A Novel UV Barrier Poly(lactic acid)/Poly(butylene succinate) Composite Biodegradable Film Enhanced by Cellulose Extracted from Coconut Shell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Cellulose from Coconut Shells
2.3. Preparation of PLA/PBS/Cellulose Blended Films
2.4. Characterization of Primary Components of Coconut Shell and Composite Films
3. Results and Discussion
3.1. Morphology and Yield of Cellulose
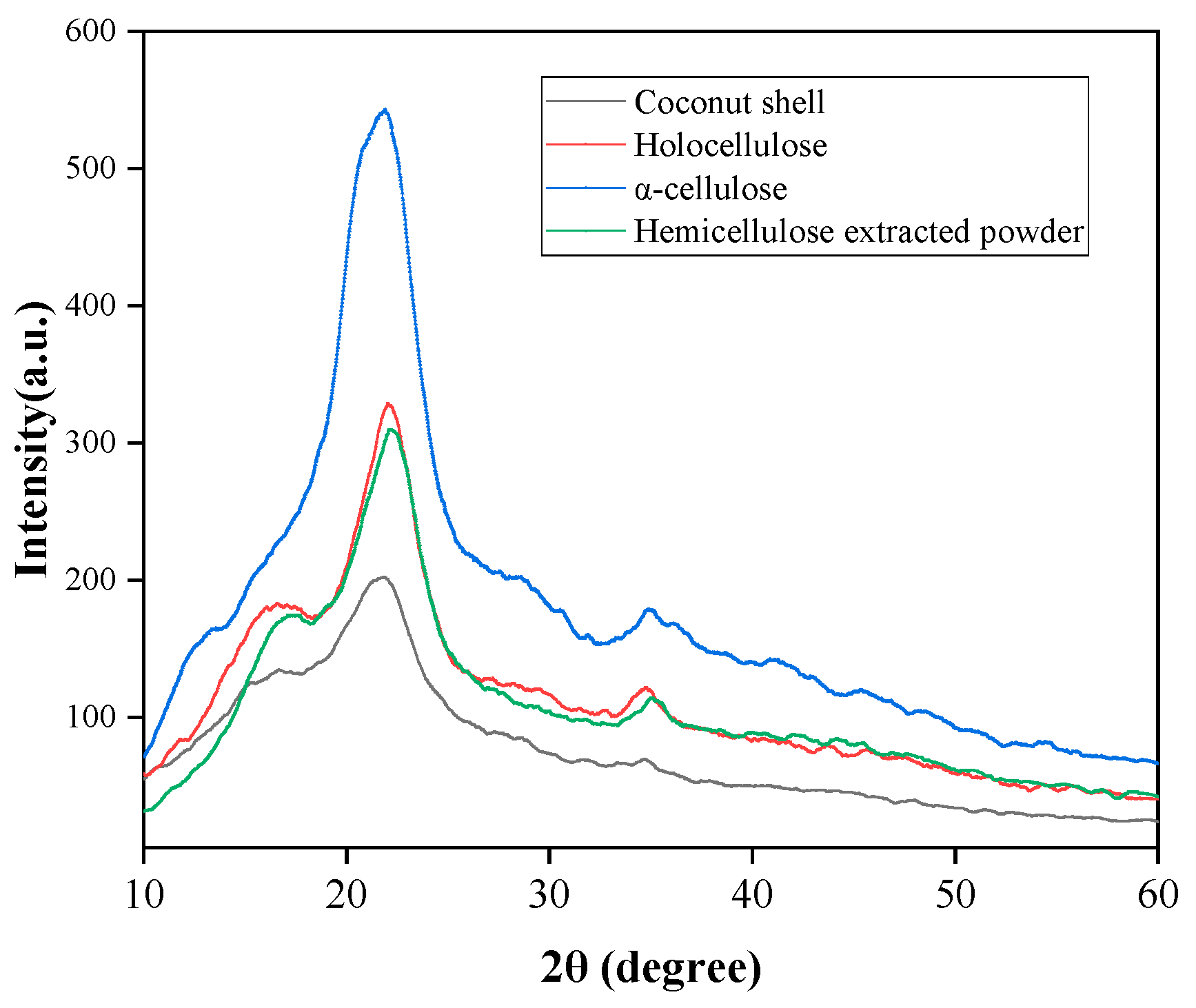
3.2. Crystallinity of CSP and Primary Components
3.3. Spectroscopic Characterization
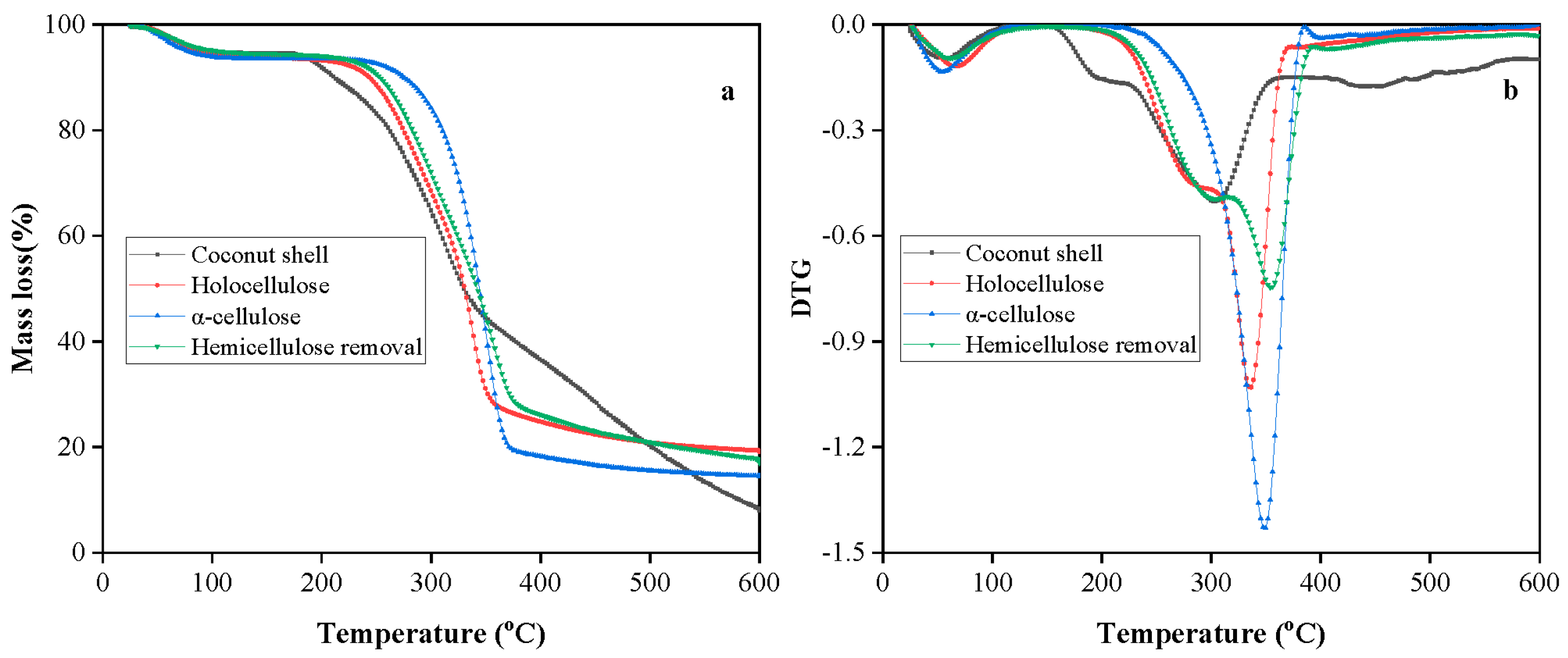
3.4. Thermal Analysis of Fibers
3.5. Morphology of Films
3.6. Effect of Cellulose on Crystallization Behaviour of Films
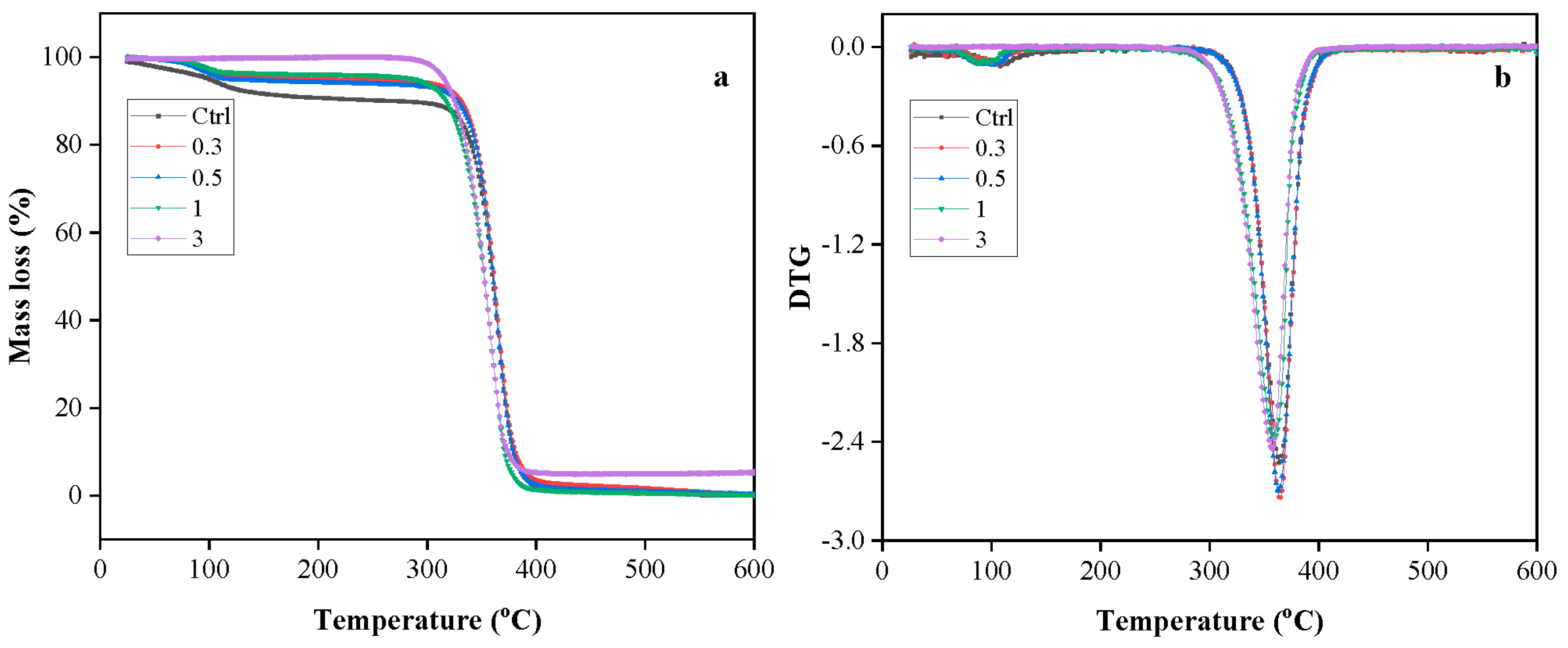
3.7. Effect of Cellulose on Thermal Stability of Films
