Manipulating Molecular Self-Assembly Process at the Solid–Liquid Interface Probed by Scanning Tunneling Microscopy
Abstract
:1. Introduction
2. Self-Assembled Structures Induced by Thermal Treatment
3. Self-Assembled Structures Tuned by Photo-Induction
4. Self-Assembled Structures Controlled by Voltage Induction
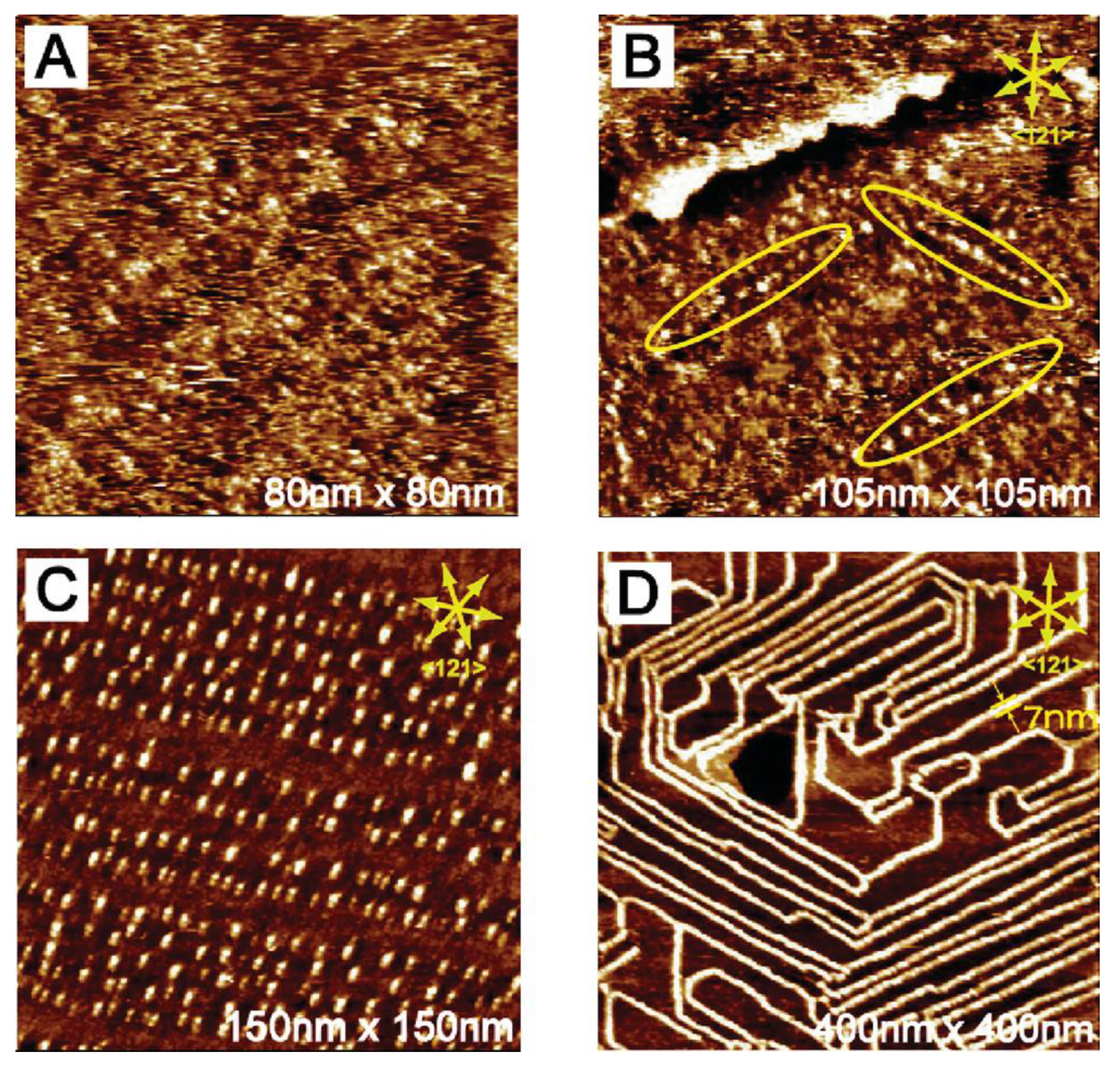
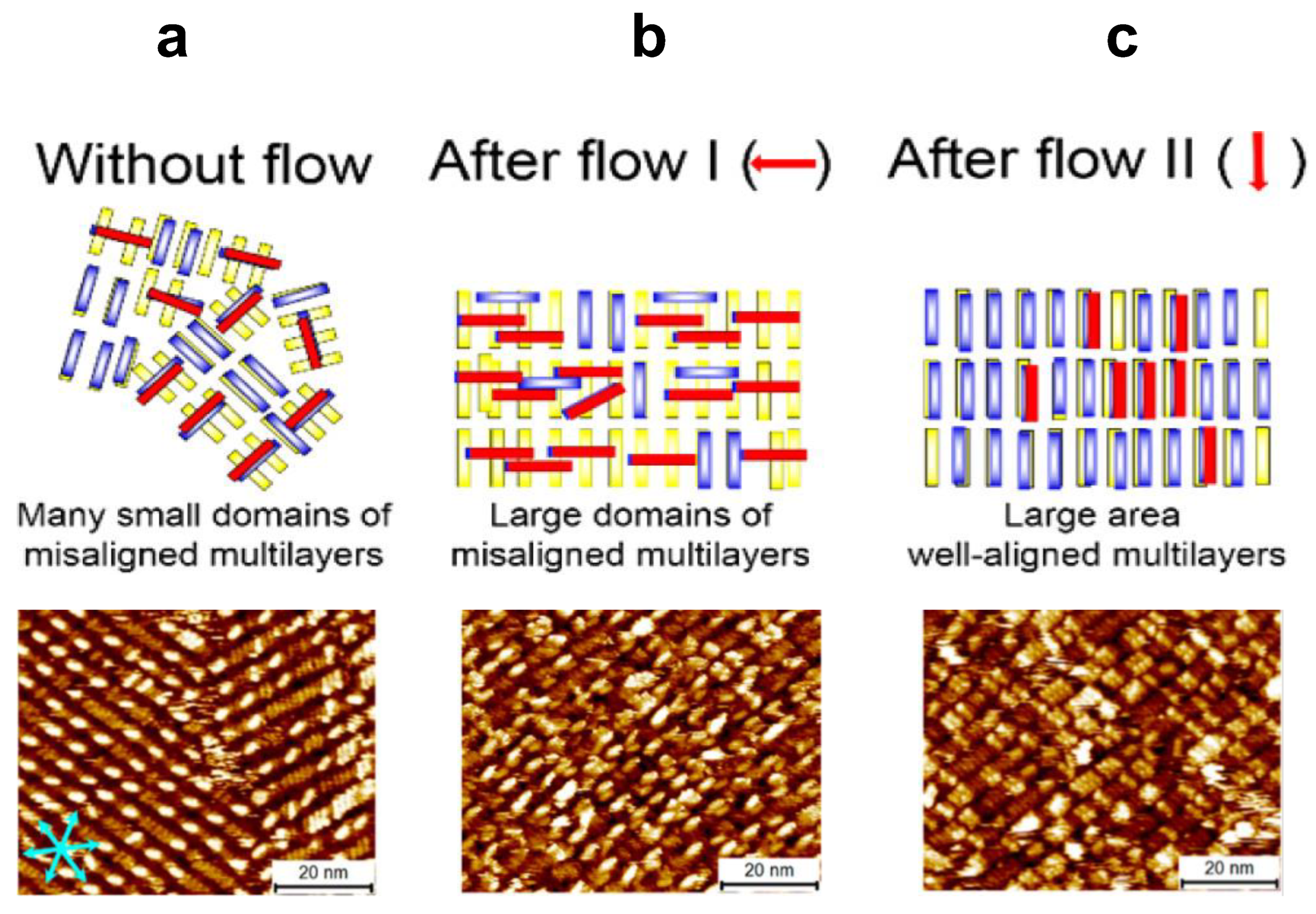
5. Self-Assembled Structures Regulated by Flow-Induction
6. Tip-Induced Self-Assembled Structures
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.; Stoddart, J. From Molecular to Supramolecular Electronics. Nat. Rev. Mater. 2021, 6, 804–828. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, X.; Soni, S.; Chiechi, R. Charge Transport through Molecular Ensembles: Recent Progress in Molecular Electronics. Chem. Phys. Rev. 2021, 2, 021303. [Google Scholar] [CrossRef]
- Zeng, K.; Tong, Z.; Ma, L.; Zhu, W.-H.; Wu, W.; Xie, Y. Molecular Engineering Strategies for Fabricating Efficient Porphyrin-Based Dye-Sensitized Solar Cells. Energy Environ. Sci. 2020, 13, 1617–1657. [Google Scholar] [CrossRef]
- Ji, D.; Li, T.; Hu, W.; Fuchs, H. Recent Progress in Aromatic Polyimide Dielectrics for Organic Electronic Devices and Circuits. Adv. Mater. 2019, 31, 1806070. [Google Scholar] [CrossRef]
- Huang, X.; Li, T. Recent Progress in the Development of Molecular-Scale Electronics Based on Photoswitchable Molecules. J. Mater. Chem. C 2020, 8, 821–848. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J. Self-Assembly as a Key Player for Materials Nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Mathias, J.P.; Seto, C. Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Pochan, D.; Scherman, O. Introduction: Molecular Self-Assembly. Chem. Rev. 2021, 121, 13699–13700. [Google Scholar] [CrossRef]
- Otero, R.; Gallego, J.M.; de Parga, A.L.V.; Martín, N.; Miranda, R. Molecular Self-Assembly at Solid Surfaces. Adv. Mater. 2011, 23, 5148–5176. [Google Scholar] [CrossRef]
- Song, Q.; Cheng, Z.; Kariuki, M.; Hall, S.C.; Hill, S.K.; Rho, J.Y.; Perrier, S. Molecular Self-Assembly and Supramolecular Chemistry of Cyclic Peptides. Chem. Rev. 2021, 121, 13936–13995. [Google Scholar] [CrossRef]
