Hydrogelation of Regenerated Silk Fibroin via Gamma Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silk Fibroin Hydrogel
2.3. Insoluble Gel Fraction Test
2.4. Free Amino Group Evaluation of Silk Fibroin
2.5. Thermal Stability Test
2.6. Transition Temperature Determination
2.7. Structural and Conformational Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Gelation of Silk Fibroin Solution
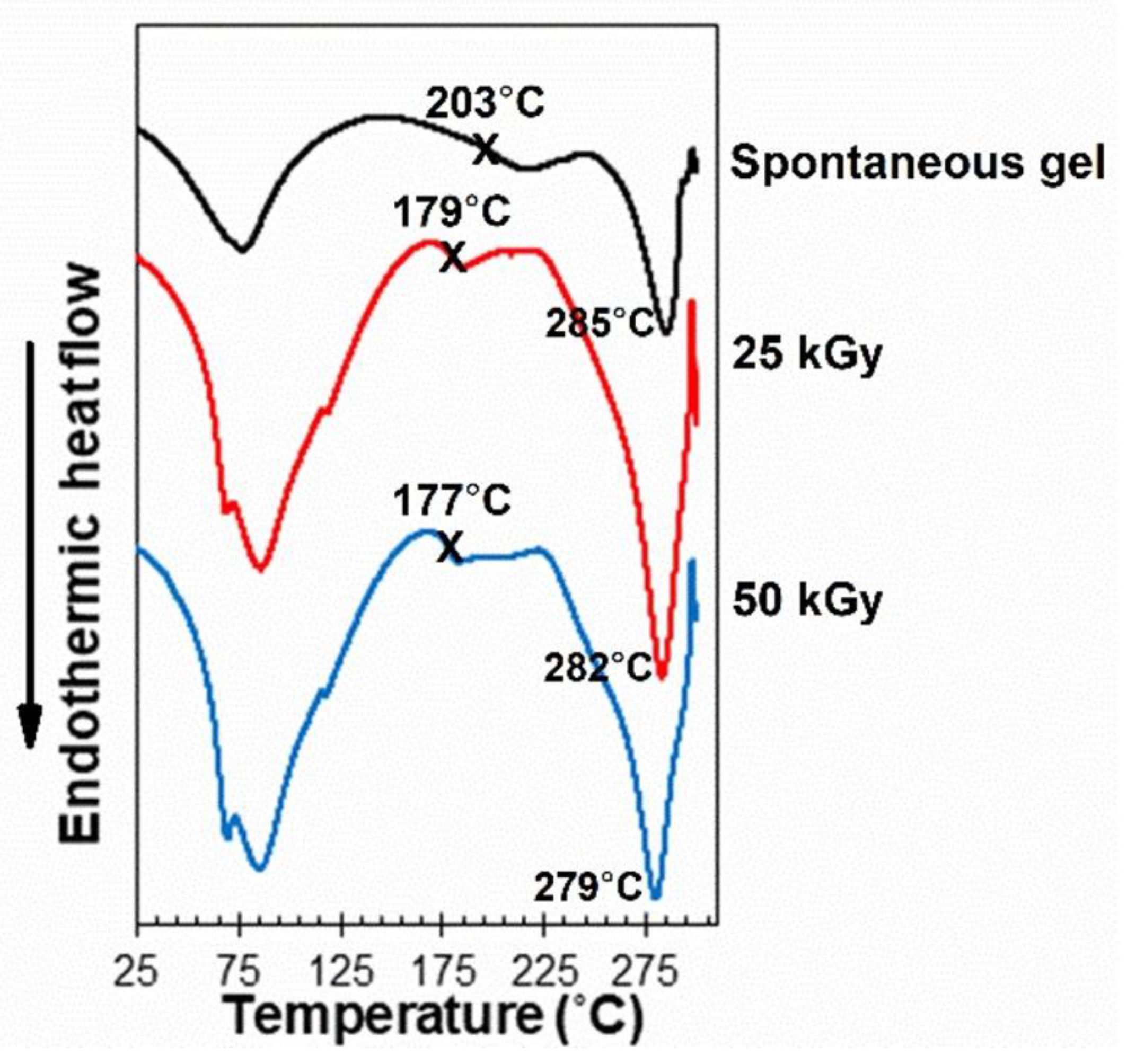
3.2. Thermal Stability
3.3. Thermal Responses of the Hydrogel
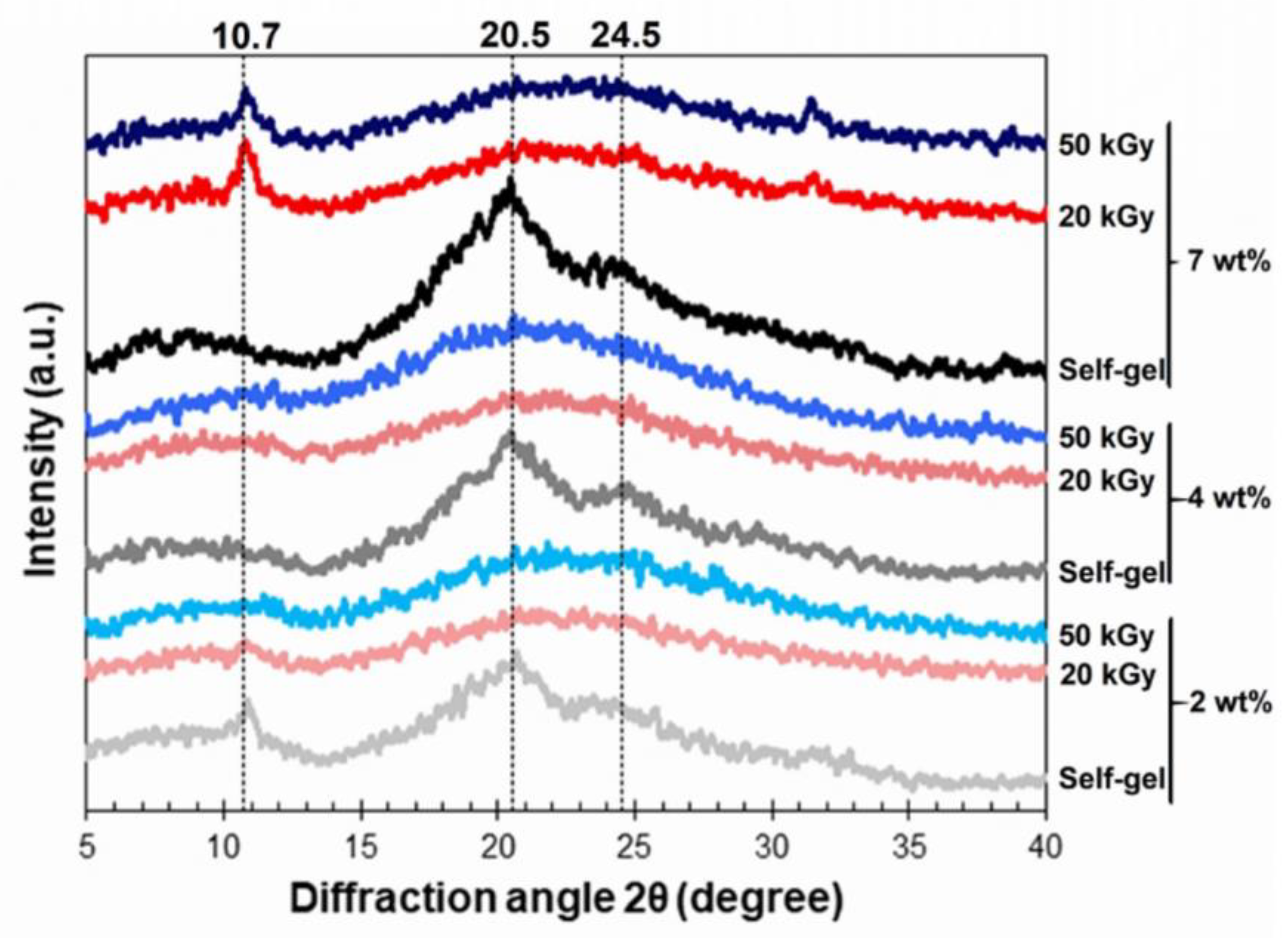
3.4. Chemical Structures of the Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Tungtasana, H.; Damrongsakkul, S.; Shuangshoti, S.; Kanokpanont, S.; Kaplan, D.L.; Bunaprasert, T. Tissue response and biodegradation of composite scaffolds prepared from Thai silk fibroin, gelatin and hydroxyapatite. J. Mater. Sci. Mater. Med. 2010, 21, 3151–3162. [Google Scholar] [CrossRef]
- Sapudom, J.; Kongsema, M.; Methachittipan, A.; Damrongsakkul, S.; Kanokpanont, S.; Teo, J.C.M.; Khongkow, M.; Tonsomboon, K.; Thongnuek, P. Degradation products of crosslinked silk fibroin scaffolds modulate