An Investigation of PPy@1T/2H MoS2 Composites with Durable Photothermal-Promoted Effect in Photo-Fenton Degradation of Methylene Blue and in Water Evaporation
Abstract
:1. Introduction
2. Experimental
2.1. Chemical Sample Reagents
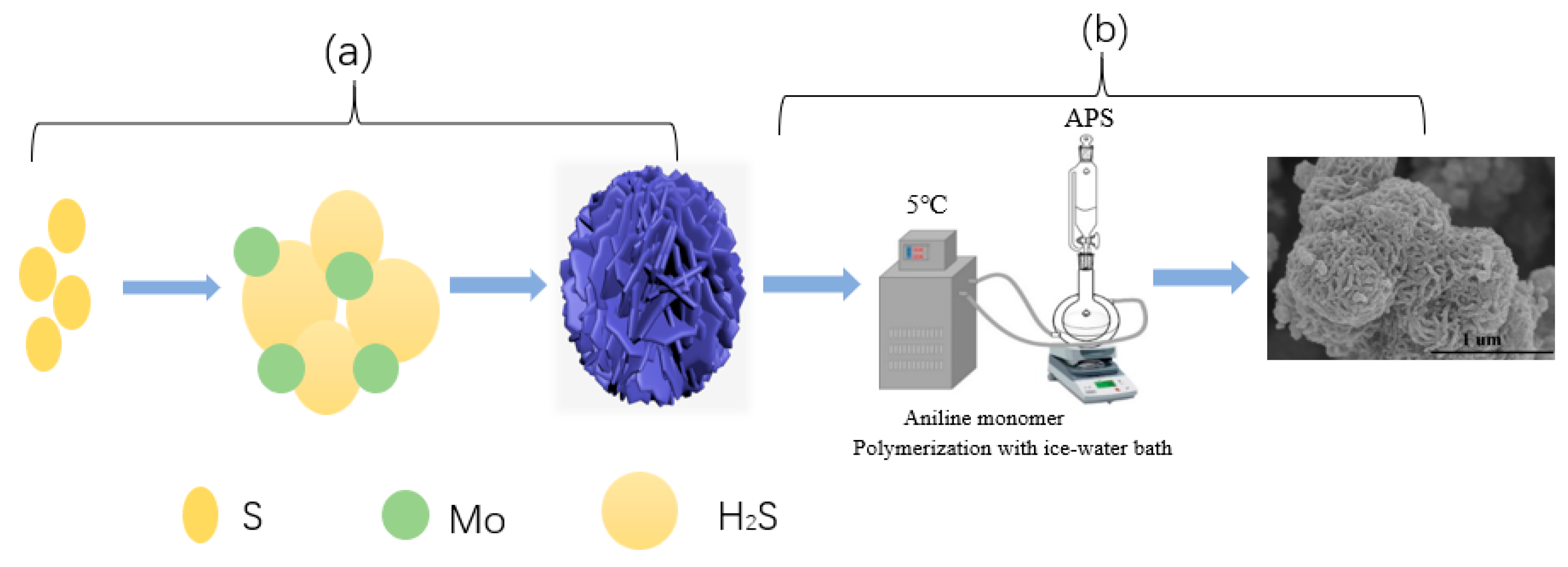
2.2. Preparation of 1T-2H MoS2 and PPy@1T-2H MoS2
2.3. Preparation of the 1T-2H MoS2 and PPy@1T-2H MoS2 Membranes
2.4. Methods and Characterization
2.5. Fenton-like Catalytic Experiments
2.6. Solar Water Evaporation Experiment
3. Results
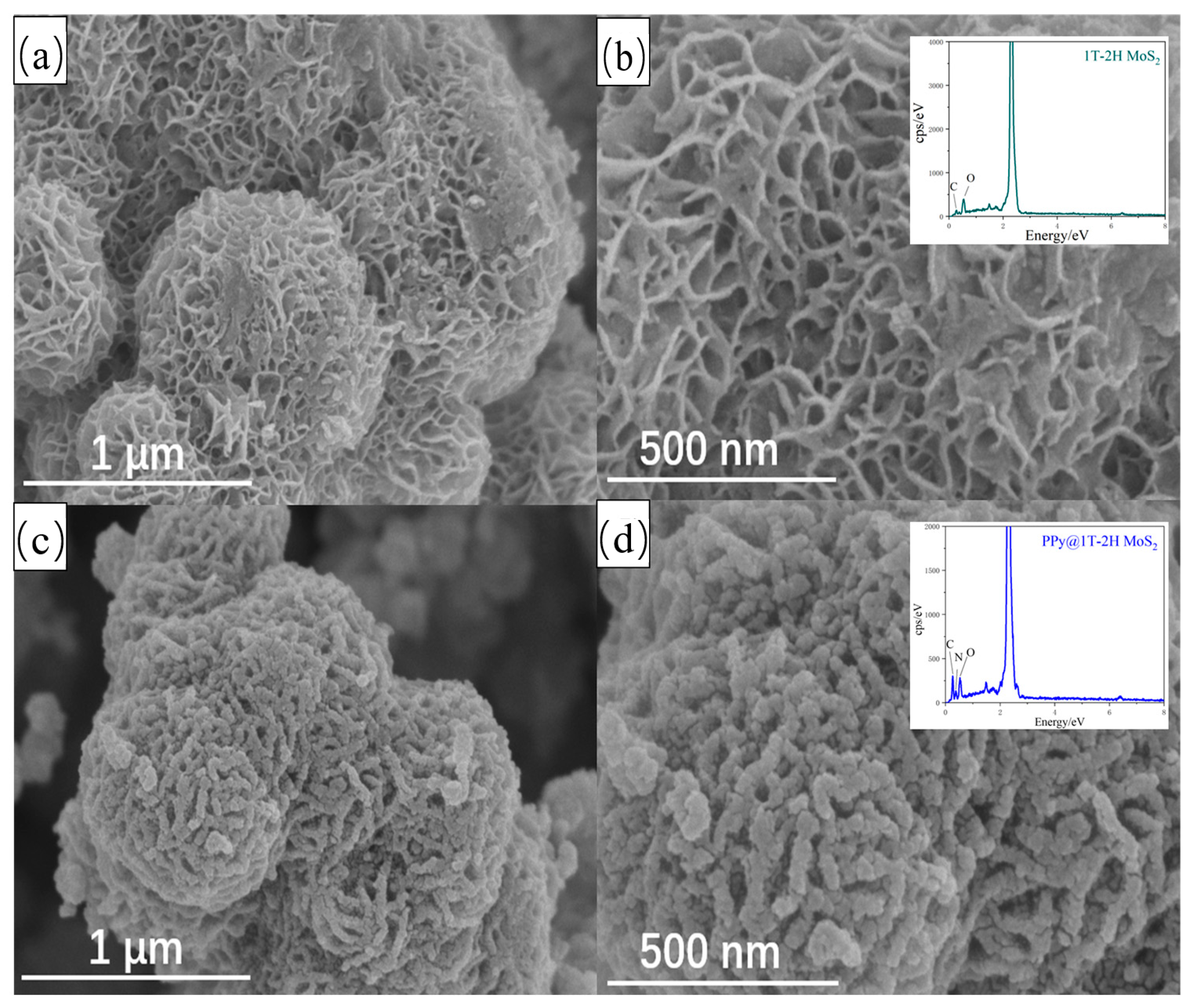
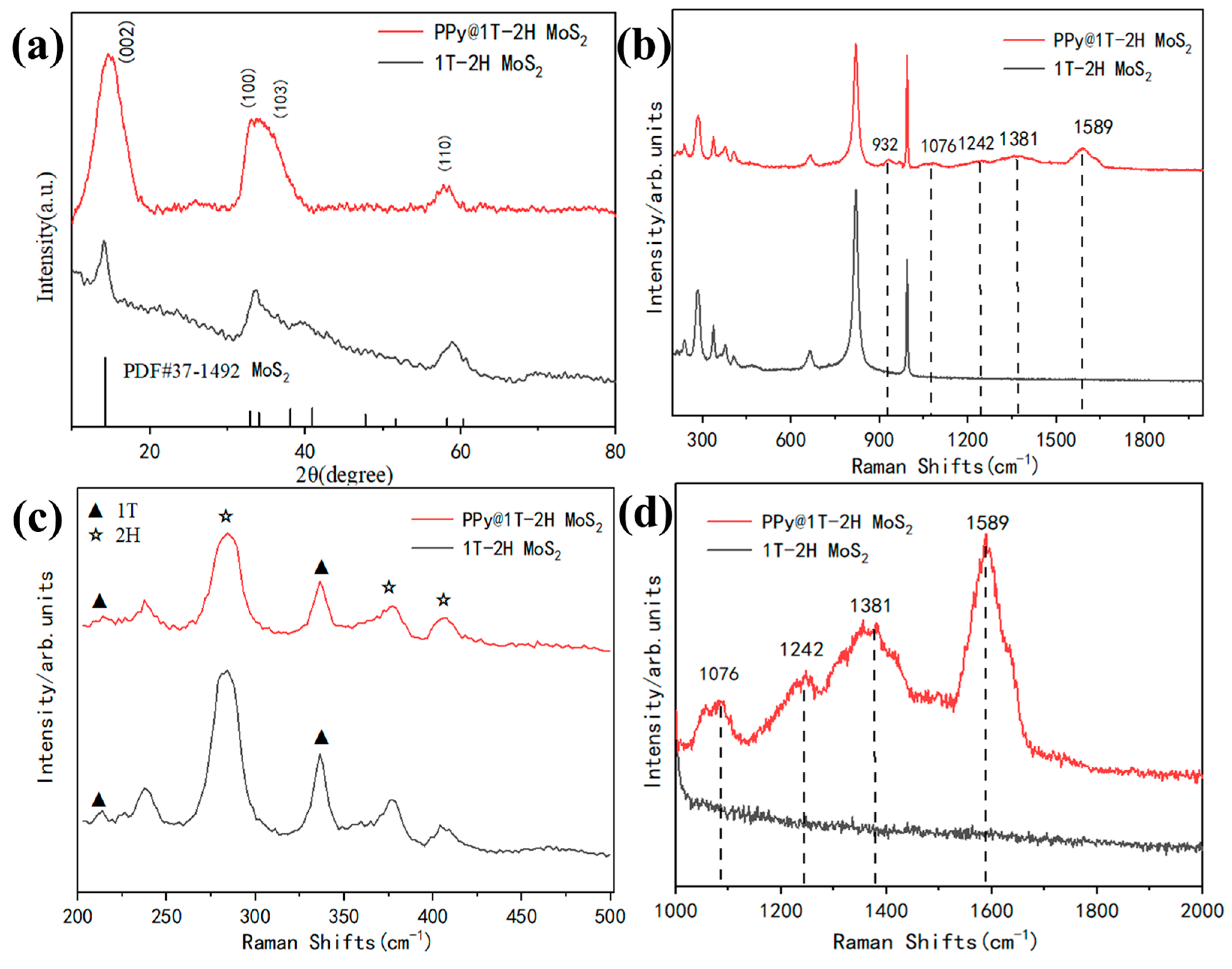
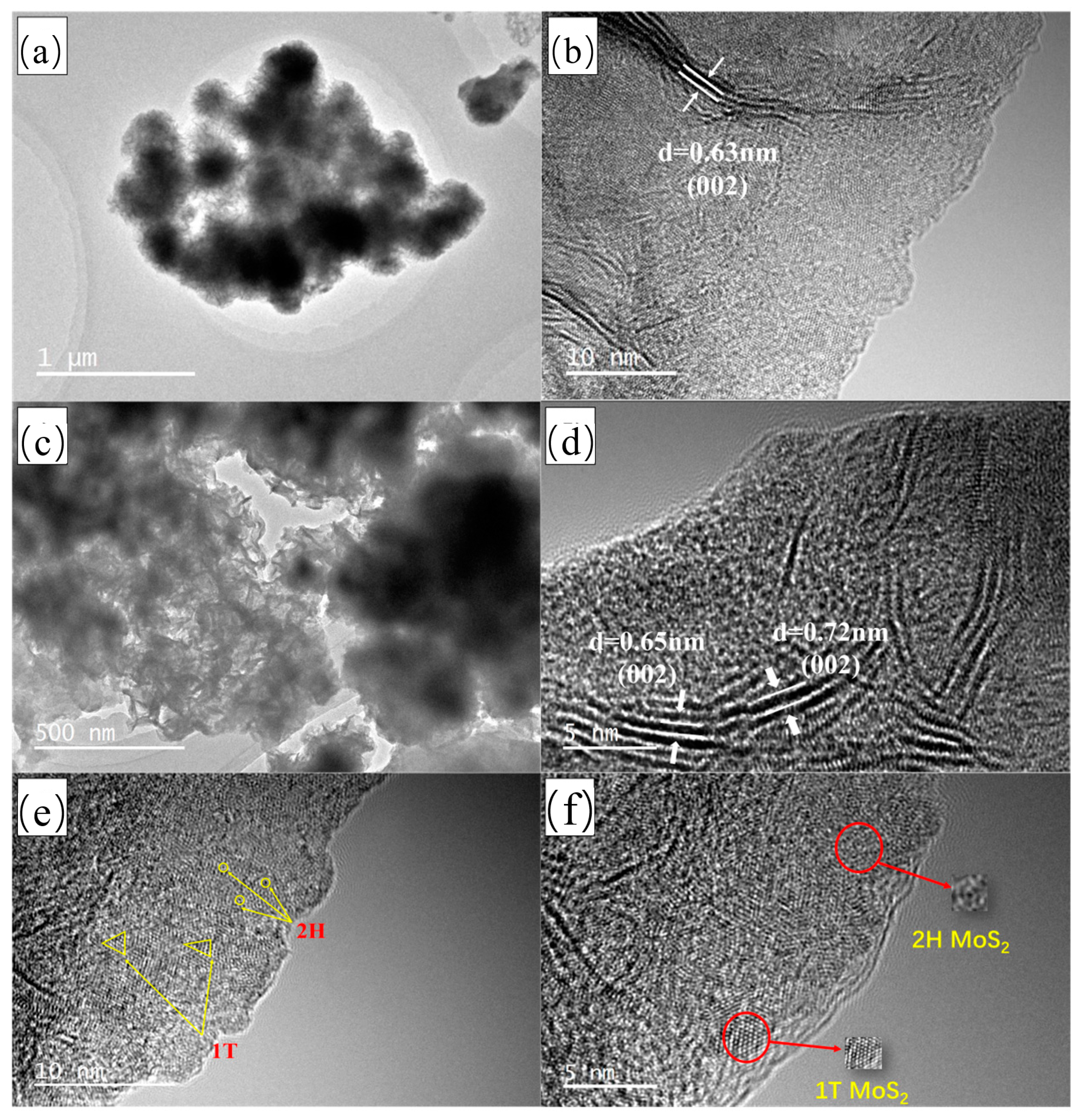
3.1. Phase Structures of 1T-2H MoS2 and PPy@1T-2H MoS2
3.2. Photocatalytic Activity of the Catalysts
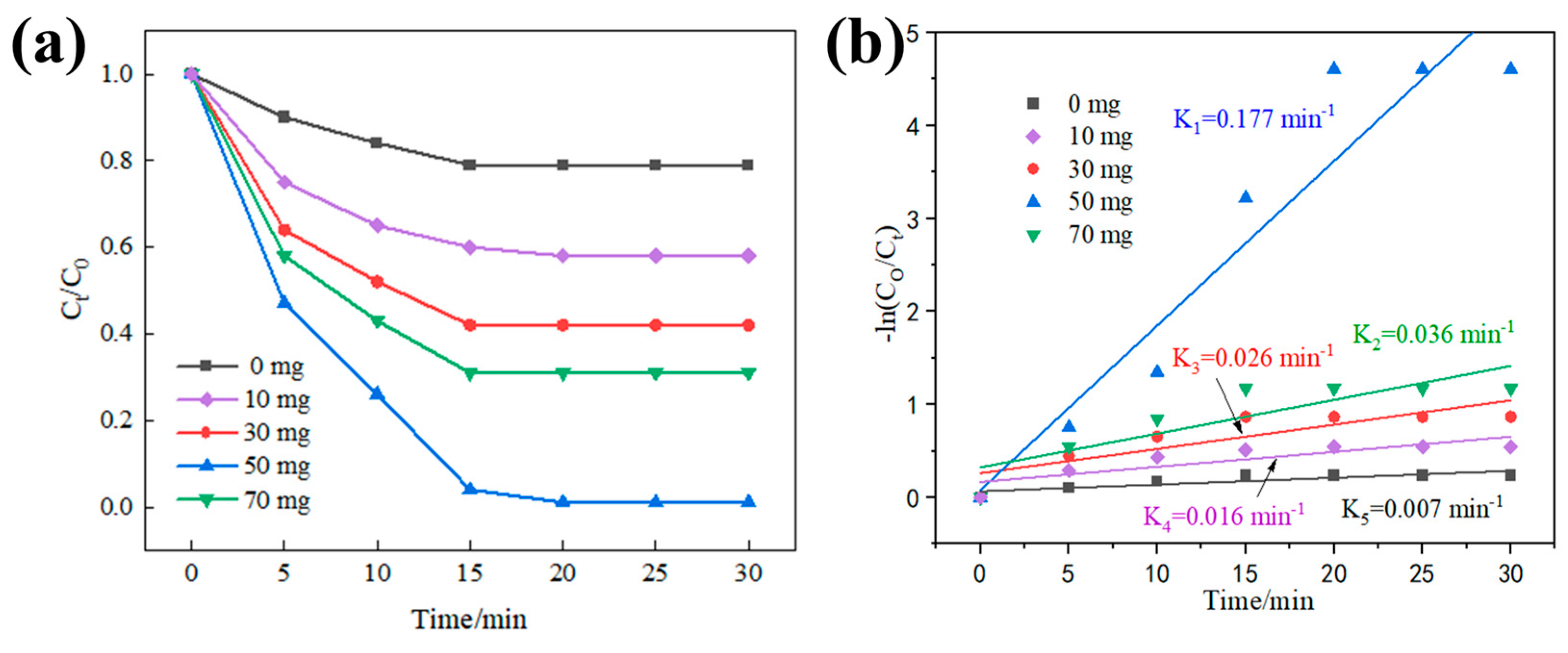
3.2.1. Effect of Catalyst Dosage
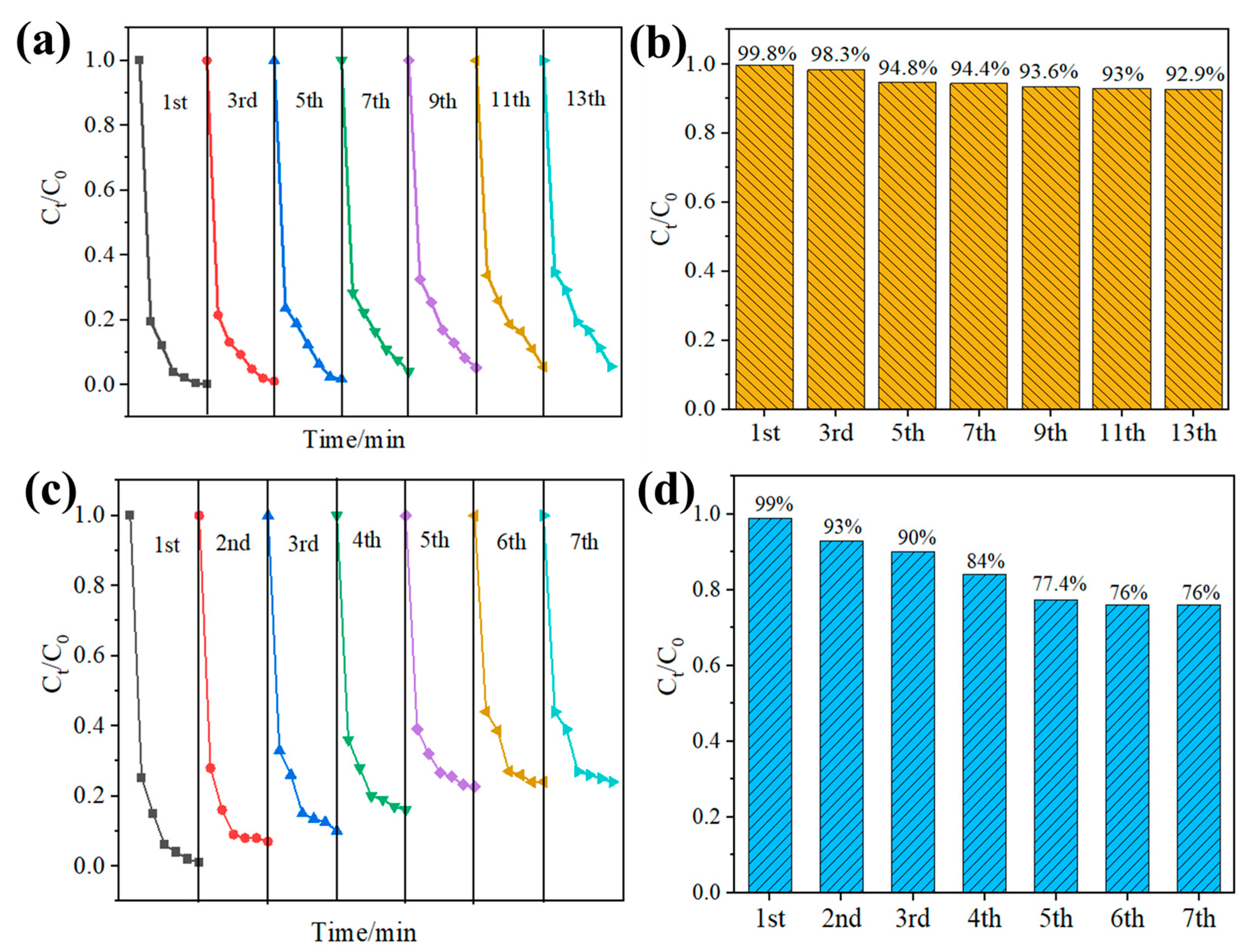
3.2.2. Catalytic Activity of Hybrid Nanoparticles
3.3. Application of Solar Energy in Water Evaporation
4. Discussion
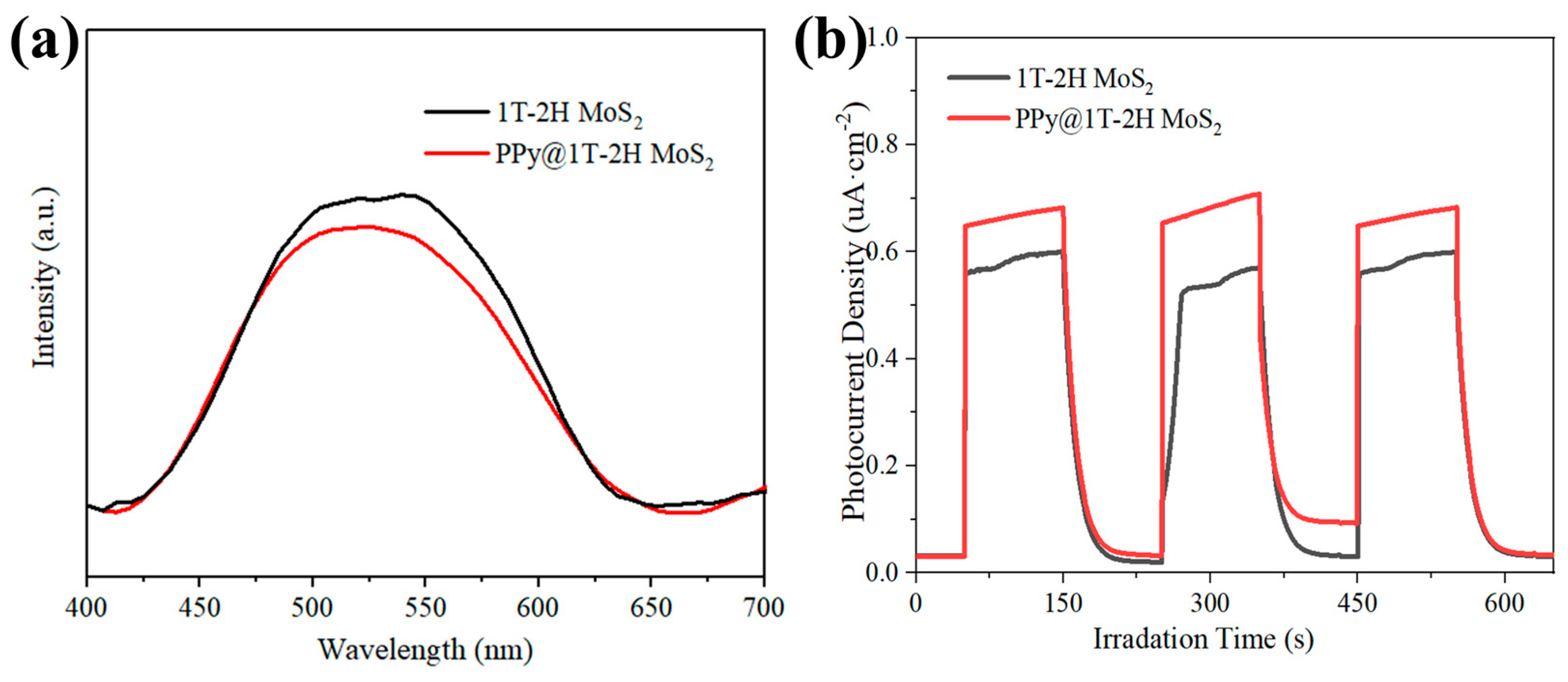
4.1. The Synergistic Effect of Photocatalytic Degradation and the Fenton Reaction
4.2. Photothermal Enhanced Photo-Fenton Catalytic Degradation
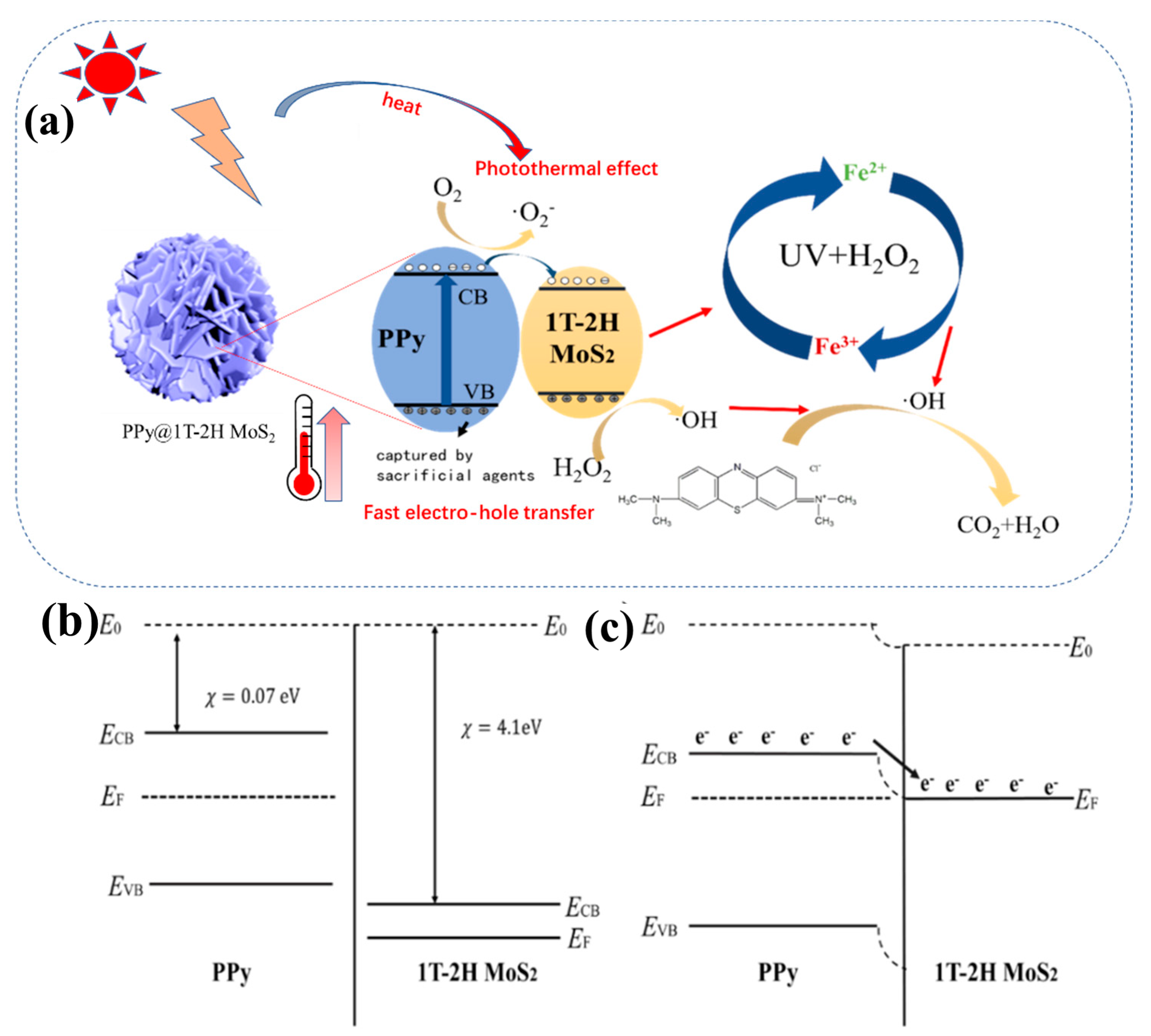
4.3. Photothermal-Fenton Reaction Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, M.; Chen, P.; Liu, M. Graphene Oxide Enwrapped Ag/AgX (X = Br, Cl) Nanocomposite as a Highly Efficient Visible-Light Plasmonic Photocatalyst. ACS Nano 2011, 5, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Li, M.; Fan, J.; Zhao, G. Magnetic ordered mesoporous copper ferrite as a heterogeneous Fenton catalyst for the degradation of imidacloprid. Appl. Catal. B Environ. 2014, 147, 534–545. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Wang, Q.; Cheng, H. High-efficiency photo-Fenton Fe/g-C3N4/kaolinite catalyst for tetracycline hydrochloride degradation. Appl. Clay Sci. 2021, 212, 106213. [Google Scholar] [CrossRef]
