Experimental and Environmental Analysis of High-Strength Geopolymer Based on Waste Bricks and Blast Furnace Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Used Materials
2.2. Employed Experimental Methods
2.3. Carbon Dioxide Emissions Assessment
3. Results and Discussion
3.1. Phase Composition and SEM Analysis
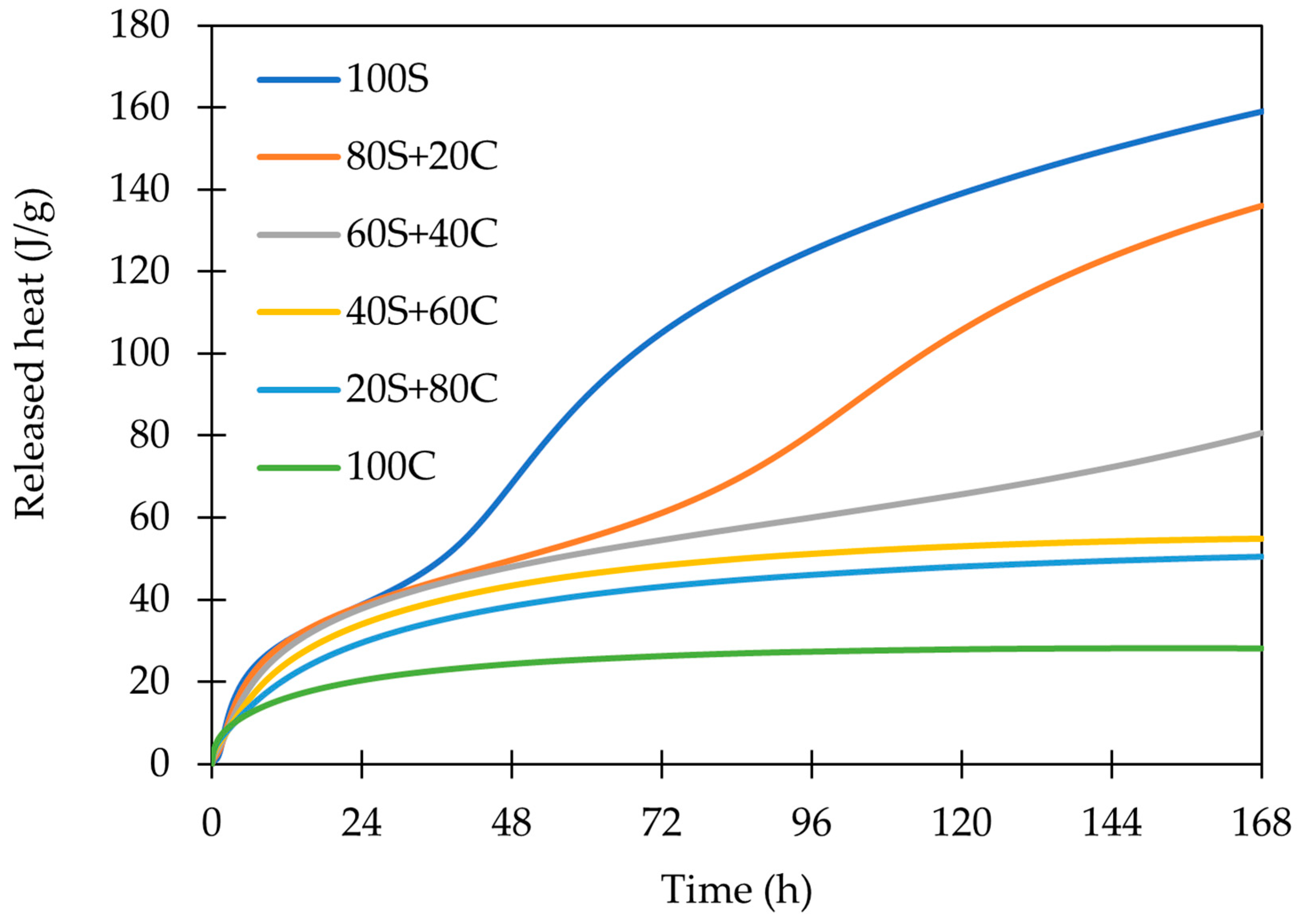
3.2. Isothermal Calorimetry
3.3. Basic Materials Properties
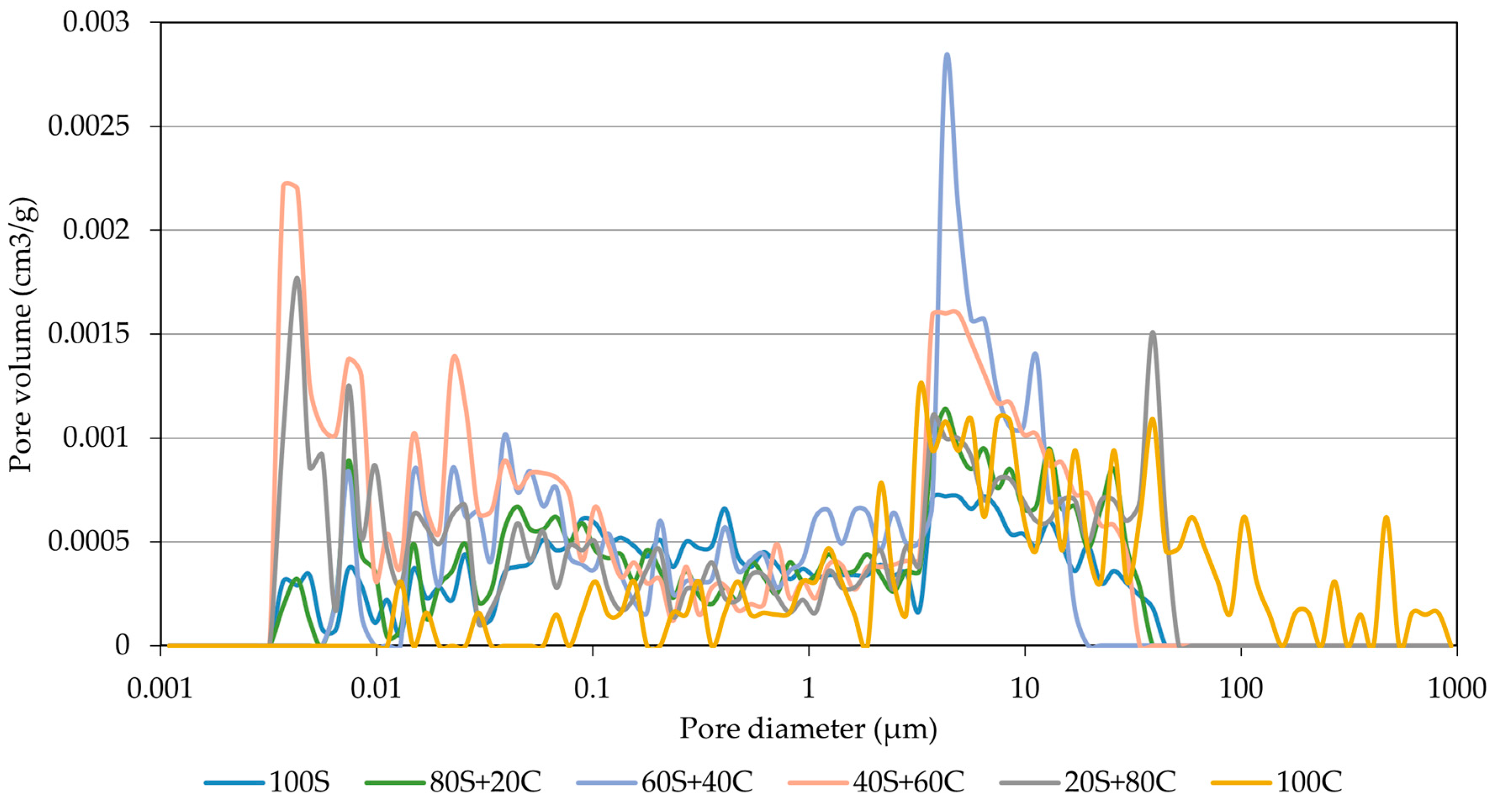
3.4. MIP Analysis
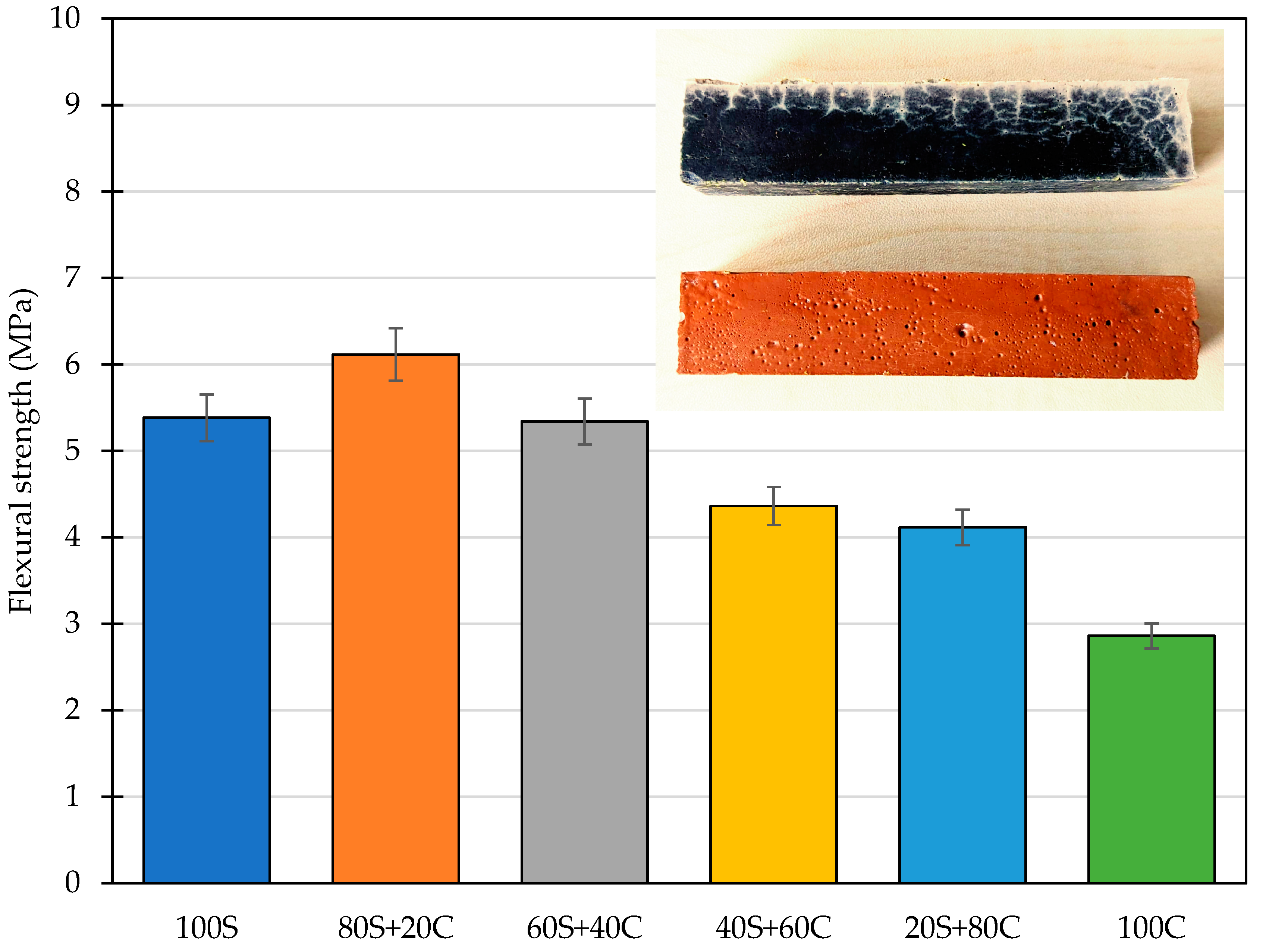
3.5. Mechanical Properties
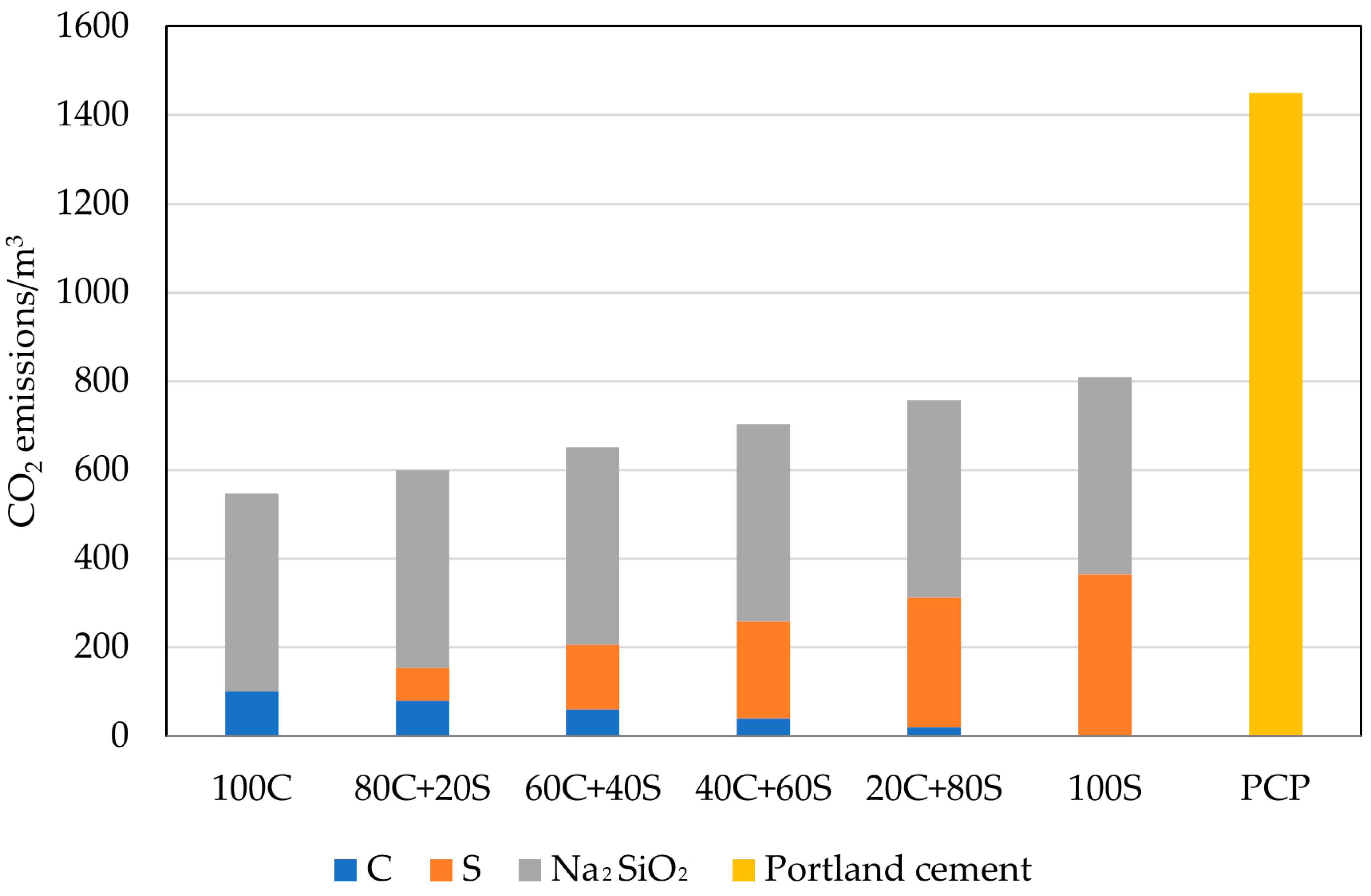
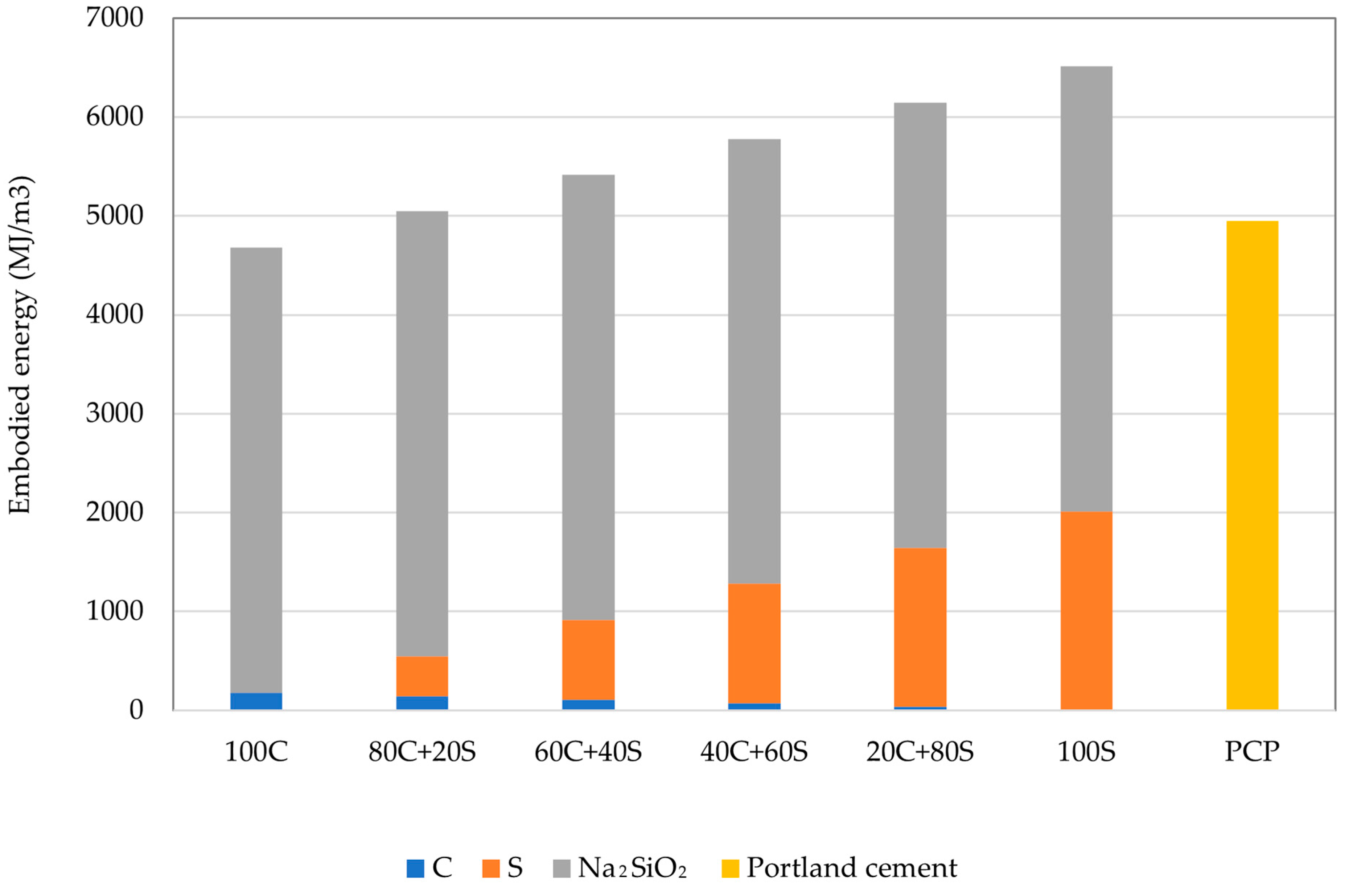
3.6. Carbon Dioxide Emissions
4. Conclusions
- WBP with lowered content of the amorphous portion compared to GBFS impaired the compressive strength of designed mixtures proportionally. However, even pure WBP can be used as the sole precursor despite the negative results described in the available literature [21]. A high level of compressive strength with WBP content up to 60 wt.% shows a way for the replacement of also high-performance building materials and significant valorization of waste materials.
- The flexural strength was substantially reduced for mixtures with a dominant portion of GBFS to values around 4–5 MPa. These findings correspond to the crack formation as the result of drying shrinkage. On the other hand, this phenomenon was mitigated by the addition of WBP and the dual role of the low-amorphous precursors.
- The employed calorimetry analysis revealed significant differences in the evolution of the hydration heat of both precursors. While the GBFS clearly shows the dissolving, polycondensation, and rearrangements peaks, only the dissolution peak was observed for WBP. Notwithstanding, at a very early age, this peak was significantly more intensive; thus, it may imply the very fact occurrence of all steps (dissolution, polycondensation, rearrangement).