the immune response but not cell toxicity. J. Mater. Chem. B 2023, 11, 3607–3616. [Google Scholar] [CrossRef]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of silk fibroin sol-gel transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef]
- Manissorn, J.; Wattanachai, P.; Tonsomboon, K.; Bumroongsakulsawat, P.; Damrongsakkul, S.; Thongnuek, P. Osteogenic enhancement of silk fibroin-based bone scaffolds by forming hybrid composites with bioactive glass through GPTMS during sol-gel process. Mater. Today Commun. 2020, 26, 101730. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, Y.; Li, M.; Zuo, B.; Lu, S.; Wang, J.; Zhu, H.; Kaplan, D.L. Silk fibroin electrogelation mechanisms. Acta Biomater. 2011, 7, 2394–2400. [Google Scholar] [CrossRef]
- Onder, O.C.; Batool, S.R.; Nazeer, M.A. Self-assembled silk fibroin hydrogels: From preparation to biomedical applications. Mater. Adv. 2022, 3, 6920–6949. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.; Leisk, G.G.; Kaplan, D.L. Sonication-Induced Gelation of Silk Fibroin for Cell Encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef]
- Yucel, T.; Cebe, P.; Kaplan, D.L. Vortex-induced injectable silk fibroin hydrogels. Biophys. J. 2009, 97, 2044–2050. [Google Scholar] [CrossRef]
- Bressner, J.E.; Marelli, B.; Qin, G.; Klinker, L.E.; Zhang, Y.; Kaplan, D.L.; Omenetto, F.G. Rapid fabrication of silk films with controlled architectures via electrogelation. J. Mater. Chem. B 2014, 2, 4983. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, W.H. Chemically cross-linked silk fibroin hydrogel with enhanced elastic properties, Biodegradability, and biocompatibility. Int. J. Nanomed. 2016, 11, 2967–2978. [Google Scholar] [CrossRef]
- Bhattacharya, A. Radiation and industrial polymers. Prog. Polym. Sci. 2000, 25, 371–401. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, B.S.; Lee, J.; Cho, D.; Kwon, O.H.; Park, W.H. Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering. Biomater. Res. 2017, 21, 12. [Google Scholar] [CrossRef]
- Nikolic, V.M.; Krkljes, A.; Popovic, Z.K.; Lausevic, Z.V.; Miljanic, S.S. On the use of gamma irradiation crosslinked PVA membranes in hydrogen fuel cells. Electrochem. Commun. 2007, 9, 2661–2665. [Google Scholar] [CrossRef]