- Li, C.; Wei, Z.; Lu, Q.; Ma, J.; Li, L. Photoelectrochemical and photo-Fenton mechanism of enhanced visible light-driven nanocatalyst synthesis of ZnFe2O4/BiOI. Environ. Sci. Pollut. Res. 2022, 29, 34930–34942. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Yu, Y.; Yu, Z.; Lai, L.; Liu, F.; Wei, L.; Chen, Y. Bifunctional catalysts for heterogeneous electro-Fenton processes: A review. Environ. Chem. Lett. 2022, 20, 3837–3859. [Google Scholar] [CrossRef]
- Liang, J.; Xiang, Q.; Lei, W.; Zhang, Y.; Sun, J.; Zhu, H.; Wang, S. Ferric iron reduction reaction electro-Fenton with gas diffusion device: A novel strategy for improvement of comprehensive efficiency in electro-Fenton. J. Hazard. Mater. 2021, 412, 125195. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Ge, M.; Liu, B.; Chen, S.; Zhang, D.; Gao, L. Converting lignin into long-chain fatty acids with the electro-Fenton reaction. GCB Bioenergy 2021, 13, 1290–1302. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Chen, C.; Hu, X.; Zhao, J.; Yu, J.C. Fe3+/Fe2+ cycling promoted by Ta3N5 under visible irradiation in Fenton degradation of organic pollutants. Appl. Catal. B Environ. 2007, 75, 256–263. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.T.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Jodat, A.; Moghiman, M.; Rad, E.Y. An Experimental Study of the Ability of Similarity Theory to predict Water Evaporation Rate for Different Convection Regimes. Arab. J. Sci. Eng. 2013, 38, 3505–3513. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Shirzadi, A. Enhancement of the photocatalytic activity of Ferrous Oxide by doping onto the nano-clinoptilolite particles towards photodegradation of tetracycline. Chemosphere 2014, 107, 136–144. [Google Scholar] [CrossRef]
- El-Kemary, M.; Abdel-Moneam, Y.; Madkour, M.; El-Mehasseb, I. Enhanced photocatalytic degradation of Safranin-O by heterogeneous nanoparticles for environmental applications. J. Lumin. 2011, 131, 570–576. [Google Scholar] [CrossRef]