- Despite the fact that geopolymers are investigated mainly with the aim of greening the construction industry, significant environmental savings were obtained only for the CO2 emissions. The analysis of the embodied energy revealed a huge impact of using alkaline activators that damaged the potential benefits associated with the utilization of waste or by-products. Described conclusions point to the importance of wider boundary conditions within the environmental assessment and the risk of potentially transferring the environmental burden to another area of protection apart from climate change.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 27. [Google Scholar] [CrossRef]
- Vo, D.H.; Hwang, C.L.; Thi, K.D.T.; Yehualaw, M.D.; Liao, M.C.; Chao, Y.F. HPC produced with CDW as a partial replacement for fine and coarse aggregates using the Densified Mixture Design Algorithm (DMDA) method: Mechanical properties and stability in development. Constr. Build. Mater. 2021, 270, 121441. [Google Scholar] [CrossRef]
- Colangelo, F.; Navarro, T.G.; Farina, I.; Petrillo, A. Comparative LCA of concrete with recycled aggregates: A circular economy mindset in Europe. Int. J. Life Cycle Assess. 2020, 25, 1790–1804. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Valencia-Saavedra, W.; de Gutierrez, R.M. Construction and Demolition Waste (CDW) Recycling-As Both Binder and Aggregates-In Alkali-Activated Materials: A Novel Re-Use Concept. Sustainability 2020, 12, 5775. [Google Scholar] [CrossRef]
- Galan, B.; Viguri, J.R.; Cifrian, E.; Dosal, E.; Andres, A. Influence of input streams on the construction and demolition waste (CDW) recycling performance of basic and advanced treatment plants. J. Clean. Prod. 2019, 236, 117523. [Google Scholar] [CrossRef]
- Zeng, Y.S.; Quek, S.T.; Tang, A.P.; Zhou, X.Y. Review of Residual Properties of Concrete under Freezing-and-Thawing Loading. ACI Mater. J. 2020, 117, 93–103. [Google Scholar] [CrossRef]
- de Brito, J.; Kurda, R. The past and future of sustainable concrete: A critical review and new strategies on cement-based materials. J. Clean. Prod. 2021, 281, 72. [Google Scholar] [CrossRef]
- Park, S.; Wu, S.; Liu, Z.C.; Pyo, S. The Role of Supplementary Cementitious Materials (SCMs) in Ultra High Performance Concrete (UHPC): A Review. Materials 2021, 14, 1472. [Google Scholar] [CrossRef]
- Vejmelkova, E.; Konakova, D.; Dolezelova, M.; Scheinherrova, L.; Svora, P.; Keppert, M.; Reiterman, P.; Cerny, R. Effect of calcined Czech claystone on the properties of high performance concrete: Microstructure, strength and durability. Constr. Build. Mater. 2018, 168, 966–974. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H.J.H. Development of an eco-friendly Ultra-High Performance Concrete (UHPC) with efficient cement and mineral admixtures uses. Cem. Concr. Compos. 2015, 55, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Guo, R.H. Applications of Steel Slag Powder and Steel Slag Aggregate in Ultra-High Performance Concrete. Adv. Civ. Eng. 2018, 2018, 1426037. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Yu, R.; Shui, Z.H.; Gao, X.; Xiao, X.G.; Zhang, X.B.; Wang, Y.Y.; He, Y.J. Low carbon design of an Ultra-High Performance Concrete (UHPC) incorporating phosphorous slag. J. Clean. Prod. 2019, 240, 118157. [Google Scholar] [CrossRef]