- Oral, E.; Muratoglu, O.K. Radiation cross-linking in ultra-high molecular weight polyethylene for orthopaedic applications. Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 265, 18–22. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Effect of γ-irradiation on the physical and mechanical properties of kefiran biopolymer film. Int. J. Biol. Macromol. 2015, 74, 343–350. [Google Scholar] [CrossRef]
- Haji-saeid, M.; Safrany, A.; Sampa, M.H.D.O.; Ramamoorthy, N. Radiation processing of natural polymers: The IAEA contribution. Radiat. Phys. Chem. 2010, 79, 255–260. [Google Scholar] [CrossRef]
- Okhawilai, M.; Rangkupan, R.; Kanokpanont, S.; Damrongsakkul, S. Preparation of Thai silk fibroin/gelatin electrospun fiber mats for controlled release applications. Int. J. Biol. Macromol. 2010, 46, 544–550. [Google Scholar] [CrossRef]
- Watchararot, T.; Prasongchean, W.; Thongnuek, P. Angiogenic property of silk fibroin scaffolds with adipose-derived stem cells on chick chorioallantoic membrane. R. Soc. Open Sci. 2021, 8, 201618. [Google Scholar] [CrossRef] [PubMed]
- Bubnis, W.A.; Ofner, C.M. The determination of ϵ-amino groups in soluble and poorly soluble proteinaceous materials by a spectrophotometrie method using trinitrobenzenesulfonic acid. Anal. Biochem. 1992, 207, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kaewprasit, K.; Kobayashi, T.; Damrongsakkul, S. Thai silk fibroin gelation process enhancing by monohydric and polyhydric alcohols. Int. J. Biol. Macromol. 2018, 118, 1726–1735. [Google Scholar] [CrossRef]
- Lugao, A.B.; Malmonge, S.M. Use of radiation in the production of hydrogels. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 185, 37–42. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. Prog. Mol. Environ. Bioeng. 2003, 51, 117–150. [Google Scholar] [CrossRef]
- Ming, J.; Li, M.; Han, Y.; Chen, Y.; Li, H.; Zuo, B.; Pan, F. Novel two-step method to form silk fibroin fibrous hydrogel. Mater. Sci. Eng. C 2016, 59, 185–192. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable Materials Based on Silk Fibroin and Keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef]
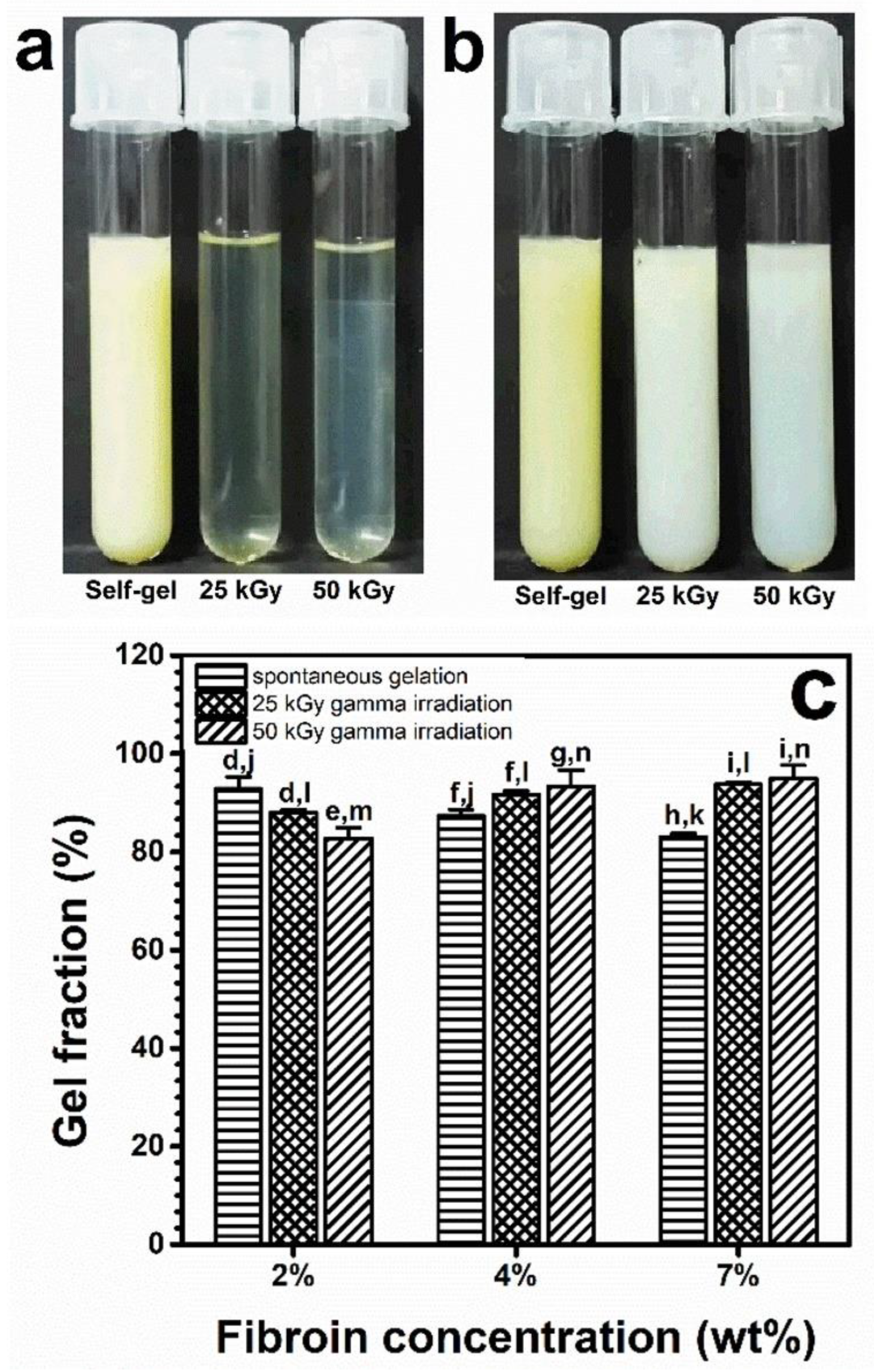

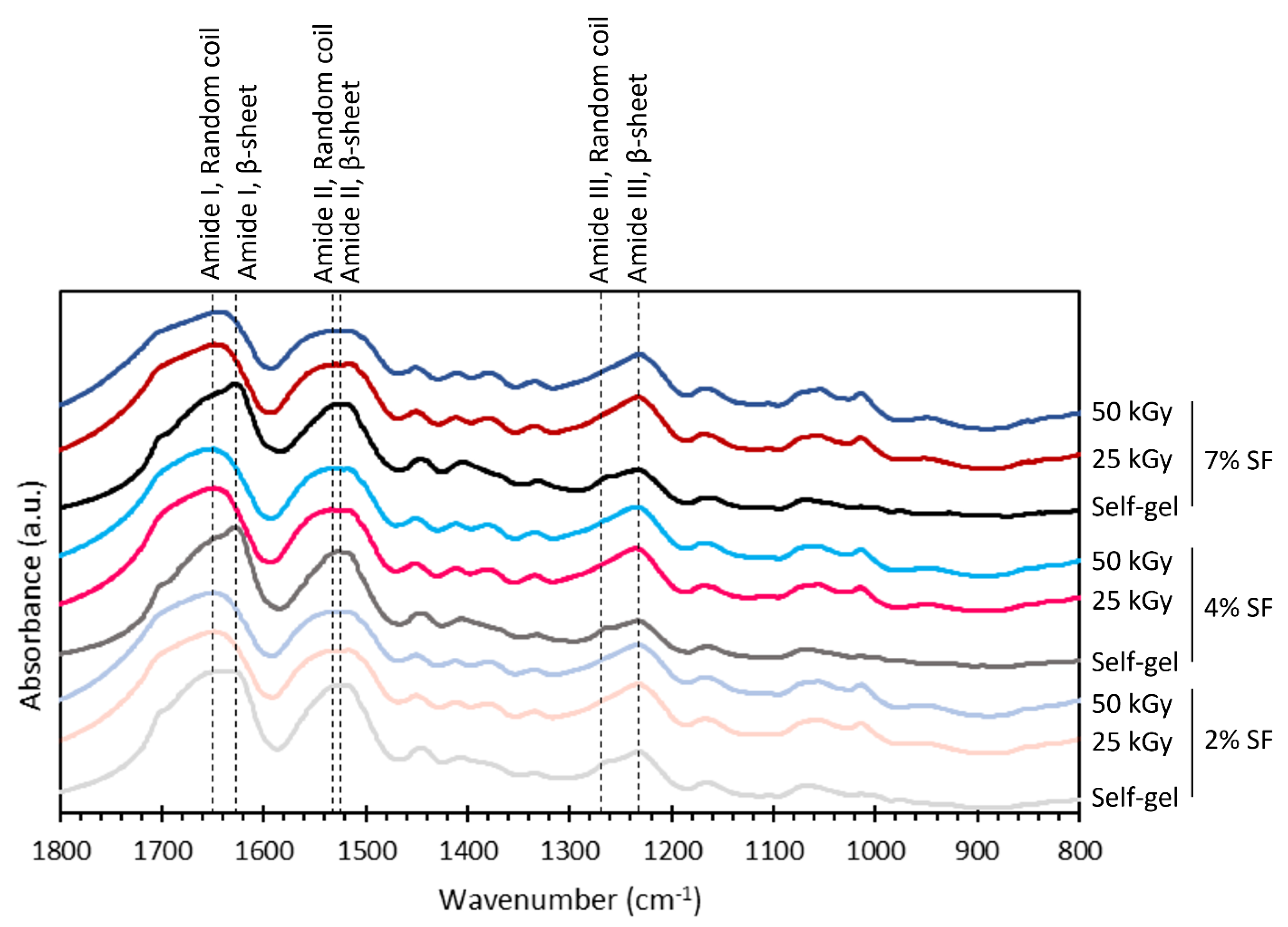
 2 wt%,
2 wt%,
 4 wt%, and
4 wt%, and  7 wt%.
