Development of Poly(vinyl alcohol) Grafted Glycidyl Methacrylate/Cellulose Nanofiber Injectable Hydrogels for Meniscus Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNF
2.3. Grafting of PVA-g-GMA
2.4. Characterization of PVA-g-GMA
2.4.1. Chemical Structure
2.4.2. Degree of Methacrylate Substitution of PVA
2.4.3. Solubility of PVA-g-GMA
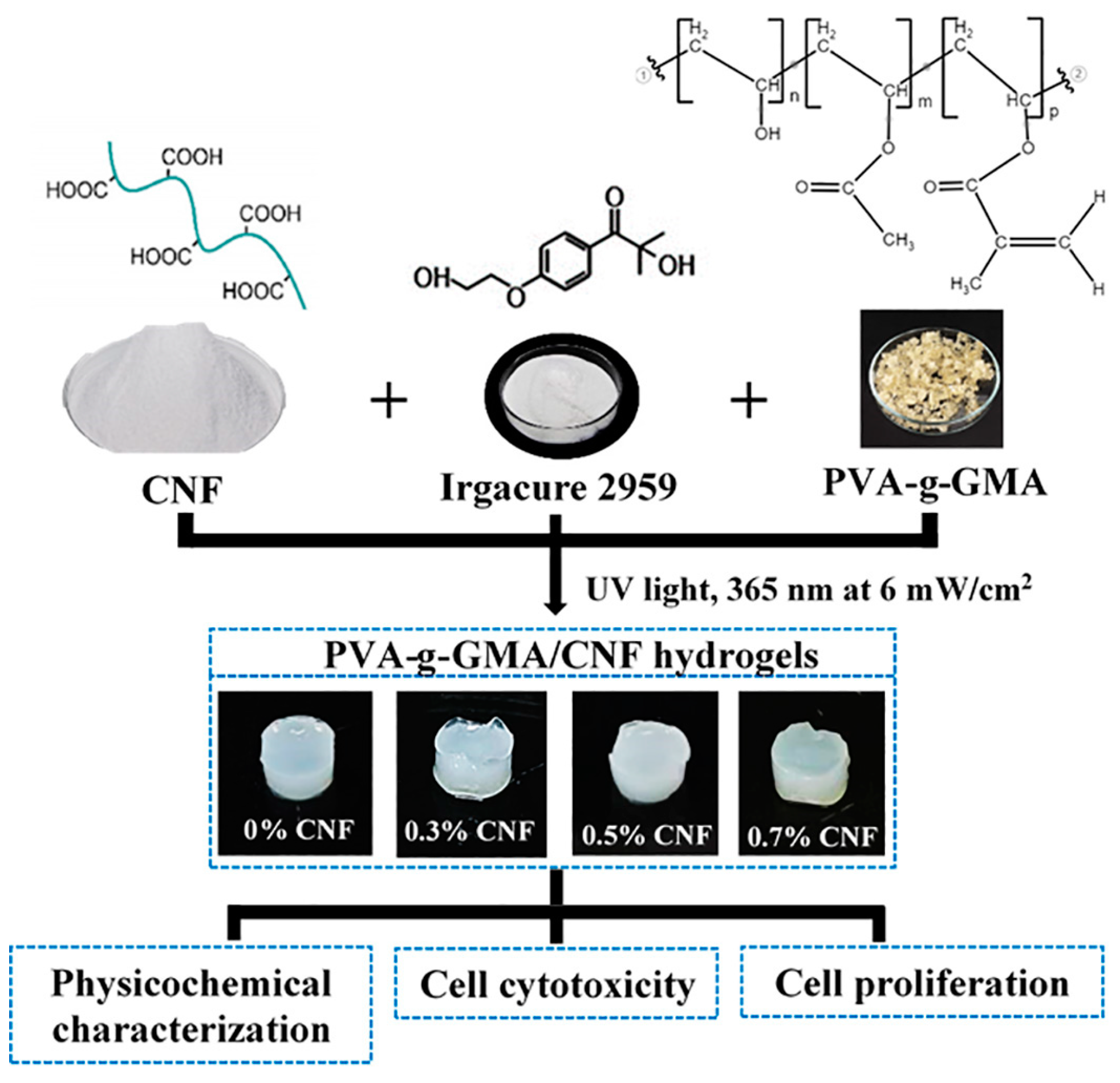
2.5. Preparation of PVA-g-GMA/CNF Injectable Hydrogels
2.6. Characterization of Injectable Hydrogel
2.6.1. Morphology and Pore Size
2.6.2. Porosity
2.6.3. Gel Fraction
2.6.4. Water Content and Swelling
2.6.5. Compressive Properties
2.6.6. Chemical Structure and Interaction
2.7. In Vitro Cell Cytotoxicity
2.8. Cell Proliferation
2.9. Statistical Analysis
3. Results and Discussions
3.1. Characterization of PVA-g-GMA
3.1.1. Chemical Structure
3.1.2. Degree of Methacrylate Substitution in PVA
3.1.3. Solubility of PVA-g-GMA
3.2. Effect of UV Radiation Time on Gelation and Gel Fraction of Hydrogel
3.3. Characterization of CNF/PVA-g-GMA Injectable Hydrogels
3.3.1. Physicochemical Characterization
Appearances
Morphology, Pore Size Diameter, and Porosity
Gel Fraction, Water Content, and Swelling
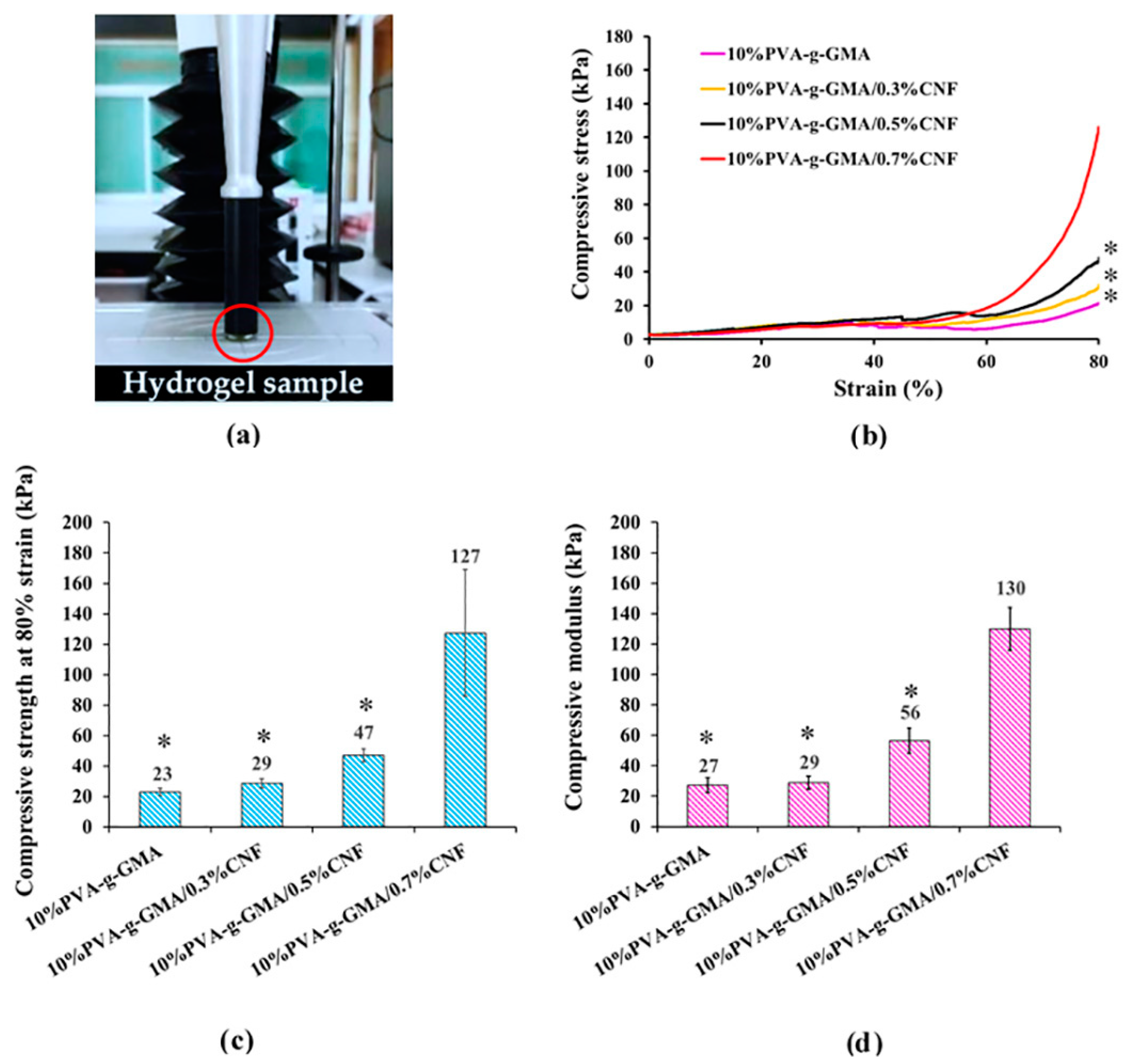
Compressive Properties
Chemical Structure and Interaction
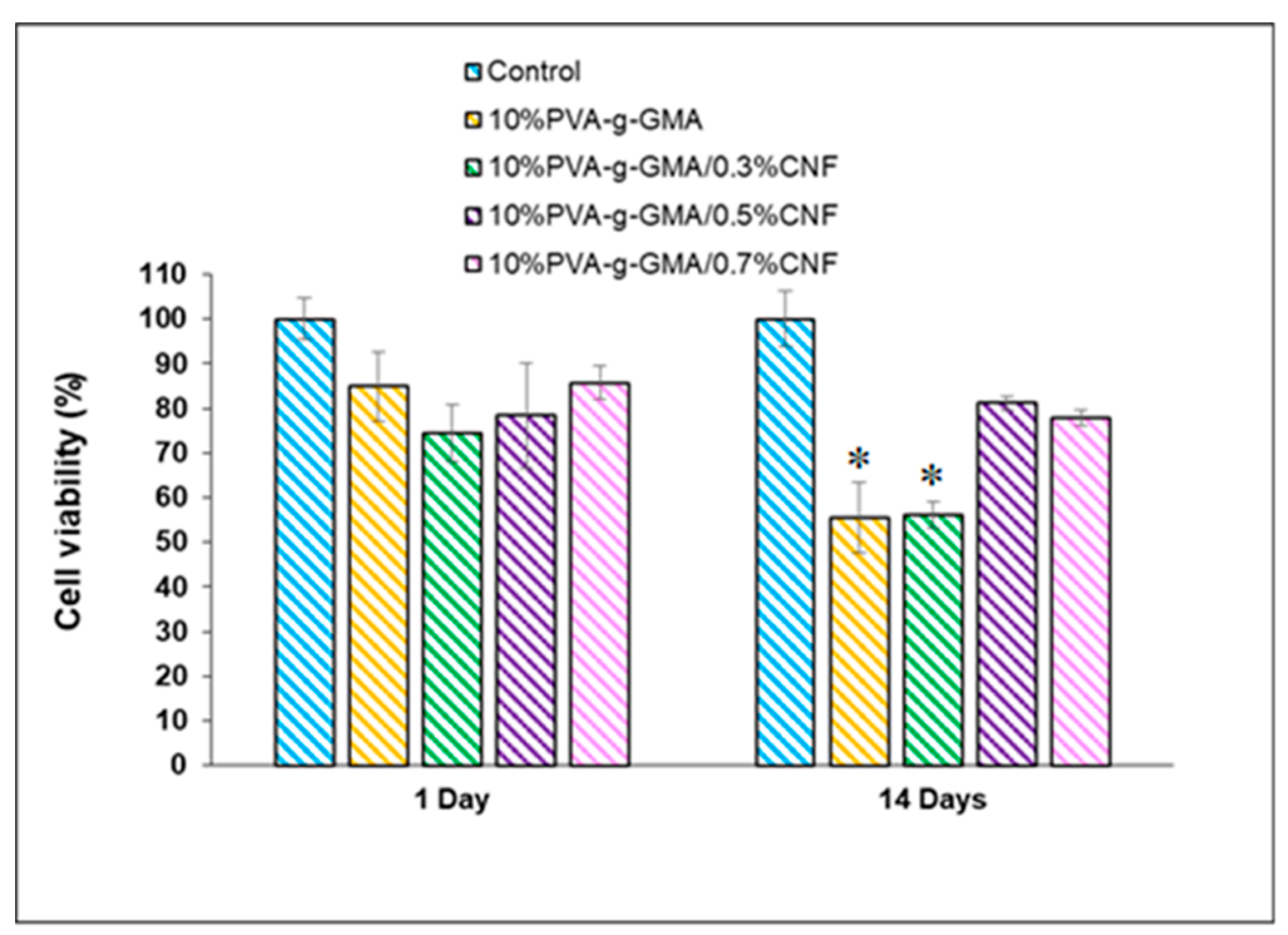
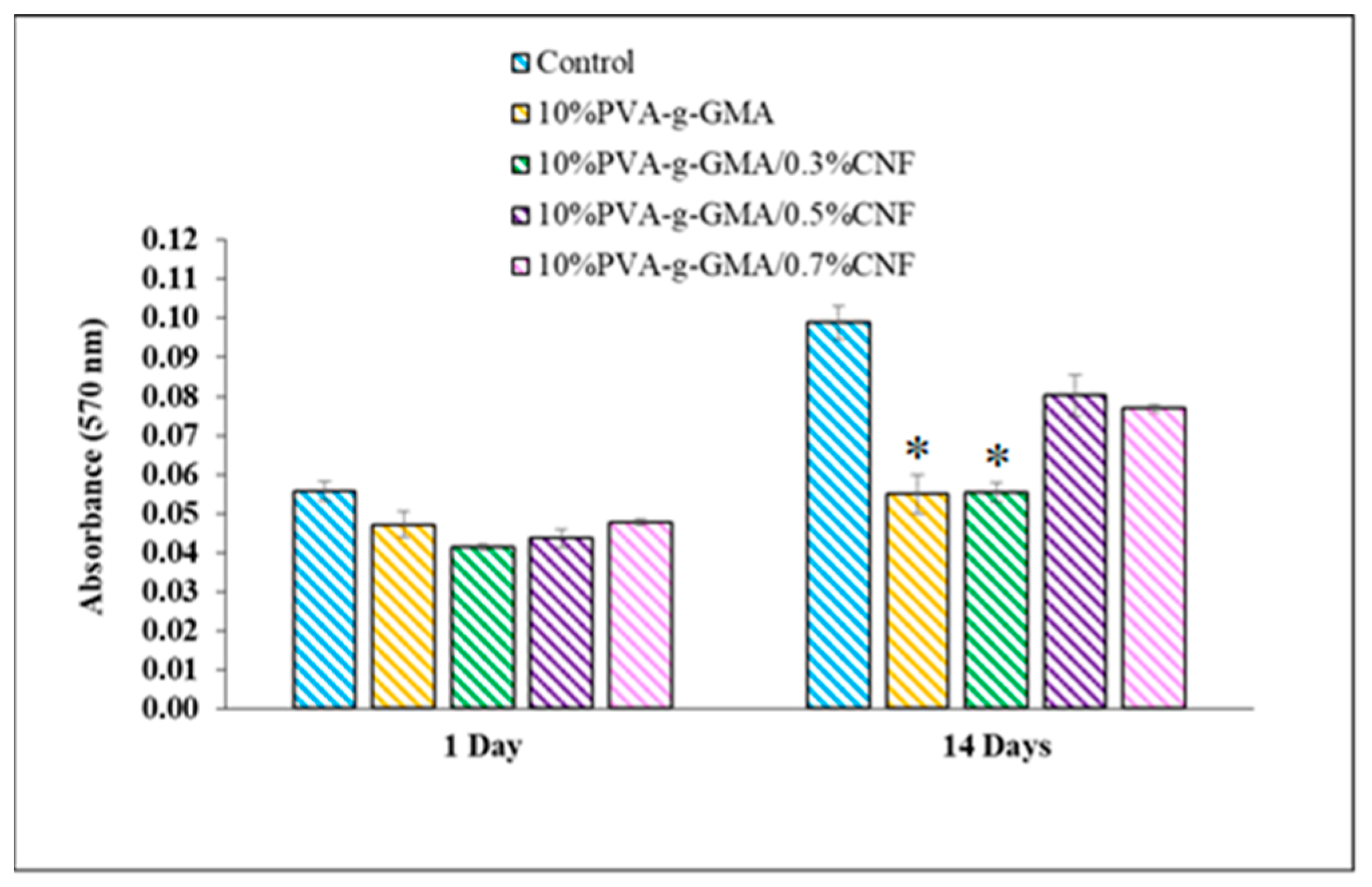
3.3.2. Cell Cytotoxicity
3.3.3. Cell Proliferation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of human knee menisci: Structure, composition, and function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Drosos, G.I.; Pozo, J.L. The causes and mechanisms of meniscal injuries