; Zhang, H.; Zeng, H. Stretchable, compressible, and conductive hydrogel for sensitive wearable soft sensors. J. Colloid Interface Sci. 2022, 618, 111–120. [Google Scholar] [CrossRef]
- Fu, Q.; Hao, S.; Meng, L.; Xu, F.; Yang, J. Engineering Self-Adhesive Polyzwitterionic Hydrogel Electrolytes for Flexible Zinc-Ion Hybrid Capacitors with Superior Low-Temperature Adaptability. ACS Nano 2021, 15, 18469–18482. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional hydrogel as wound dressing for intelligent wound monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Huang, H.-S.; Wang, Y.-Y.; Zhang, Y.-S.; Chakravarthy, R.D.; Yeh, M.-Y.; Lin, H.-C.; Wei, J. Stretchable, Adhesive, and Biocompatible Hydrogel Based on Iron–Dopamine Complexes. Polymers 2023, 15, 4378. https://doi.org/10.3390/polym15224378
Lee C, Huang H-S, Wang Y-Y, Zhang Y-S, Chakravarthy RD, Yeh M-Y, Lin H-C, Wei J. Stretchable, Adhesive, and Biocompatible Hydrogel Based on Iron–Dopamine Complexes. Polymers. 2023; 15(22):4378. https://doi.org/10.3390/polym15224378
Chicago/Turabian StyleLee, Celine, He-Shin Huang, Yun-Ying Wang, You-Sheng Zhang, Rajan Deepan Chakravarthy, Mei-Yu Yeh, Hsin-Chieh Lin, and Jeng Wei. 2023. "Stretchable, Adhesive, and Biocompatible Hydrogel Based on Iron–Dopamine Complexes" Polymers 15, no. 22: 4378. https://doi.org/10.3390/polym15224378