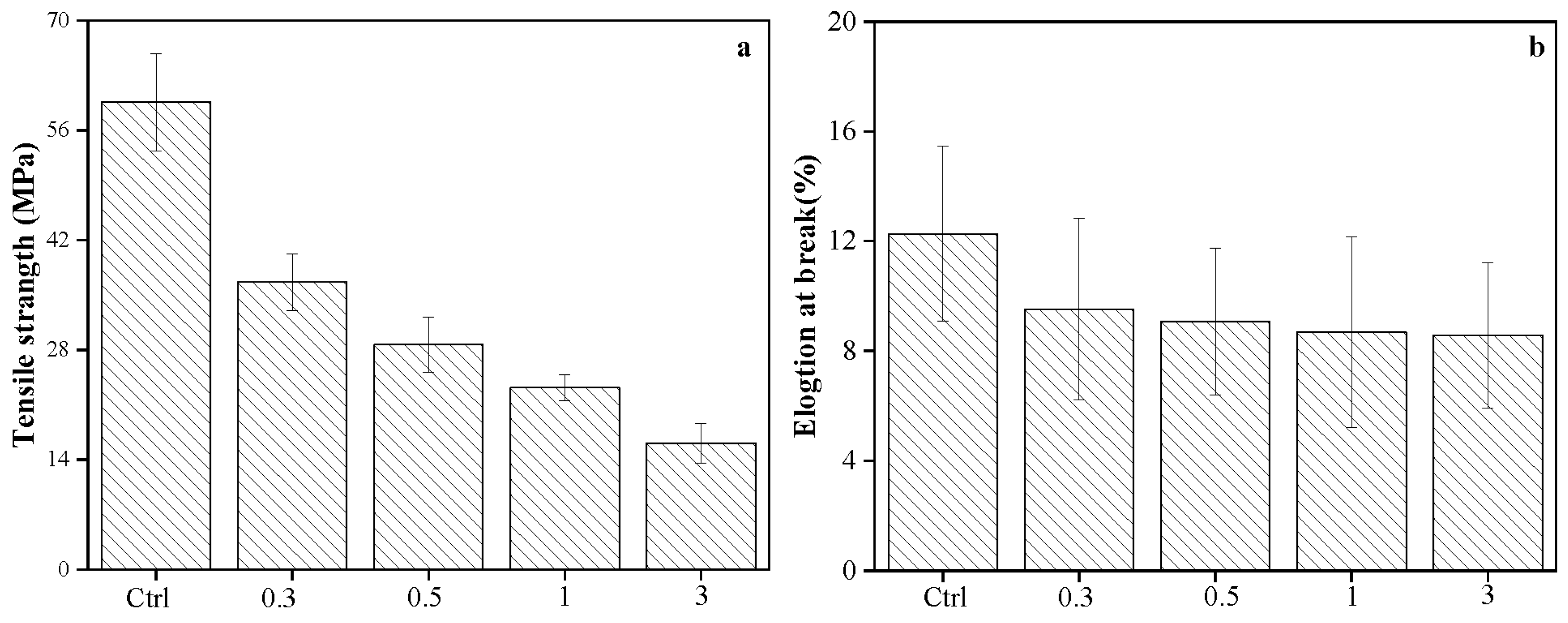
3.8. Effect of Cellulose on Mechanical Property of Films
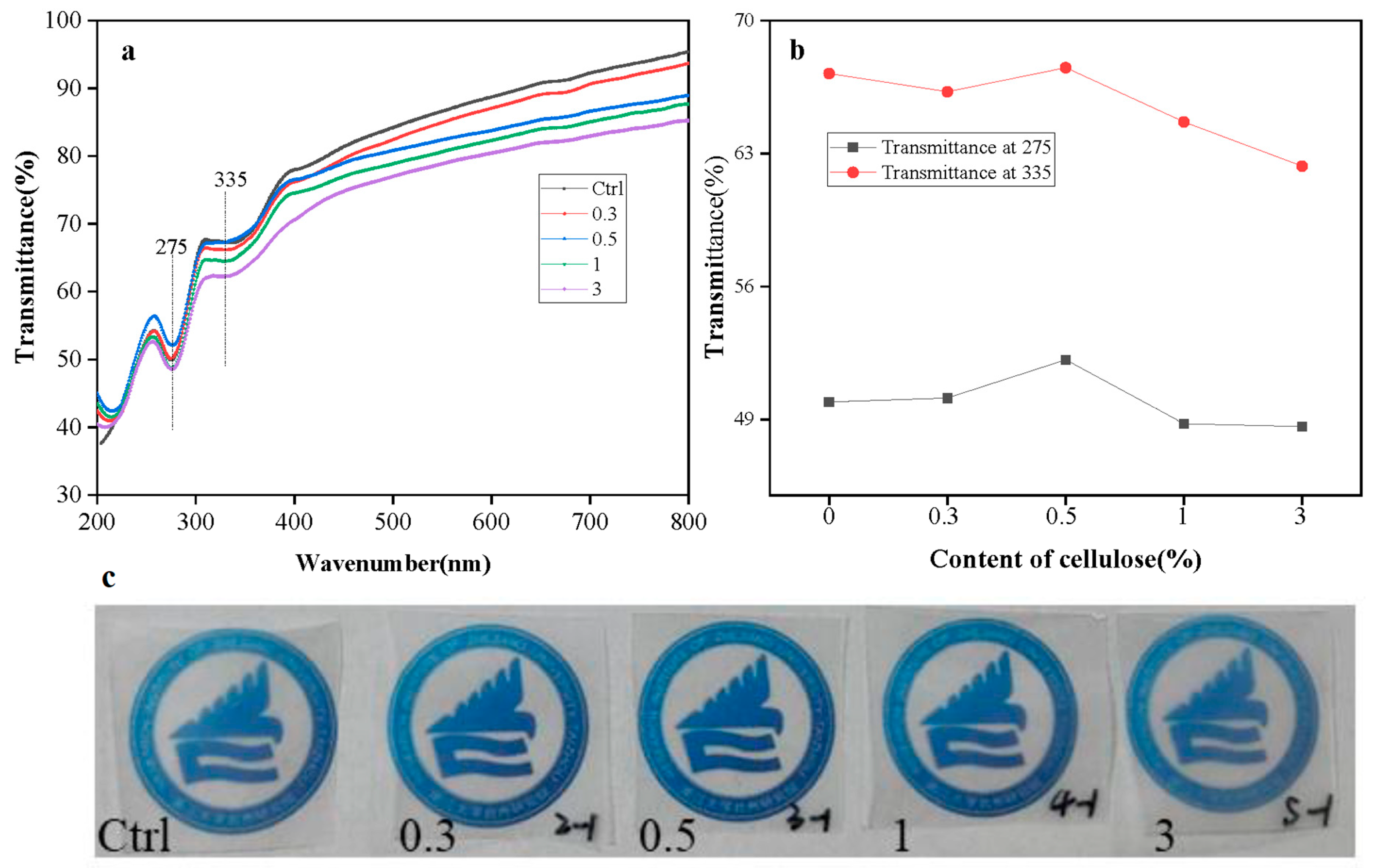
3.9. Effect of Cellulose on UV Transmittance of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Da Costa, F.A.T.; Parra, D.F.; Cardoso, E.C.L.; Güven, O. PLA, PBAT, Cellulose nanocrystals (CNCs), and their Blends: Biodegradation, Ccompatibilization, and nanoparticle interactions. J. Polym. Environ. 2023, 1–29. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Pol. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; López-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A comprehensive review on polylactic acid (PLA)–Synthesis, processing and application in food packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Li, J.; Liang, X.; Zhou, W.; Peng, S. Strategies