in the sporting and non-sporting environment in an unselected population. Knee 2004, 11, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hu, G.; Dong, Y.; Hu, Y.; Xu, Q. The effect of meniscal tears and resultant partial meniscectomies on the knee contact stresses: A finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Seil, R.; Krych, A.J.; Koga, H. Surgical treatment of complex meniscus tear and disease: State of the art. J. ISAKOS 2021, 6, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rijk, P.C. Meniscal allograft transplantation—Part I: Background, results, graft selection and preservation, and surgical considerations. Arthroscopy 2004, 20, 728–743. [Google Scholar] [CrossRef]
- Bilgen, B.; Jayasuriya, C.T.; Owens, B.D. Current concepts in meniscus tissue engineering and repair. Adv. Healthc. Mater. 2018, 7, 1701407. [Google Scholar] [CrossRef]
- Doblado, L.R.; Ramos, C.M.; Pradas, M.M. Biomaterials for neural tissue engineering. Front. Nanotechnol. 2021, 3, 643507. [Google Scholar] [CrossRef]
- Niu, W.; Guo, W.; Han, S.; Zhu, Y.; Liu, S.; Guo, Q. Cell-based strategies for meniscus tissue engineering. Stem Cells Int. 2016, 2016, 4717184. [Google Scholar] [CrossRef]
- Baker, B.M.; Nathan, A.S.; Huffman, G.R.; Mauck, R.L. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthr. Cartil. 2009, 17, 336–345. [Google Scholar] [CrossRef]
- Liang, Q.; Lu, Y.; Zhang, Q. Hydrogels-based electronic devices for biosensing applications. Smart Stimuli-Responsive Polym. Films Gels 2022, 339–373. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Fibrin sealant: The only approved hemostat, sealant, and adhesive-a laboratory and clinical perspective. ISRN Surg. 2014, 2014, 203943. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Fibrin glue. In Meyler’s Side Effects of Drugs, 16th ed.; Elsevier: Oxford, UK, 2016; pp. 312–313. [Google Scholar]