- Wu, Z.M.; Shi, C.J.; He, W. Comparative study on flexural properties of ultra-high performance concrete with supplementary cementitious materials under different curing regimes. Constr. Build. Mater. 2017, 136, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Koci, V.; Petrikova, M.; Fort, J.; Fiala, L.; Cerny, R. Preparation of self-heating alkali-activated materials using industrial waste products. J. Clean. Prod. 2020, 260, 8. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, B.; Zhou, H.; Ma, Y.; Jiang, X. A state-of-the-art review of crushed urban waste glass used in OPC and AAMs (geopolymer): Progress and challenges. Clean. Mater. 2022, 4, 100083. [Google Scholar] [CrossRef]
- Mills, J.; Mondal, P.; Wagner, N. Structure-property relationships and state behavior of alkali-activated aluminosilicate gels. Cem. Concr. Res. 2022, 151, 106618. [Google Scholar] [CrossRef]
- Fiala, L.; Pommer, V.; Bohm, M.; Scheinherrova, L.; Cerny, R. Self-heating alkali activated materials: Microstructure and its effect on electrical, thermal and mechanical properties. Constr. Build. Mater. 2022, 335, 361–368. [Google Scholar] [CrossRef]
- Blanco, I.; D’Angelo, A.; Viola, V.; Vertuccio, L.; Catauro, M. Metakaolin-based geopolymers filled with volcanic fly ashes: FT-IR, thermal characterization, and antibacterial property. Sci. Eng. Compos. Mater. 2023, 30, 20220192. [Google Scholar] [CrossRef]
- Fort, J.; Novotny, R.; Vejmelkova, E.; Trnik, A.; Rovnanikova, P.; Keppert, M.; Pommer, V.; Cerny, R. Characterization of geopolymers prepared using powdered brick. J. Mater. Res. Technol. 2019, 8, 6253–6261. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzo, J.; Cheeseman, C.R.; Paya, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.L.; Yehualaw, M.D.; Vo, D.H.; Huyn, T.P. Development of high-strength alkali-activated pastes containing high volumes of waste brick and ceramic powders. Constr. Build. Mater. 2019, 218, 519–529. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.A.; Vasquez-Garcia, S.R.; Israde-Alcantara, I.; Flores-Ramirez, N.; Rico, J.L.; Mohammed, M.S. Cleaner production of one-part white geopolymer cement using pre-treated wood biomass ash and diatomite. J. Clean. Prod. 2019, 209, 1420–1428. [Google Scholar] [CrossRef]