- Lei, P.; Ma, L.; Zhang, S.; Li, J.; Gan, L.; Deng, K.; Duan, W.; Li, W.; Zeng, Q. The Self-Assembly and Structural Regulation of a Hydrogen-Bonded Dimeric Building Block Formed by Two NH··· O Hydrogen Bonds on HOPG. Chin. Chem. Lett. 2023, 34, 108005. [Google Scholar] [CrossRef]
- Gao, H.-Y.; Wagner, H.; Held, P.A.; Du, S.; Gao, H.-J.; Studer, A.; Fuchs, H. In-Plane Van Der Waals Interactions of Molecular Self-Assembly Monolayer. Appl. Phys. Lett. 2015, 106, 081606. [Google Scholar] [CrossRef]
- Bera, S.; Basu, S.; Jana, B.; Dastidar, P. Real-Time Observation of Macroscopic Helical Morphologies under Optical Microscope: A Curious Case of π-π Stacking Driven Molecular Self-Assembly of an Organic Gelator Devoid of Hydrogen Bonding. Angew. Chem. Int. Ed. 2023, 62, e202216447. [Google Scholar] [CrossRef]
- Yang, D.; Gao, S.; Fang, Y.; Lin, X.; Jin, X.; Wang, X.; Ke, L.; Shi, K. The π–π Stacking-Guided Supramolecular Self-Assembly of Nanomedicine for Effective Delivery of Antineoplastic Therapies. Nanomedicine 2018, 13, 3159–3177. [Google Scholar] [CrossRef]
- Halter, M.; Liao, Y.; Plocinik, R.M.; Coffey, D.C.; Bhattacharjee, S.; Mazur, U.; Simpson, G.J.; Robinson, B.H.; Keller, S. Molecular Self-Assembly of Mixed High-Beta Zwitterionic and Neutral Ground-State NLO Chromophores. Chem. Mater. 2008, 20, 1778–1787. [Google Scholar] [CrossRef]
- Hu, Y.; Miao, K.; Zha, B.; Xu, L.; Miao, X.; Deng, W. Fabrication of Chiral Networks for a Tri-Substituted Anthraquinone Derivative Using Molecular Self-Assembly. Phys. Chem. Chem. Phys. 2016, 18, 13164–13168. [Google Scholar] [CrossRef]
- Sirtl, T.; Song, W.; Eder, G.; Neogi, S.; Schmittel, M.; Heckl, W.M.; Lackinger, M. Solvent-Dependent Stabilization of Metastable Monolayer Polymorphs at the Liquid–Solid Interface. ACS Nano 2013, 7, 6711–6718. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Yan, X.; Su, Y.; Yang, Y.; Li, J. Solvent-Induced Structural Transition of Self-Assembled Dipeptide: From Organogels to Microcrystals. Chem. Eur. J. 2010, 16, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Katsonis, N.; Xu, H.; Haak, R.M.; Kudernac, T.; Tomović, Ž.; George, S.; Van der Auweraer, M.; Schenning, A.P.; Meijer, E.W.; Feringa, B. Emerging Solvent-Induced Homochirality by the Confinement of Achiral Molecules against a Solid Surface. Angew. Chem. Int. Ed. 2008, 47, 4997–5001. [Google Scholar] [CrossRef] [PubMed]