- Wang, B.; Liu, J.; Niu, D.; Wu, N.; Yun, W.; Wang, W.; Zhang, K.; Li, G.; Yan, S.; Xu, G.; et al. Mussel-inspired bisphosphonated injectable nanocomposite hydrogels with adhesive, self-healing, and osteogenic properties for bone regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Shao, C.; Cui, C.; Xu, F.; Lei, J.; Yang, J. Autonomous self-healing silk fibroin injectable hydrogels formed via surfactant-free hydrophobic association. ACS Appl. Mater. Interfaces 2020, 12, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, H.; Wang, X.; Yu, X.; Li, H.; Tang, K.; Li, Q. Preparation of gelatin-based hydrogels with tunable mechanical properties and modulation on cell-matrix interactions. J. Biomater. Appl. 2021, 36, 902–911. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Omer, A.M.; Abu-Serie, M.M.; Khattab, S.N.; Ahmed, H.M.; Elbardan, A.A. Photopolymerized pva-g-gma hydrogels for biomedical applications: Factors affecting hydrogel formation and bioevaluation tests. Arab. J. Sci. Eng. 2018, 43, 3565–3575. [Google Scholar] [CrossRef]
- Crispim, E. Hydrogels Based on chemically modified poly (vinyl alcohol) (pva-gma) and pva-gma/chondroitin sulfate: Preparation and characterization. Express Polym. Lett. 2012, 6, 383–395. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Kitaoka, T. Surface-carboxylated nanocellulose-based bioadaptive scaffolds for cell culture. Cellulose 2022, 29, 2869–2883. [Google Scholar] [CrossRef]