- Rakhimova, N.R.; Rakhimov, R.Z. Alkali-activated cements and mortars based on blast furnace slag and red clay brick waste. Mater. Des. 2015, 85, 324–331. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Vasic, M.V.; Terzic, A.; Radovanovic, Z.; Radojevic, Z.; Warr, L.N. Alkali-activated geopolymerization of a low illitic raw clay and waste brick mixture. An alternative to traditional ceramics. Appl. Clay Sci. 2022, 218, 106410. [Google Scholar] [CrossRef]
- Fort, J.; Cerny, R. Transition to circular economy in the construction industry: Environmental aspects of waste brick recycling scenarios. Waste Manag. 2020, 118, 510–520. [Google Scholar] [CrossRef]
- Caldas, L.R.; Da Gloria, M.Y.R.; Pittau, F.; Andreola, V.M.; Habert, G.; Toledo, R.D. Environmental impact assessment of wood bio-concretes: Evaluation of the influence of different supplementary cementitious materials. Constr. Build. Mater. 2021, 268, 13. [Google Scholar] [CrossRef]
- Fort, J.; Mildner, M.; Keppert, M.; Cerny, R. Waste solidified alkalis as activators of aluminosilicate precursors: Functional and environmental evaluation. J. Build. Eng. 2022, 54, 104598. [Google Scholar] [CrossRef]
- Fort, J.; Vejmelkova, E.; Konakova, D.; Alblova, N.; Cachova, M.; Keppert, M.; Rovnanikova, P.; Cerny, R. Application of waste brick powder in alkali activated aluminosilicates: Functional and environmental aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
- Vaclavik, V.; Ondova, M.; Dvorsky, T.; Estokova, A.; Fabianova, M.; Gola, L. Sustainability Potential Evaluation of Concrete with Steel Slag Aggregates by the LCA Method. Sustainability 2020, 12, 9873. [Google Scholar] [CrossRef]
- Palod, R.; Deo, S.V.; Ramtekkar, G.D. Utilization of waste from steel and iron industry as replacement of cement in mortars. J. Mater. Cycles Waste Manag. 2019, 21, 1361–1375. [Google Scholar] [CrossRef]
- Najimi, M.; Ghafoori, N.; Sharbaf, M. Alkali-activated natural pozzolan/slag mortars: A parametric study. Constr. Build. Mater. 2018, 164, 625–643. [Google Scholar] [CrossRef]
- Shen, J.L.; Li, Y.; Lin, H.; Lv, J.F.; Feng, S.; Ci, J.C. Early properties and chemical structure analysis of alkali-activated brick geopolymer with varied alkali dosage. J. Build. Eng. 2022, 60, 105186. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J.; Magrinho, M. Red clay brick and tungsten mining waste-based alkali-activated binder: Microstructural and mechanical properties. Constr. Build. Mater. 2018, 190, 1034–1048. [Google Scholar] [CrossRef]
- Soultana, A.; Valouma, A.; Bartzas, G.; Komnitsas, K. Properties of Inorganic Polymers Produced from Brick Waste and Metallurgical Slag. Minerals 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, G.; Kul, A.; Ozcelikci, E.; Sahmaran, M.; Aldemir, A.; Figueira, D.; Ashour, A. Development of alkali-activated binders from recycled mixed masonry-originated waste. J. Build. Eng. 2021, 33, 12. [Google Scholar] [CrossRef]