- Rao, Y.F.; Chu, W.; Wang, Y.R. Photocatalytic oxidation of carbamazepine in triclinic-WO3 suspension: Role of alcohol and sulfate radicals in the degradation pathway. Appl. Catal. A-Gen. 2013, 468, 240–249. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Vázquez, A.; Sanchez-Martinez, D.; Obregón, S. Performance of the polymeric g-C3N4 photocatalyst through the degradation of pharmaceutical pollutants under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2016, 324, 47–52. [Google Scholar] [CrossRef]
- Wu, W.; Ruan, Z.; Li, J.; Li, Y.; Jiang, Y.; Xu, X.; Li, D.; Yuan, Y.; Lin, K. In Situ Preparation and Analysis of Bimetal Co-doped Mesoporous Graphitic Carbon Nitride with Enhanced Photocatalytic Activity. Nano-Micro Lett. 2019, 11, 10. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, R.; Fan, Y.; Hu, L.; Chen, Q. In situ synthesis of C-doped BiVO4 with natural leaf as a template under different calcination temperatures. RSC Adv. 2019, 9, 14004–14010. [Google Scholar] [CrossRef]
- Marks, R.; Doudrick, K. Photocatalytic reduction of chlorite in water using bismuth vanadate (BiVO4): Effect of irradiance conditions and presence of oxalate on the reactivity and by-product selectivity. Environ. Sci. Water Res. Technol. 2019, 5, 2015–2026. [Google Scholar] [CrossRef]
- Li, Y.; Bando, Y.; Golberg, D. MoS2 nanoflowers and their field-emission properties. Appl. Phys. Lett. 2003, 82, 1962–1964. [Google Scholar] [CrossRef]
- Thamankar, R.; Yap, T.L.; Goh, K.E.J.; Troadec, C.; Joachim, C. Low Temperature Nanoscale Electronic Transport on the MoS2 surface. Physics 2013, 103, 083106. [Google Scholar] [CrossRef]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.-T.; Hong, L.-F.; Ji, X.-Y.; Li, Z.-S.; Lin, Z.-D.; Pan, W.-G. Recent advances and perspectives of MoS2-based materials for photocatalytic dyes degradation: A review. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125836. [Google Scholar] [CrossRef]