- Doench, I.; Tran, T.A.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose nanofiber-reinforced chitosan hydrogel composites for intervertebral disc tissue repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef]
- Aarstad, O.; Heggset, E.B.; Pedersen, I.S.; Bjørnøy, S.H.; Syverud, K.; Strand, B.L. Mechanical properties of composite hydrogels of alginate and cellulose nanofibrils. Polymers 2017, 9, 378. [Google Scholar] [CrossRef]
- Qingxiu, L.; Liu, J.; Qin, S.; Pei, S.; Zheng, X.; Tang, K. High mechanical strength gelatin composite hydrogels reinforced by cellulose nanofibrils with unique beads-on-a-string morphology. Int. J. Biol. Macromol. 2020, 164, 1776–1784. [Google Scholar]
- Cai, H.; Sudhir, S.; Wenying, L.; Wei, M.; Xiaodan, Z.; Yulin, D. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Oh, D.X.; Choy, S.; Nguyen, H.-L.; Cha, H.J.; Hwang, D.S. 3D cellulose nanofiber scaffold with homogeneous cell population and long-term proliferation. Cellulose 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Taguchi, T. Engineering an injectable tough tissue adhesive through nanocellulose reinforcement. ACS Appl. Bio Mater. 2020, 3, 9093–9100. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, Y.; Zhang, S.; Ma, P.; Dong, W. Photo-crosslinkable poly (vinyl alcohol)/nanocrystalline cellulose composites with controllable performance and exceptional water vapor barrier property for packaging application. Cellulose 2022, 29, 7721–7734. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, K.; Zhou, D.; Yang, H.; Liu, X.; Yin, X.; Xu, W.; Zhou, Y.; Xiao, P. High-performance photopolymerized poly(vinyl alcohol)/silica nanocomposite hydrogels with enhanced cell adhesion. ACS Appl. Mater. Interfaces 2018, 10, 27692–27700. [Google Scholar] [CrossRef]