- Li, Y.; Shen, J.L.; Lin, H.; Lv, J.F.; Feng, S.; Ci, J.C. Properties and environmental assessment of eco-friendly brick powder geopolymer binders with varied alkali dosage. J. Build. Eng. 2022, 58, 105020. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Yap, S.P.; Alengaram, U.J.; Ling, T.C. Potential use of brick waste as alternate concrete-making materials: A review. J. Clean. Prod. 2018, 195, 226–239. [Google Scholar] [CrossRef]
- Ye, T.H.; Xiao, J.Z.; Duan, Z.H.; Li, S.S. Geopolymers made of recycled brick and concrete powder—A critical review. Constr. Build. Mater. 2022, 330, 127232. [Google Scholar] [CrossRef]
- Fort, J.; Vejmelkova, E.; Keppert, M.; Rovnanikova, P.; Bezdicka, P.; Cerny, R. Alkaline activation of low-reactivity ceramics: Peculiarities induced by the precursors’ dual character. Cem. Concr. Compos. 2020, 105, 103440. [Google Scholar] [CrossRef]
- Koci, V.; Konakova, D.; Pommer, V.; Keppert, M.; Vejmelkova, E.; Cerny, R. Exploiting advantages of empirical and optimization approaches to design alkali activated materials in a more efficient way. Constr. Build. Mater. 2021, 292, 123460. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, T.A. Effects of Composition Type and Activator on Fly Ash-Based Alkali Activated Materials. Polymers 2022, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.Y.; Bernal, S.A.; Provis, J.L.; Lothenbach, B. Thermodynamic modelling of phase evolution in alkali-activated slag cements exposed to carbon dioxide. Cem. Concr. Res. 2020, 136, 106158. [Google Scholar] [CrossRef]
- Ke, X.Y.; Duan, Y. Coupling machine learning with thermodynamic modelling to develop a composition-property model for alkali-activated materials. Compos. Part B Eng. 2021, 216, 108801. [Google Scholar] [CrossRef]
- Salas, D.A.; Ramirez, A.D.; Ulloa, N.; Baykara, H.; Boero, A.J. Life cycle assessment of geopolymer concrete. Constr. Build. Mater. 2018, 190, 170–177. [Google Scholar] [CrossRef]
- Bianco, I.; Tomos, B.A.; Vinai, R. Analysis of the environmental impacts of alkali-activated concrete produced with waste glass-derived silicate activator-A LCA study. J. Clean. Prod. 2021, 316, 12. [Google Scholar] [CrossRef]
- Sandanayake, M.; Law, D.; Sargent, P. A new framework for assessing the environmental impacts of circular economy friendly soil waste-based geopolymer cements. Build. Environ. 2022, 210, 108702. [Google Scholar] [CrossRef]
- Kavlak, G.; McNerney, J.; Trancik, J.E. Evaluating the causes of cost reduction in photovoltaic modules. Energy Policy 2018, 123, 700–710. [Google Scholar] [CrossRef] [Green Version]
- Fawer, M.; Concannon, M.; Rieber, W. Life cycle inventories for the production of sodium silicates. Int. J. Life Cycle Assess. 1999, 4, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Fort, J.; Mildner, M.; Keppert, M.; Abed, M.; Cerny, R. Potential of industrial waste as alternative alkaline activator for development of eco-efficient mortars. Case Stud. Constr. Mater. 2023, 18, e01716. [Google Scholar] [CrossRef]