- Blunt, M.O.; Adisoejoso, J.; Tahara, K.; Katayama, K.; Van der Auweraer, M.; Tobe, Y.; De Feyter, S. Temperature-Induced Structural Phase Transitions in a Two-Dimensional Self-Assembled Network. J. Am. Chem. Soc. 2013, 135, 12068–12075. [Google Scholar] [CrossRef] [PubMed]
- Gutzler, R.; Sirtl, T.; Dienstmaier, J.; Mahata, K.; Heckl, W.M.; Schmittel, M.; Lackinger, M. Reversible Phase Transitions in Self-Assembled Monolayers at the Liquid—Solid Interface: Temperature-Controlled Opening and Closing of Nanopores. J. Am. Chem. Soc. 2010, 132, 5084–5090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, Y.; Cao, K.; Zeng, S.; Jiang, G.; Liu, Y.; Cheng, W.; Bai, W.; Weng, X.; Chen, W. A General Strategy for Synthesizing Biomacromolecular Ionogel Membranes via Solvent-Induced Self-Assembly. Nat. Synth. 2023, 2, 864–872. [Google Scholar] [CrossRef]
- Shen, X.; Wei, X.; Tan, P.; Yu, Y.; Yang, B.; Gong, Z.; Zhang, H.; Lin, H.; Li, Y.; Li, Q. Concentration-Controlled Reversible Phase Transitions in Self-Assembled Monolayers on HOPG Surfaces. Small 2015, 11, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Shi, Y.; Maclennan, J.E.; Clark, N.A.; Farrow, M.J.; Walba, D. Photo-Reversible Liquid Crystal Alignment Using Azobenzene-Based Self-Assembled Monolayers: Comparison of the Bare Monolayer and Liquid Crystal Reorientation Dynamics. Langmuir 2010, 26, 17482–17488. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.-X.; Wang, H.; Duan, S.; Zhang, H.-M.; Xu, X.; Chi, L.-F. Potential-Induced Phase Transition of N-Isobutyryl-L-Cysteine Monolayers on Au (111) Surfaces. Acta Phys. Chim. Sin. 2017, 33, 1010–1016. [Google Scholar] [CrossRef]
- Mahmood, A.; Zeng, X.; Saleemi, A.S.; Cheng, K.-Y.; Lee, S.-L. Electric-Field-Induced Supramolecular Phase Transitions at the Liquid/Solid Interface: Cat-Assembly from Solvent Additives. Chem. Commun. 2020, 56, 8790–8793. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Wang, Z.X.; Han, X.B.; Sun, Y.-L.; Pryor, D. Orientational Ordering of Guest Induced Structural Phase Transition Coupled with Switchable Dielectric Properties in a Host–Guest Crystal: Bis(Thiourea) Thiazolium Chloride. RSC Adv. 2016, 6, 108028–108033. [Google Scholar] [CrossRef]