- Yan, Q.; Lian, C.; Huang, K.; Liang, L.; Yu, H.; Yin, P.; Zhang, J.; Xing, M. Constructing an Acidic Microenvironment by MoS2 in Heterogeneous Fenton Reaction for Pollutant Control. Angew. Chem. Int. Ed. 2021, 60, 17155–17163. [Google Scholar] [CrossRef]
- Li, Z.; Gu, Y.; Li, F. Heterogeneous Fenton system with dual working mechanisms for aqueous pollutants degradation. J. Environ. Chem. Eng. 2022, 10, 107686. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, L.; Chen, J.; Zhang, Y.; Liu, M.; Han, Y.; Chen, Y.; Zeng, H.; Shi, Z. How MoS2 assisted sulfur vacancies featured Cu2S in hollow Cu2S@MoS2 nanoboxes to activate H2O2 for efficient sulfadiazine degradation? Chem. Eng. J. 2022, 446, 137364. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Liu, J.; Mine, S.; Matsuoka, M.; Zhang, J.; Xing, M. Designing 3D-MoS2 Sponge as Excellent Cocatalysts in Advanced Oxidation Processes for Pollutant Control. Angew. Chem. Int. Ed. 2020, 59, 13968–13976. [Google Scholar] [CrossRef]
- Jun Mao, Y.W.Z.Z.D.D. The rise of two-dimensional MoS2 for catalysis. Front. Phys. 2018, 13, 138118. [Google Scholar]
- Yan, Y.; Xia, B.; Ge, X.; Liu, Z.; Wang, J.-Y.; Wang, X. Ultrathin MoS2 Nanoplates with Rich Active Sites as Highly Efficient Catalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2013, 5, 12794–12798. [Google Scholar] [CrossRef]
- Yin, Q.; Qiao, R.; Tong, G. Preparation and Photocatalytic Application of Ion-Doped ZnO Functional Nanomaterials. Prog. Chem. 2014, 26, 1619–1632. [Google Scholar]
- Xianjun, T.; Wenhui, D.; Zhenying, J.; Linxiao, S.; Yuxiong, H. Reinventing MoS2 Co-catalytic Fenton reaction: Oxygen-incorporation mediating surface superoxide radical generation. Nano Res. 2022, 15, 1973–1982. [Google Scholar]