- Vinai, R.; Soutsos, M. Production of sodium silicate powder from waste glass cullet for alkali activation of alternative binders. Cem. Concr. Res. 2019, 116, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Tong, K.T.; Vinai, R.; Soutsos, M.N. Use of Vietnamese rice husk ash for the production of sodium silicate as the activator for alkali-activated binders. J. Clean. Prod. 2018, 201, 272–286. [Google Scholar] [CrossRef] [Green Version]
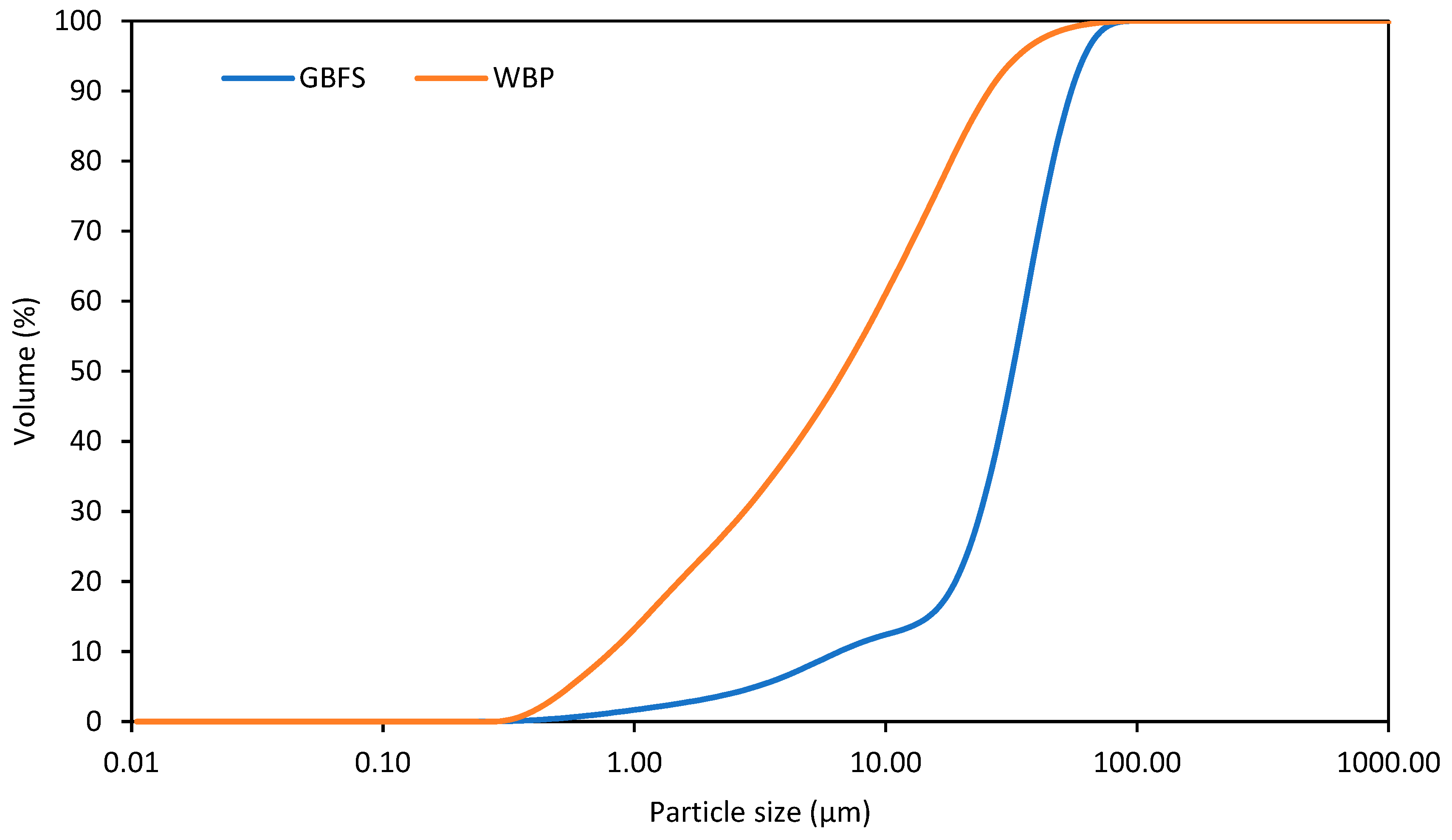

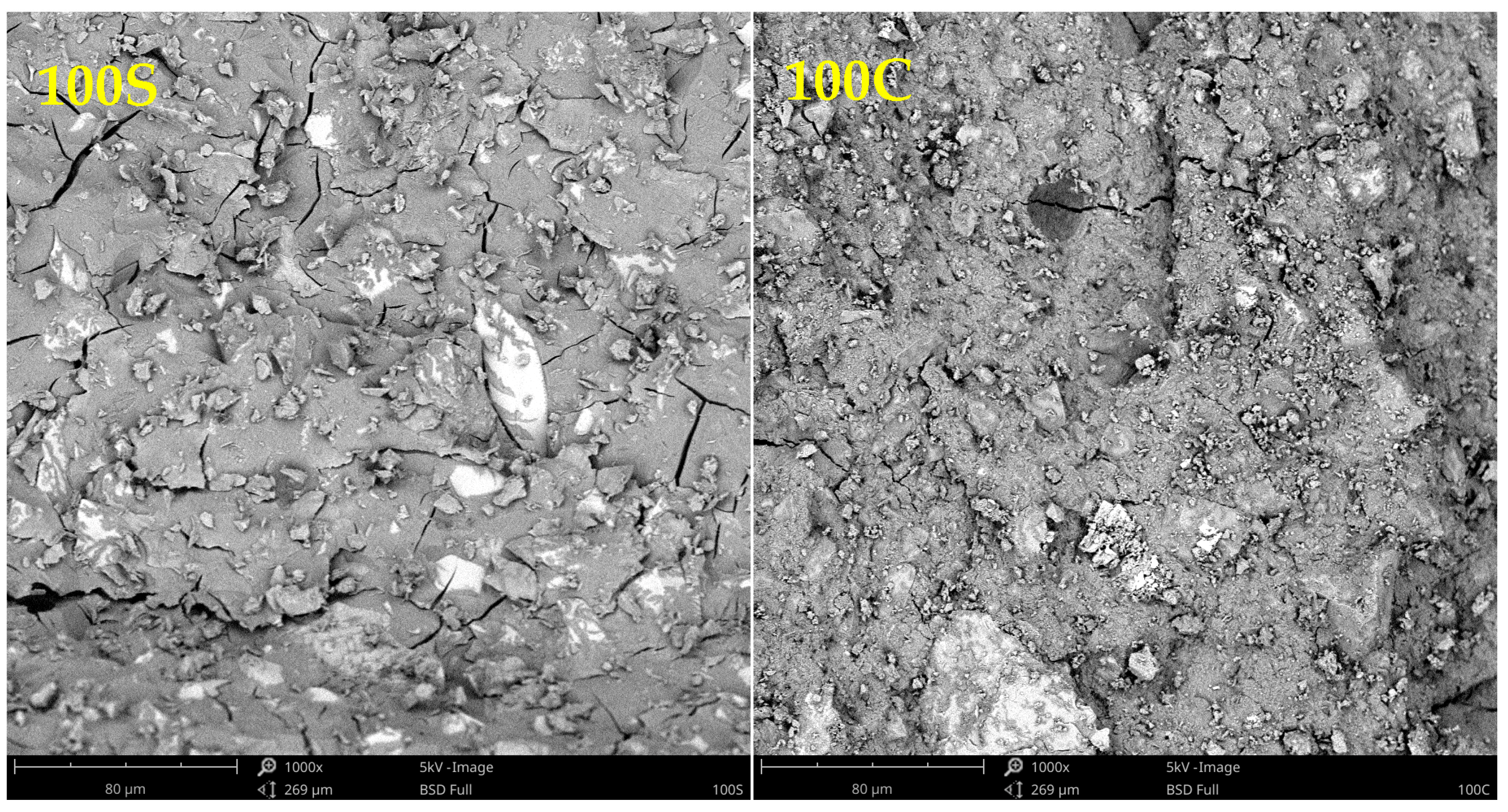
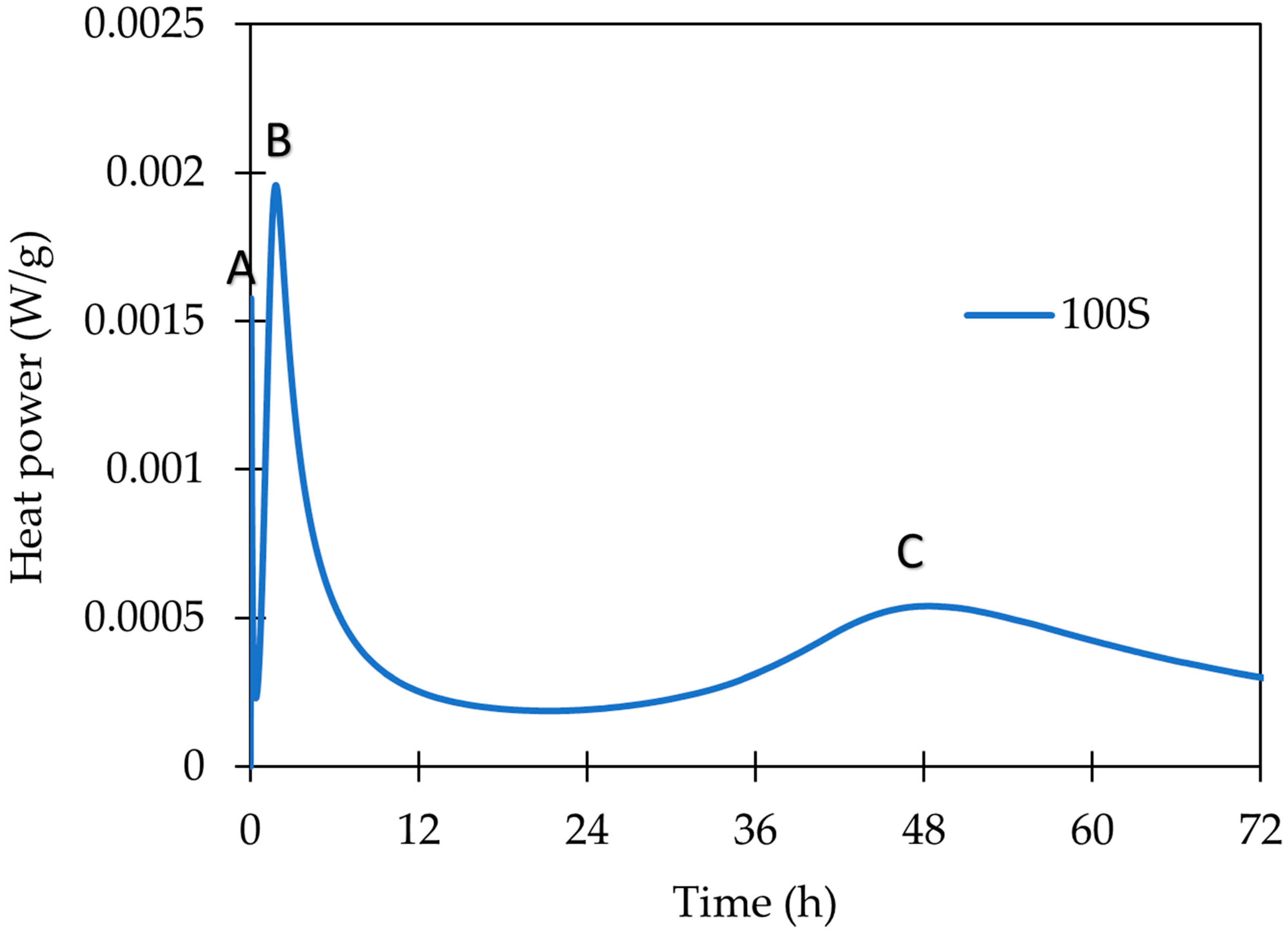
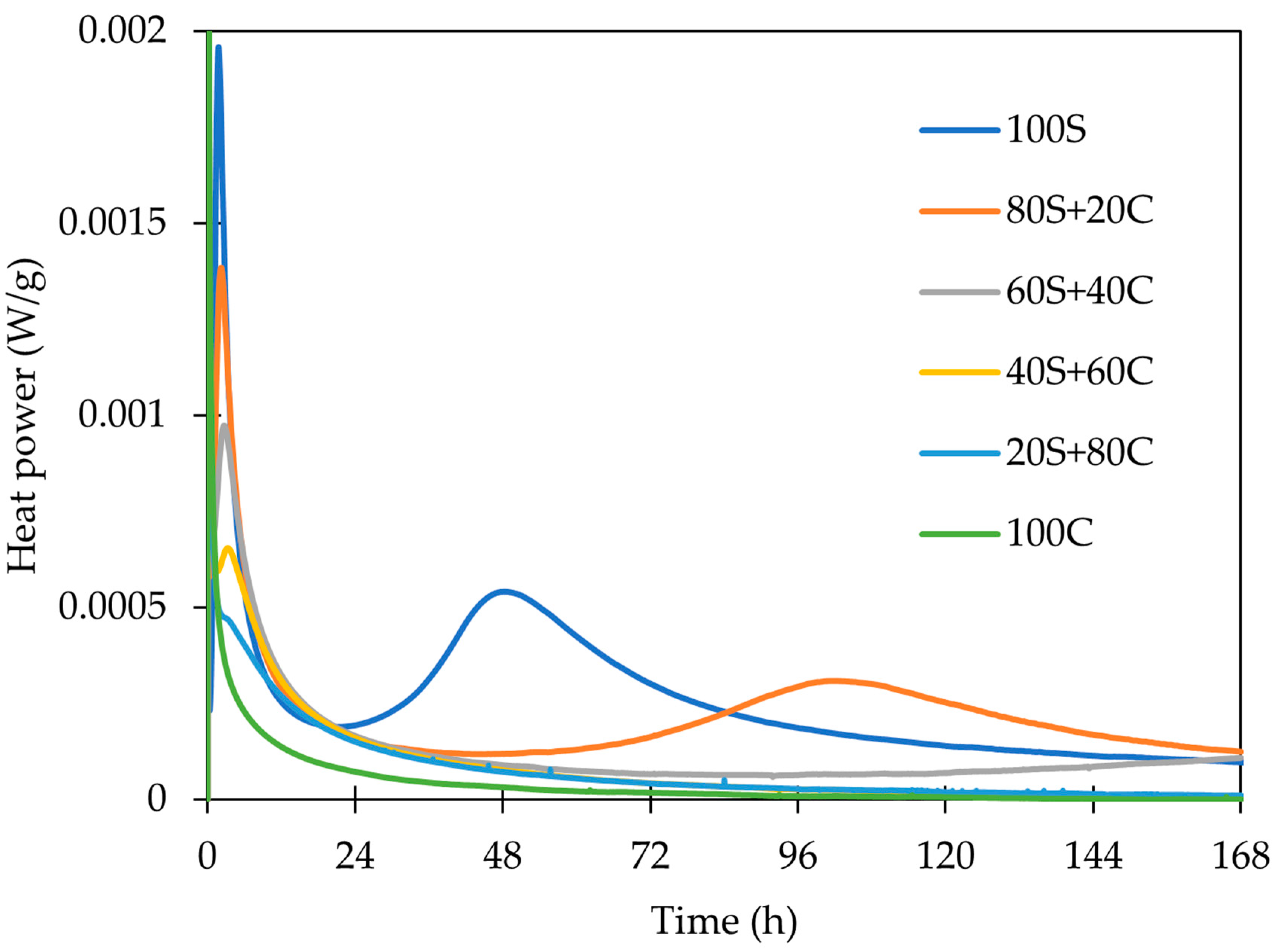


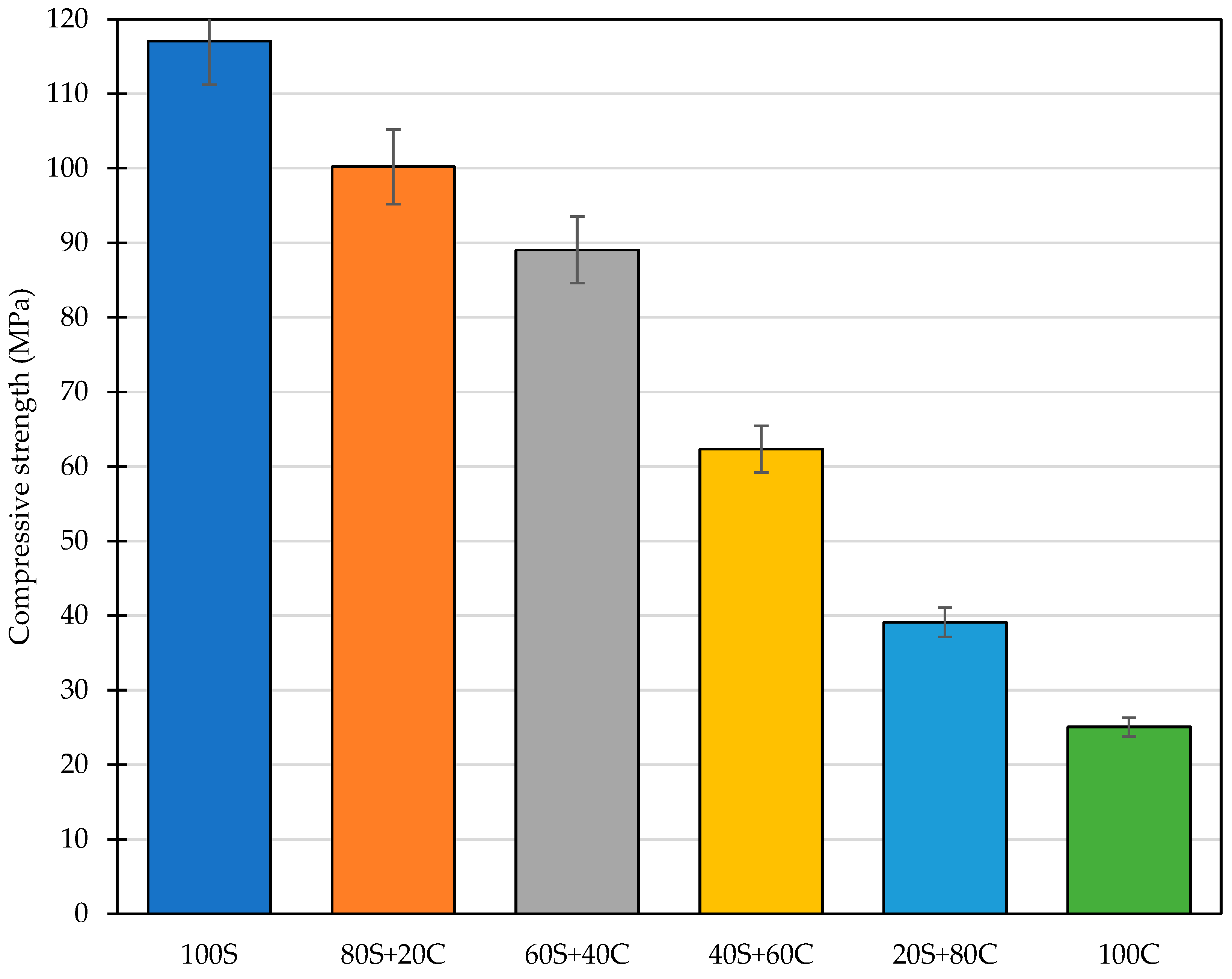
| GBFS | WBP | ||
|---|---|---|---|
| Amorphous phase | - | 82 | 30 |
| Akermanite | Ca2Mg(Si2O7) | 12 | - |
| Calcite | CaCO3 | 5 | - |
| Quartz | SiO2 | 1 | 53 |
| Anorthite | CaAl2Si2O8 | - | 8 |
| Microcline | KAlSi3O8 | - | 4 |
| Orthoclase | KAlSi3O8 | - | 1 |
| Muscovite | KAl2(AlSi3O10)(F, OH)2 | - | 4 |
| SiO2 | CaO | Al2O3 | MgO | MnO | K2O | SO3 | Fe2O3 | Na2O | TiO2 | BaO | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GBFS | 39.1 | 38.8 | 9.8 | 8.7 | 0.9 | 0.7 | 0.6 | 0.5 | 0.4 | 0.3 | 0.1 |
| WBP | 58.8 | 6.9 | 19.6 | 2.8 | - | 2.9 | 0.7 | 5.7 | 1.5 | 0.8 | - |
| Mixture | WBP (g) | GBFS (g) | Sodium Silicate (M = 1.6) (g) | WA (g) | Water (g) | Precursor Si/Al Ratio |
|---|---|---|---|---|---|---|
| 100C | 900 | 0 | 375 | 30 | 0 | 2.64 |
| 80C+20S | 720 | 180 | 375 | 30 | 0 | 2.87 |
| 60C+40S | 540 | 360 | 375 | 30 | 0 | 3.06 |
| 40C+60S | 360 | 180 | 375 | 30 | 5 | 3.23 |
| 20C+80S | 180 | 720 | 375 | 30 | 10 | 3.38 |
| 100S | 0 | 900 | 375 | 30 | 15 | 3.52 |
| Mixture | Bulk Density (kg/m3) | Matrix Density (kg/m3) |
|---|---|---|
| 100S | 2040.9 | 2496.0 |
| 80S+20C | 1952.6 | 2333.0 |
| 60S+40S | 1902.3 | 2381.8 |
| 40S+60C | 1900.8 | 2490.0 |
| 20S+80C | 1916.5 | 2520.2 |
| 100C | 1905.3 | 2486.5 |
| Mixture | Ci (kg CO2/MPa) | Ei (MJ/MPa) |
|---|---|---|
| 100C | 21.83 | 187.29 |
| 80C+20S | 15.15 | 127.81 |
| 60C+40S | 10.50 | 87.32 |
| 40C+60S | 7.91 | 64.94 |
| 20C+80S | 7.56 | 61.46 |
| 100S | 6.91 | 55.65 |
| PCP | 22.31 | 76.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fořt, J.; Mildner, M.; Keppert, M.; Pommer, V.; Černý, R. Experimental and Environmental Analysis of High-Strength Geopolymer Based on Waste Bricks and Blast Furnace Slag. Polymers 2023, 15, 3092. https://doi.org/10.3390/polym15143092
Fořt J, Mildner M, Keppert M, Pommer V, Černý R. Experimental and Environmental Analysis of High-Strength Geopolymer Based on Waste Bricks and Blast Furnace Slag. Polymers. 2023; 15(14):3092. https://doi.org/10.3390/polym15143092
Chicago/Turabian StyleFořt, Jan, Martin Mildner, Martin Keppert, Vojtěch Pommer, and Robert Černý. 2023. "Experimental and Environmental Analysis of High-Strength Geopolymer Based on Waste Bricks and Blast Furnace Slag" Polymers 15, no. 14: 3092. https://doi.org/10.3390/polym15143092