- Crispim, E.G.; Piai, J.F.; Schüquel, I.T.; Rubira, A.F.; Muniz, E.C. Functionalization of poly(vinyl alcohol) by addition of methacryloyl groups: Characterization by FTIR and NMR and optimization of reaction conditions by RSM. e-Polymers 2006, 6, 062. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Liu, Z. Facile fabrication of tough photocrosslinked polyvinyl alcohol hydrogels with cellulose nanofibrils reinforcement. Polymer 2019, 173, 103–109. [Google Scholar] [CrossRef]
- Wong, D.Y.; Ranganath, T.; Kasko, A.M. Low-dose, long-wave UV light does not affect gene expression of human mesenchymal stem cells. PLoS ONE 2015, 29, e0139307. [Google Scholar] [CrossRef]
- Jeencham, R.; Tawonsawatruk, T.; Numpaisal, P.; Ruksakulpiwat, Y. Reinforcement of Injectable Hydrogel for Meniscus Tissue Engineering by Using Cellulose Nanofiber from Cassava Pulp. Polymers 2023, 15, 2092. [Google Scholar] [CrossRef]
- Lu, J.; Huang, J.; Jin, J.; Xie, C.; Xue, B.; Lai, J.; Cheng, B.; Li, L.; Jiang, Q. The design and characterization of a strong bio-ink for meniscus regeneration. Int. J. Bioprint. 2022, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Q.; Fan, C.; Xiao, M.; Yang, R.; Yao, Y.; Wu, Y.; Nie, X.; Wang, H.; Liu, W. A gel microparticle-based self-thickening strategy for 3D printing high-modulus hydrogels skeleton cushioned with PNAGA hydrogel mimicking anisotropic mechanics of meniscus. Bioact. Mater. 