- Chava, R.K.; Do, J.Y.; Kang, M. Smart Hybridization of Au Coupled CdS Nanorods with Few Layered MoS2 Nanosheets for High Performance Photocatalytic Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 6445–6457. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Li, D.D.; Lei, Y.H.; Tan, N.; Liu, T.; Chang, X.T.; Fan, R.H.; Gao, G.H. One-Step Hydrothermal Synthesis of 1T@2H MoS2 for Enhanced Photocatalytic Degradation Performance of Methyl Blue. Mater. Sci. Forum. 2020, 993, 1496–1501. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, S.; Qiu, Z.; Yin, K.; Lei, Y.; Sun, K.; Chang, X.; Li, X.; Fan, R. In situ chemo-polymerized polypyrrole-coated filter paper for high-efficient solar vapor generation. Int. J. Energy Res. 2020, 44, 1191–1204. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, L.; Cao, S.; Wang, Z.; Du, H.; Sun, K.; Wang, D.; Zhang, Y. Application of wooden arrays in solar water evaporation and desalination. Mater. Today Commun. 2021, 29, 102819. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, Y.; Zhang, F.; Wang, K.; Chang, X.; An, Y.; Dong, L.; Yin, Y. From CdS to Cu7S4 Nanorods via a Cation Exchange Route and Their Applications: Environmental Pollution Removal, Photothermal Conversion and Light-Induced Water Evaporation. ChemistrySelect 2017, 2, 3039–3048. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Dang, Y.; Han, P.; Li, Y.; Zhang, Y.; Zhou, Y. Low-crystalline mixed Fe-Co-MOFs for efficient oxygen evolution electrocatalysis. J. Mater. Sci. 2020, 55, 13951–13963. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Wu, X.; Song, L. Gram-Scale Aqueous Synthesis of Stable Few-Layered 1T-MoS2: Applications for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Sandoval, S.; Yang, D.; Frindt, R.F.; Irwin, J.C. Raman study and lattice dynamics of single molecular layers of MoS2. Phys. Rev. B Condens. Matter 1991, 44, 3955–3962. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Meng, L.; Chen, H.; Luo, S.; Li, W.; Jiao, K. Synthesis of Thin-Layered Molybdenum Disulfide-Based Polyaniline Nanointerfaces for Enhanced Direct Electrochemical DNA Detection. Adv. Mater. Interfaces 2016, 3, 1500700. [Google Scholar] [CrossRef]