- Park, K.-W.; Adisoejoso, J.; Plas, J.; Hong, J.; Müllen, K.; De Feyter, S. Self-Assembly Behavior of Alkylated Isophthalic Acids Revisited: Concentration in Control and Guest-Induced Phase Transformation. Langmuir 2014, 30, 15206–15211. [Google Scholar] [CrossRef] [PubMed]
- Binning, G.; Rohrer, H.; Gerber, C.; Weibel, E. Surface Studies by Scanning Tunneling Microscopy. Phys. Rev. Lett. 1982, 49, 57–61. [Google Scholar] [CrossRef]
- Williams, R.J.; Smith, A.M.; Collins, R.; Hodson, N.; Das, A.K.; Ulijn, R. Enzyme-Assisted Self-Assembly under Thermodynamic Control. Nat. Nanotech. 2009, 4, 19–24. [Google Scholar] [CrossRef]
- Packwood, D.M.; Han, P.; Hitosugi, T. Chemical and Entropic Control on the Molecular Self-Assembly Process. Nat. Commun. 2017, 8, 14463. [Google Scholar] [CrossRef]
- Xu, P.; Li, X.; Yu, H. Thermodynamic Phase-Like Transition Effect of Molecular Self-Assembly. J. Phys. Chem. Lett. 2020, 12, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Su, G.J.; Aguilar-Sanchez, R.; Li, Z.; Pobelov, I.; Homberger, M.; Simon, U.; Wandlowski, T. Scanning Tunneling Microscopy and Spectroscopy Studies of 4-Methyl-4’-(N-Mercaptoalkyl) Biphenyls on Au (111)-(1×1). Chem. Eur. J. 2007, 8, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Azzam, W.; Al-Rashdi, A.; Subaihi, A.; Rohwerder, M.; Zharnikov, M.; Bashir, A. Annealing Effect for Self-Assembled Monolayers Formed from Terphenylethanethiol on Au (111). Phys. Chem. Chem. Phys. 2020, 22, 13580–13591. [Google Scholar] [CrossRef]
- Fang, Y.; Ghijsens, E.; Ivasenko, O.; Cao, H.; Noguchi, A.; Mali, K.S.; Tahara, K.; Tobe, Y.; De Feyter, S. Dynamic Control over Supramolecular Handedness by Selecting Chiral Induction Pathways at the Solution–Solid Interface. Nat. Chem. 2016, 8, 711–717. [Google Scholar] [CrossRef]
- Rohde, D.; Yan, C.J.; Yan, H.J.; Wan, L.J. From a Lamellar to Hexagonal Self-Assembly of Bis (4,4’-(M,M’-Di(Dodecyloxy)Phenyl)-2,2’-Difluoro-1,3,2-Dioxaborin) Molecules: A Trans-to-Cis-Isomerization-Induced Structural Transition Studied with STM. Angew. Chem. Int. Ed. 2006, 118, 4100–4104. [Google Scholar] [CrossRef]
- Shi, K.J.; Zhang, X.; Shu, C.H.; Li, D.Y.; Wu, X.Y.; Liu, P. Ullmann Coupling Reaction of Aryl Chlorides on Au (111) Using Dosed Cu as a Catalyst and the Programmed Growth of 2D Covalent Organic Frameworks. Chem. Commun. 2016, 52, 8726–8729. [Google Scholar] [CrossRef]