2023, 26, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Shadi, M.; Talaei-Khozani, T.; Sani, M.; Hosseinie, R.; Parsaei, H.; Vojdani, Z. Optimizing artificial meniscus by mechanical stimulation of the chondrocyte-laden acellular meniscus using ad hoc bioreactor. Stem Cell Res. Ther. 2022, 13, 382. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, C.; Zhang, Q.; Liu, Y.; Cui, C.; Liu, B.; Wu, T.; Zhang, X.; Liu, W. A self-thickening and self-strengthening strategy for 3D printing high-strength and antiswelling supramolecular polymer hydrogels as meniscus substitutes. Adv. Funct. Mater. 2021, 31, 2100462. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Ozmen, M.M.; Okay, O. Superfast responsive ionic hydrogels: Effect of the monomer concentration. J. Macromol. Sci. Phys. 2006, 43, 1215–1225. [Google Scholar] [CrossRef]
- Kramschuster, A.; Turng, L.S. 17-Fabrication of Tissue Engineering Scaffolds. In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 427–446. [Google Scholar]
- Cui, S.; Zhang, S.; Coseri, S. An injectable and self-healing cellulose nanofiber-reinforced alginate hydrogel for bone repair. Carbohydr. Polym. 2023, 300, 120243. [Google Scholar] [CrossRef] [PubMed]
- Baghbadorani, N.B.; Behzad, T.; Karimi Darvanjooghi, M.H.; Etesamin, N. Modelling of water absorption kinetics and biocompatibility study of synthesized cellulose nanofiber-assisted starch-graft-poly (acrylic acid) hydrogel nanocomposites. Cellulose 2020, 27, 9927–9945. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Patent Summary for US-7008635-B1, Hyrogels for Orthopedic Repair. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-7008635-B1 (accessed on 25 December 2022).