- Ye, R.; del Angel-Vicente, P.; Liu, Y.; Arellano-Jimenez, M.J.; Peng, Z.; Wang, T.; Li, Y.; Yakobson, B.I.; Wei, S.-H.; Yacaman, M.J.; et al. High-Performance Hydrogen Evolution from MoS2(1–x)Px Solid Solution. Adv. Mater. 2016, 28, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Miao, N.; Wen, Y.; Zhang, S.; Ghosez, P.; Sun, Z.; Allwood, D.A. Sulfur-Depleted Monolayered Molybdenum Disulfide Nanocrystals for Superelectrochemical Hydrogen Evolution Reaction. ACS Nano 2016, 10, 8929–8937. [Google Scholar] [CrossRef]
- Geng, X.; Jiao, Y.; Han, Y.; Mukhopadhyay, A.; Yang, L.; Zhu, H. Batteries: Freestanding Metallic 1T MoS2 with Dual Ion Diffusion Paths as High Rate Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702998. [Google Scholar] [CrossRef]
- Xianhong, W.; Wang, Z.; Yu, M.; Xiu, L.; Qiu, J. Stabilizing the MXenes by Carbon Nanoplating for Developing Hierarchical Nanohybrids with Efficient Lithium Storage and Hydrogen Evolution Capability. Adv. Mater. 2017, 29, 1607017. [Google Scholar]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, G.; Wang, H.; Bai, J. Half-cell and full-cell investigations of 3D hierarchical MoS2/graphene composite on anode performance in lithium-ion batteries. J. Alloys Compd. 2016, 660, 62–72. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Thenuwara, A.C.; Patra, A.; Aulin, Y.V.; Tran, T.M.; Chakraborty, H.; Borguet, E.; Klein, M.L.; Perdew, J.P.; Strongin, D.R. Effect of Intercalated Metals on the Electrocatalytic Activity of 1T-MoS2 for the Hydrogen Evolution Reaction. ACS Energy Lett. 2018, 3, 7–13. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.; Valova, E.; Armyanov, S.; Tatchev, D.; Sotiropoulos, S.; Avramova, I.; Dimitrova, N.; Hubin, A.; Steebhaut, O. A simple Preparation and Characterization of B and N co-Doped TiO2 Nanotube arrays with Enhanced Photoelectrochemical Performance. Appl. Surf. Sci. 2017, 413, 284–291. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Zhang, Y.; Zhang, L. Novel low-cost Fenton-like layered Fe-titanate catalyst: Preparation, characterization and application for degradation of organic colorants. J. Colloid Interface Sci. 2014, 422, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Millero, F.J.; Johnson, R.L.; Vega, C.A.; Sharma, V.K.; Sotolongo, S. Effect of ionic interactions on the rates of reduction of Cu(II) with H2O2 in aqueous solutions. J. Solut. Chem. 1992, 21, 1271–1287. [Google Scholar] [CrossRef]
- Fan, X.; Xu, P.; Zhou, D.; Sun, Y.; Li, Y.C.; Nguyen, M.A.; Terrones, M.; Mallouk, T.E. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef]
- Farias, J.; Albizzati, E.D.; Alfano, O.M. Kinetic study of the photo-Fenton degradation of formic acid: Combined effects of temperature and iron concentration. Catal. Today 2009, 144, 117–123. [Google Scholar] [CrossRef]
- Zapata, A.; Oller, I.; Bizani, E.; Sánchez-Pérez, J.; Maldonado, M.; Malato, S. Evaluation of operational parameters involved in solar photo-Fenton degradation of a commercial pesticide mixture. Catal. Today 2009, 144, 94–99. [Google Scholar] [CrossRef]
- Hu, R.; Fang, Y.; Huo, M.; Yao, H.; Wang, C.; Chen, Y.; Wu, R. Ultrasmall Cu2−xS nanodots as photothermal-enhanced Fenton nanocatalysts for synergistic tumor therapy at NIR-II biowindow. Biomaterials 2009, 206, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Han, X.; Liu, J.; Lan, X.; Feng, J.; Li, Y.; Zhang, W.; Wang, J. Photothermal-boosted effect of binary CuFe bimetallic magnetic MOF heterojunction for high-performance photo-Fenton degradation of organic pollutants. Sci. Total Environ. 2021, 795, 148883. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Deng, L.; Wei, N.; Weng, Y.; Dong, S.; Qi, D.; Qiu, J.; Chen, X.; Wu, T. High-performance photothermal conversion of narrow-bandgap Ti2O3 nanoparticles. Adv. Mater. 