- Dienstmaier, J.F.; Gigler, A.M.; Goetz, A.J.; Knochel, P.; Bein, T.; Lyapin, A.; Reichlmaier, S.; Heckl, W.M.; Lackinger, M. Synthesis of Well-Ordered Cof Monolayers: Surface Growth of Nanocrystalline Precursors Versus Direct on-Surface Polycondensation. ACS Nano 2011, 5, 9737–9745. [Google Scholar] [CrossRef]
- Ruffieux, P.; Wang, S.; Yang, B.; Sánchez-Sánchez, C.; Liu, J.; Dienel, T.; Talirz, L.; Shinde, P.; Pignedoli, C.A.; Passerone, D. On-Surface Synthesis of Graphene Nanoribbons with Zigzag Edge Topology. Nature 2016, 531, 489–492. [Google Scholar] [CrossRef]
- Chen, Z.; Narita, A.; Müllen, K. Graphene Nanoribbons: On-Surface Synthesis and Integration into Electronic Devices. Adv. Mater. 2020, 32, 2001893. [Google Scholar] [CrossRef] [PubMed]
- Zuzak, R.; Castro-Esteban, J.; Engelund, M.; Pérez, D.; Peña, D.; Godlewski, S. On-Surface Synthesis of Nanographenes and Graphene Nanoribbons on Titanium Dioxide. ACS Nano 2023, 17, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, R.; Hoang, N.V.; Moghaddam, K.G.; Crespi, S.; Pooler, D.R.; Faraji, S.; Pshenichnikov, M.S.; Feringa, B. Synergistic Interplay between Photoisomerization and Photoluminescence in a Light-Driven Rotary Molecular Motor. Nat. Commun. 2022, 13, 5765. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-Y.; Niu, Q.; Chen, Y.; Lu, M.; Zhang, M.; Shi, J.-W.; Liu, J.; Yan, Y.; Li, S.-L.; Lan, Y.-Q. Interpenetrating 3D Covalent Organic Framework for Selective Stilbene Photoisomerization and Photocyclization. J. Am. Chem. Soc. 2023, 145, 8860–8870. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Yin, J.; Jiang, X. Self-Wrinkling Induced by the Photopolymerization and Self-Assembly of Fluorinated Polymer at Air/Liquid Interface. J. Mater. Chem. A 2014, 2, 18574–18582. [Google Scholar] [CrossRef]
- Li, Y.; Wong, K.M.-C.; Wong, H.-L.; Yam, V.W.-W. Interfaces, Helical Self-Assembly and Photopolymerization Properties of Achiral Amphiphilic Platinum (Ii) Diacetylene Complexes of Tridentate 2,6-Bis (1-Alkylpyrazol-3-Yl) Pyridines. ACS Appl. Mater. Interfaces 2016, 8, 17445–17453. [Google Scholar] [CrossRef]
- Gromov, S.P.; Vedernikov, A.I.; Lobova, N.A.; Kuz’mina, L.G.; Basok, S.S.; Strelenko, Y.A.; Alfimov, M.V.; Howard, J. Controlled Self-Assembly of Bis (Crown) Stilbenes into Unusual Bis-Sandwich Complexes: Structure and Stereoselective [2+2] Photocycloaddition. New J. Chem. 2011, 35, 724–737. [Google Scholar] [CrossRef]