7 wt%.
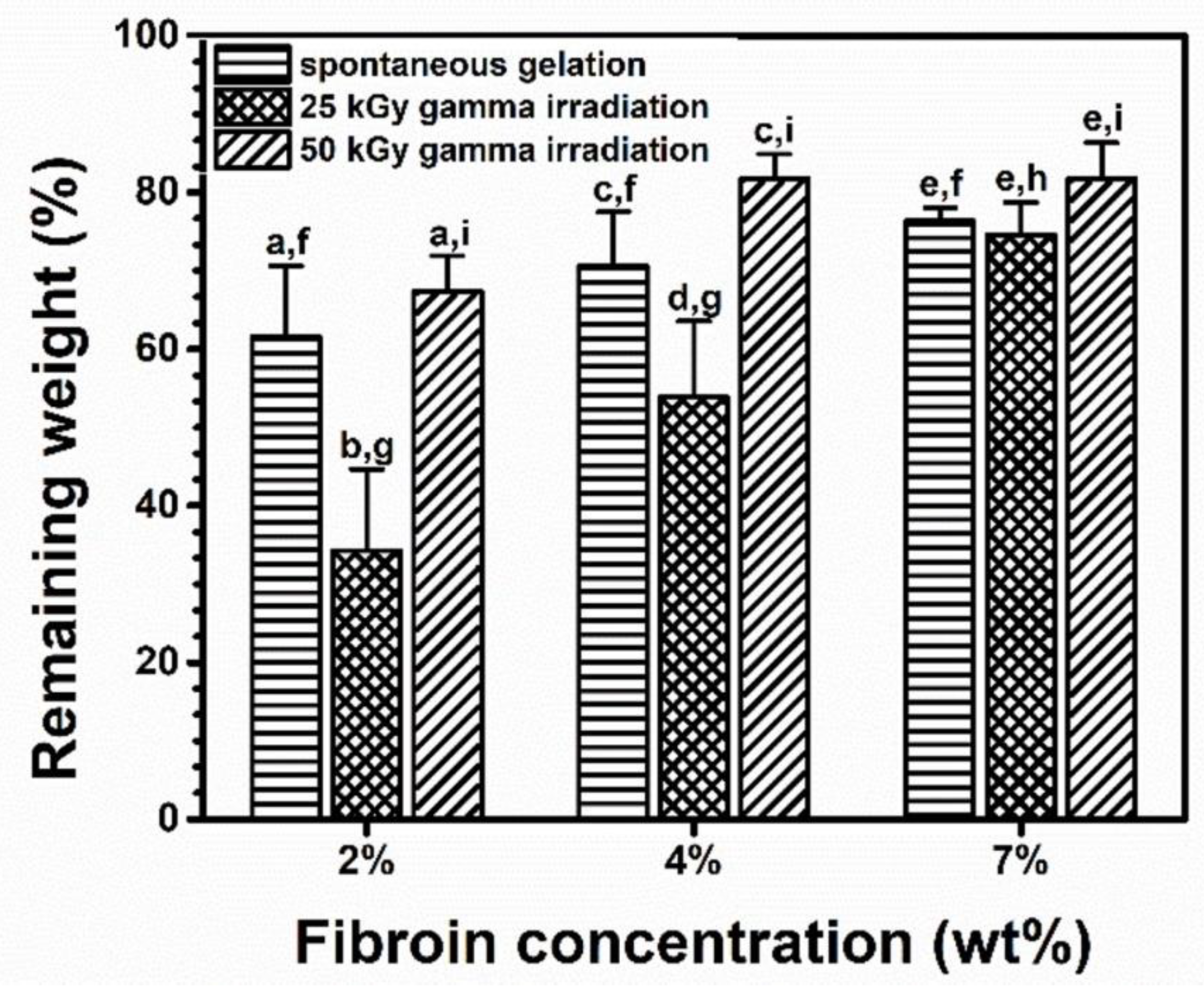
 2 wt%,
2 wt%,
 4 wt%, and
4 wt%, and  7 wt%.