- Wicaksono, R.; Syamsu, K.; Yuliasih, I.; Nasir, M. Cellulose nanofibers from cassava bagasse: Characterization and application on tapioca-film. Chem. Mater. Res. 2013, 3, 79–87. [Google Scholar]
- Widiarto, S.; Pramono, E.; Rochliadi, A.; Arcana, I.M. Cellulose nanofibers preparation from cassava peels via mechanical disruption. Fibers 2019, 7, 44. [Google Scholar] [CrossRef]



| Time of UV Radiation | Gel Fraction of 10%PVA-g-GMA (%) |
|---|---|
| 5 min | 66.60 ± 3.50 |
| 10 min | 81.37 ± 1.61 |
| 15 min | 80.65 ± 1.26 |
| Formulations | Pore Size Diameter (µm) | Porosity (%) | Gel Fraction (%) | Water Content (%) | Water Swelling (%) |
|---|---|---|---|---|---|
| 10%PVA-g- GMA | 5–68 | 94.31 + 0.95 | 81.37 + 1.61 | 73.12 ± 0.77 | 272.30 ± 10.32 |
| 10%PVA-g-GMA/0.3%CNF | 3–64 | 90.23 ± 0.89 | 82.34 ± 2.37 | 84.12 ± 0.69 | 530.73 ± 28.96 |
| 10%PVA-g-GMA/0.5%CNF | 5–62 | 87.01 ± 1.36 | 81.45 ± 1.99 | 85.36 ± 1.15 | 586.66 ± 54.45 |
| 10%PVA-g-GMA/0.7%CNF | 3–64 | 83.31 ± 1.10 | 82.09 ± 1.89 | 86.80 ± 0.61 | 652.19 ± 29.95 |
| Materials | Compressive Strength (kPa) | Compressive Modulus (kPa) | References |
|---|---|---|---|
| Nano-hydroxyapatite/ poly (L-glutamic acid)-dextran | 51.00 | / | [13] |
| Stearyl methacrylate/silk fibroin | 17.10 | / | [14] |
| Genipin cross-linked gelatin hydrogel | 70.00 | 300.00 ± 30.00 | [15] |
| Fibrin glue | 46.71 ± 8.87 | 11.98 ± 4.45 | [31] |
| 10%PVA-g-GMA | 22.95 ± 2.30 | 27.30 ± 5.02 | This work |
| 10%PVA-g-GMA/0.3%CNF | 28.79 ± 3.05 | 28.84 ± 4.20 | |
| 10%PVA-g-GMA/0.5%CNF | 47.14 ± 4.33 | 56.46 ± 8.29 | |
| 10%PVA-g-GMA/0.7%CNF | 127.39 ± 41.59 | 129.91 ± 14.07 | |
| Meniscus scaffold requirement | ≥100 | ≥100 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinna, J.; Jeencham, R.; Mueangkhot, P.; Sophon, S.; Noralak, P.; Raksapakdee, R.; Numpaisal, P.-o.; Ruksakulpiwat, Y. Development of Poly(vinyl alcohol) Grafted Glycidyl Methacrylate/Cellulose Nanofiber Injectable Hydrogels for Meniscus Tissue Engineering. Polymers 2023, 15, 4230. https://doi.org/10.3390/polym15214230
Sinna J, Jeencham R, Mueangkhot P, Sophon S, Noralak P, Raksapakdee R, Numpaisal P-o, Ruksakulpiwat Y. Development of Poly(vinyl alcohol) Grafted Glycidyl Methacrylate/Cellulose Nanofiber Injectable Hydrogels for Meniscus Tissue Engineering. Polymers. 2023; 15(21):4230. https://doi.org/10.3390/polym15214230
Chicago/Turabian StyleSinna, Jiraporn, Rachasit Jeencham, Priyapat Mueangkhot, Sorasak Sophon, Pornpattara Noralak, Romtira Raksapakdee, Piya-on Numpaisal, and Yupaporn Ruksakulpiwat. 2023. "Development of Poly(vinyl alcohol) Grafted Glycidyl Methacrylate/Cellulose Nanofiber Injectable Hydrogels for Meniscus Tissue Engineering" Polymers 15, no. 21: 4230. https://doi.org/10.3390/polym15214230