2017, 29, 1603730. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Xing, Z.; Zhao, T.; Qiu, Y.; Tao, B.; Li, Z.; Zhou, W. Hollow flower-like polyhedral α-Fe2O3/Defective MoS2/Ag Z-scheme heterojunctions with enhanced photocatalytic-Fenton performance via surface plasmon resonance and photothermal effects. Appl. Catal. B 2020, 272, 118978. [Google Scholar] [CrossRef]
- Wei, Y.; Han, S.; Walker, D.A.; Warren, S.C.; Grzybowski, B.A. Enhanced photocatalytic activity of hybrid Fe2O3–Pd nanoparticulate catalysts. Chem. Sci. 2012, 3, 1090–1094. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Pai, C.Y.; Wu, C.H.; Jian, M.Y. Effects of oxidant concentration and temperature on decolorization of azo dye: Comparisons of UV/Fenton and UV/Fenton-like systems. Water Sci. Technol. 2012, 65, 1970–1974. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Huo, D.; Liu, H.; Cheng, S.; Ding, M.; Jiang, B.; Zhang, F.; Zhang, Y.; Gao, G. An Investigation of PPy@1T/2H MoS2 Composites with Durable Photothermal-Promoted Effect in Photo-Fenton Degradation of Methylene Blue and in Water Evaporation. Polymers 2023, 15, 3900. https://doi.org/10.3390/polym15193900
Lei Y, Huo D, Liu H, Cheng S, Ding M, Jiang B, Zhang F, Zhang Y, Gao G. An Investigation of PPy@1T/2H MoS2 Composites with Durable Photothermal-Promoted Effect in Photo-Fenton Degradation of Methylene Blue and in Water Evaporation. Polymers. 2023; 15(19):3900. https://doi.org/10.3390/polym15193900
Chicago/Turabian StyleLei, Yanhua, Da Huo, Hui Liu, Sha Cheng, Mengchao Ding, Bochen Jiang, Fei Zhang, Yuliang Zhang, and Guanhui Gao. 2023. "An Investigation of PPy@1T/2H MoS2 Composites with Durable Photothermal-Promoted Effect in Photo-Fenton Degradation of Methylene Blue and in Water Evaporation" Polymers 15, no. 19: 3900. https://doi.org/10.3390/polym15193900