- Lakshmi, K.M.; Rival, J.V.; Sreeraj, P.; Nambiar, S.R.; Jeyabharathi, C.; Nonappa; Shibu, E. Precision Nanocluster-Based Toroidal and Supertoroidal Frameworks Using Photocycloaddition-Assisted Dynamic Covalent Chemistry. Small 2023, 19, 2207119. [Google Scholar] [CrossRef]
- Pace, G.; Ferri, V.; Grave, C.; Elbing, M.; von Hänisch, C.; Zharnikov, M.; Mayor, M.; Rampi, M.A.; Samorì, P. Cooperative Light-Induced Molecular Movements of Highly Ordered Azobenzene Self-Assembled Monolayers. Proc. Natl. Acad. Sci. USA 2007, 104, 9937–9942. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Inukai, K.; Adisoejoso, J.; Yamaga, H.; Balandina, T.; Blunt, M.O.; De Feyter, S.; Tobe, Y. Tailoring Surface-Confined Nanopores with Photoresponsive Groups. Angew. Chem. Int. Ed. 2013, 125, 8531–8534. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hirose, T.; Matsuda, K. Photoinduced Four-State Three-Step Ordering Transformation of Photochromic Terthiophene at a Liquid/Solid Interface Based on Two Principles: Photochromism and Polymorphism. Langmuir 2015, 31, 6404–6414. [Google Scholar] [CrossRef] [PubMed]
- Pijper, T.C.; Kudernac, T.; Katsonis, N.; van der Maas, M.; Feringa, B.L.; van Wees, B. Reversible Light Induced Conductance Switching of Asymmetric Diarylethenes on Gold: Surface and Electronic Studies. Nanoscale 2013, 5, 9277–9282. [Google Scholar] [CrossRef]
- Garg, M.; Martin-Jimenez, A.; Luo, Y.; Kern, K. Ultrafast Photon-Induced Tunneling Microscopy. ACS Nano 2021, 15, 18071–18084. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-L.; Fang, Y.; Velpula, G.; Cometto, F.P.; Lingenfelder, M.; Mullen, K.; Mali, K.S.; De Feyter, S. Reversible Local and Global Switching in Multicomponent Supramolecular Networks: Controlled Guest Release and Capture at the Solution/Solid Interface. ACS Nano 2015, 9, 11608–11617. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-F.; Zhan, G.; Daukiya, L.; Eyley, S.; Thielemans, W.; Severin, K.; De Feyter, S. Electric-Field-Mediated Reversible Transformation between Supramolecular Networks and Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 11404–11408. [Google Scholar] [CrossRef]