7 wt%.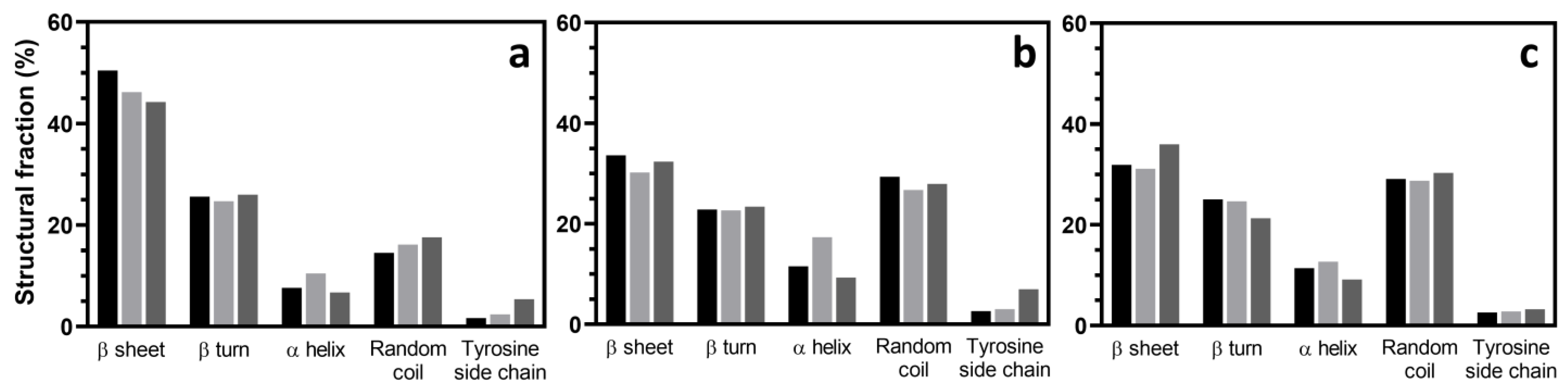
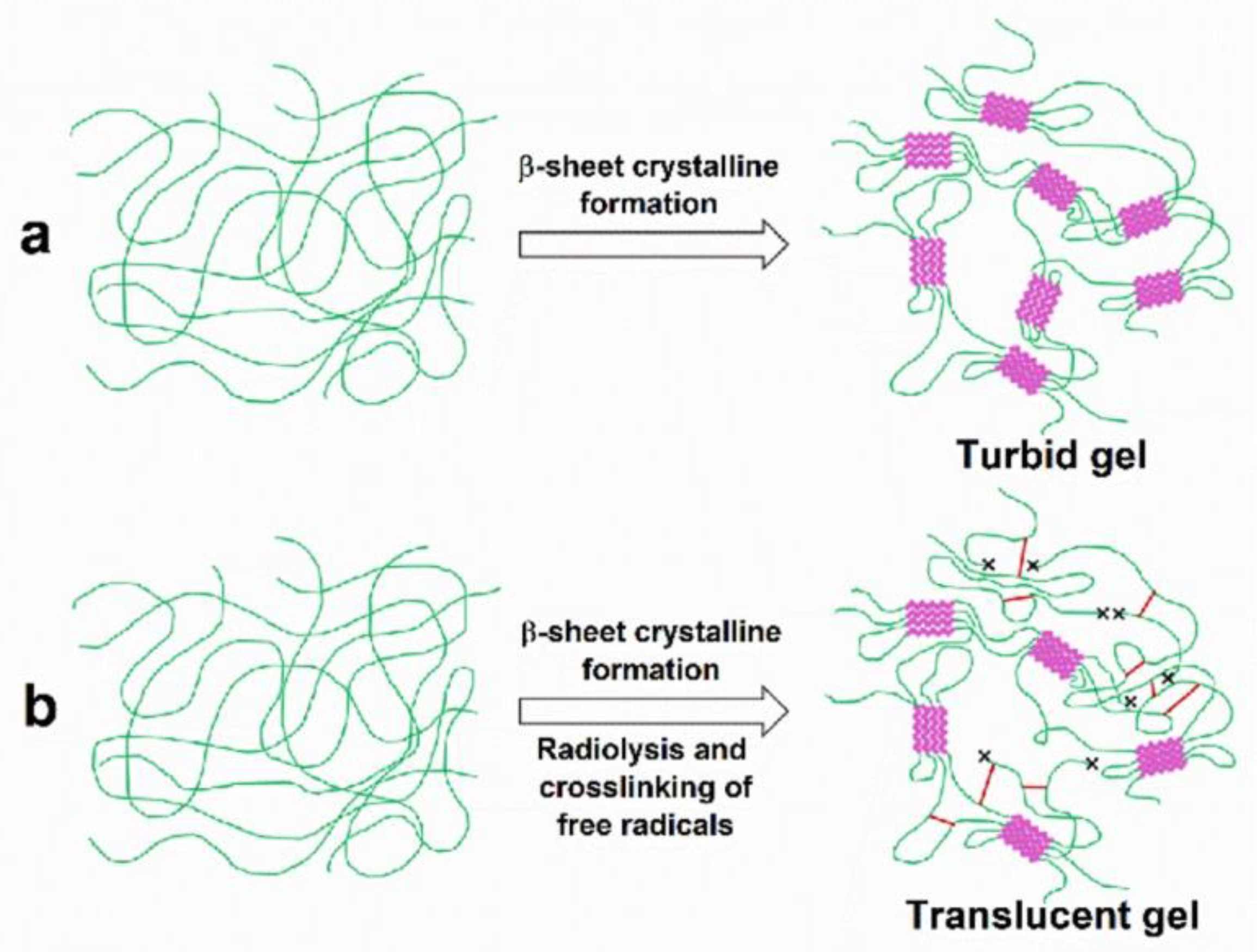
| Fibroin Concentration (wt%) | Spontaneous Gelation at 25 °C | 25 kGy | 50 kGy |
|---|---|---|---|
| 1% | Turbid gel | No gel | No gel |
| 2% | Turbid gel | Translucent gel | Translucent gel |
| 4% | Turbid gel | Translucent gel | Translucent gel |
| 7% | Turbid gel | Translucent gel | Translucent gel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongnuek, P.; Kanokpanont, S.; Uttayarat, P.; Damrongsakkul, S. Hydrogelation of Regenerated Silk Fibroin via Gamma Irradiation. Polymers 2023, 15, 3734. https://doi.org/10.3390/polym15183734
Thongnuek P, Kanokpanont S, Uttayarat P, Damrongsakkul S. Hydrogelation of Regenerated Silk Fibroin via Gamma Irradiation. Polymers. 2023; 15(18):3734. https://doi.org/10.3390/polym15183734
Chicago/Turabian StyleThongnuek, Peerapat, Sorada Kanokpanont, Pimpon Uttayarat, and Siriporn Damrongsakkul. 2023. "Hydrogelation of Regenerated Silk Fibroin via Gamma Irradiation" Polymers 15, no. 18: 3734. https://doi.org/10.3390/polym15183734