- Wen, R.; Pan, G.-B.; Wan, L.-J. Oriented Organic Islands and One-Dimensional Chains on a Au (111) Surface Fabricated by Electrodeposition: An Stm Study. J. Am. Chem. Soc. 2008, 130, 12123–12127. [Google Scholar] [CrossRef]
- Cui, K.; Mali, K.S.; Ivasenko, O.; Wu, D.; Feng, X.; Walter, M.; Müllen, K.; De Feyter, S.; Mertens, S. Squeezing, Then Stacking: From Breathing Pores to Three-Dimensional Ionic Self-Assembly under Electrochemical Control. Angew. Chem. Int. Ed. 2014, 53, 12951–12954. [Google Scholar] [CrossRef]
- Higashi, T.; Shigemitsu, Y.; Sagara, T. Faradaic Phase Transition of Dibenzyl Viologen on an HOPG Electrode Surface Studied by in Situ Electrochemical Stm and Electroreflectance Spectroscopy. Langmuir 2011, 27, 13910–13917. [Google Scholar] [CrossRef]
- Thi, M.T.H.; Thanh, H.P.; De Feyter, S. Surface Engineering of Graphite and Graphene by Viologen Self-Assembling: From Global to Local Architectures. J. Phys. Chem. C 2022, 126, 6413–6419. [Google Scholar] [CrossRef]
- Geng, J.; Zhao, X.; Zhou, E.; Li, G.; Lam, J.W.Y.; Tang, B. Shear Induced Molecular Alignments of a Side-Chain Liquid Crystalline Polyacetylene Containing Biphenyl Mesogens. Polymer 2003, 44, 8095–8102. [Google Scholar] [CrossRef]
- Rozman, M.; Urbakh, M.; Klafter, J.; Elmer, F. Atomic Scale Friction and Different Phases of Motion of Embedded Molecular Systems. J. Phys. Chem. B 1998, 102, 7924–7930. [Google Scholar] [CrossRef]
- Hoogboom, J.; Garcia, P.M.; Otten, M.B.; Elemans, J.A.; Sly, J.; Lazarenko, S.V.; Rasing, T.; Rowan, A.E.; Nolte, R. Tunable Command Layers for Liquid Crystal Alignment. J. Am. Chem. Soc. 2005, 127, 11047–11052. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-L.; Chi, C.-Y.J.; Huang, M.-J.; Chen, C.-h.; Li, C.-W.; Pati, K.; Liu, R.-S. Shear-Induced Long-Range Uniaxial Assembly of Polyaromatic Monolayers at Molecular Resolution. J. Am. Chem. Soc. 2008, 130, 10454–10455. [Google Scholar] [CrossRef]
- Lee, S.L.; Lin, N.T.; Liao, W.C.; Chen, C.H.; Yang, H.C.; Luh, T. Oligomeric Tectonics: Supramolecular Assembly of Double-Stranded Oligobisnorbornene through π-π Stacking. Chem. Eur. J. 2009, 15, 11594–11600. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-L.; Yuan, Z.; Chen, L.; Mali, K.S.; Müllen, K.; De Feyter, S. Forced to Align: Flow-Induced Long-Range Alignment of Hierarchical Molecular Assemblies from 2D to 3D. J. Am. Chem. Soc. 2014, 136, 4117–4120. [Google Scholar] [CrossRef] [PubMed]
- Van Hameren, R.; Schön, P.; Van Buul, A.M.; Hoogboom, J.; Lazarenko, S.V.; Gerritsen, J.W.; Engelkamp, H.; Christianen, P.C.; Heus, H.A.; Maan, J. Macroscopic Hierarchical Surface Patterning of Porphyrin Trimers Via Self-Assembly and Dewetting. Science 2006, 314, 1433–1436. [Google Scholar] [CrossRef]
- Alemani, M.; Peters, M.V.; Hecht, S.; Rieder, K.-H.; Moresco, F.; Grill, L. Electric Field-Induced Isomerization of Azobenzene by STM. J. Am. Chem. Soc. 2006, 128, 14446–14447. [Google Scholar] [CrossRef]
- Mali, K.S.; Wu, D.; Feng, X.; Müllen, K.; Van der Auweraer, M.; De Feyter, S. Scanning Tunneling Microscopy-Induced Reversible Phase Transformation in the Two-Dimensional Crystal of a Positively Charged Discotic Polycyclic Aromatic Hydrocarbon. J. Am. Chem. Soc. 2011, 133, 5686–5688. [Google Scholar] [CrossRef]
- Bragança, A.M.; Greenwood, J.; Ivasenko, O.; Phan, T.H.; Müllen, K.; De Feyter, S. The Impact of Grafted Surface Defects and Their Controlled Removal on Supramolecular Self-Assembly. Chem. Sci. 2016, 7, 7028–7033. [Google Scholar] [CrossRef]
- Verstraete, L.; Greenwood, J.; Hirsch, B.E.; De Feyter, S. Self-Assembly under Confinement: Nanocorrals for Understanding Fundamentals of 2D Crystallization. ACS Nano 2016, 10, 10706–10715. [Google Scholar] [CrossRef]
- Seibel, J.; Verstraete, L.; Hirsch, B.E.; Braganca, A.M.; De Feyter, S. Biasing Enantiomorph Formation Via Geometric Confinement: Nanocorrals for Chiral Induction at the Liquid–Solid Interface. J. Am. Chem. Soc. 2018, 140, 11565–11568. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Y.; Yin, C. Manipulating Molecular Self-Assembly Process at the Solid–Liquid Interface Probed by Scanning Tunneling Microscopy. Polymers 2023, 15, 4176. https://doi.org/10.3390/polym15204176
Li Z, Li Y, Yin C. Manipulating Molecular Self-Assembly Process at the Solid–Liquid Interface Probed by Scanning Tunneling Microscopy. Polymers. 2023; 15(20):4176. https://doi.org/10.3390/polym15204176
Chicago/Turabian StyleLi, Zhi, Yanan Li, and Chengjie Yin. 2023. "Manipulating Molecular Self-Assembly Process at the Solid–Liquid Interface Probed by Scanning Tunneling Microscopy" Polymers 15, no. 20: 4176. https://doi.org/10.3390/polym15204176