and techniques for improving heat resistance and mechanical performances of poly(lactic acid)(PLA) biodegradable materials. Int. J. Biol. Macromol. 2022, 218, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Jeong, Y.G. Improvement in thermal stability, elastic modulus, and impact strength of Poly(lactic acid) blends with modified polyketone. Polymer 2022, 257, 125281. [Google Scholar] [CrossRef]
- Shah, B.L.; Selke, S.E.; Walters, M.B.; Heiden, P.A. Effects of wood flour and chitosan on mechanical, chemical, and thermal properties of polylactide. Polym. Compos. 2008, 29, 655–663. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.A.; Majumdar, A.; Butola, B.S. Tailoring the mechanical and thermal properties of polylactic acid-based bionanocomposite films using halloysite nanotubes and polyethylene glycol by solvent casting process. J. Mater. Sci. 2019, 54, 8971–8983. [Google Scholar] [CrossRef]
- Supthanyakul, R.; Kaabbuathong, N.; Chirachanchai, S. Random poly (butylene succinate-co-lactic acid) as a multi-functional additive for miscibility, toughness, and clarity of PLA/PBS blends. Polymer 2016, 105, 1–9. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly (butylene succinate)(PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Wu, Q.; Ren, J. Preparation, mechanical, and thermal properties of biodegradable polyesters/poly(lactic acid) blends. J. Nanomater. 2010, 2010, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yokohara, T.; Yamaguchi, M. Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Bhatia, A.; Gupta, R.; Bhattacharya, S.; Choi, H. Compatibility of biodegradable poly (lactic acid) (PLA) and poly (butylene succinate) (PBS) blends for packaging application. Korea-Aust. Rheol. J. 2007, 19, 125–131. [Google Scholar]
- Shibata, M.; Inoue, Y.; Miyoshi, M. Mechanical properties, morphology, and crystallization behavior of blends of poly (L-lactide) with poly (butylene succinate-co-L-lactate) and poly (butylene succinate). Polymer 2006, 47, 3557–3564. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Cinelli, P.; Coltelli, M.-B.; Lazzeri, A. Essential Work of Fracture and Evaluation of the Interfacial Adhesion of Plasticized PLA/PBSA Blends with the Addition of Wheat Bran by-Product. Polymers 2022, 14, 615. [Google Scholar] [CrossRef]
- Guo, Z.; Song, W.; Wei, X.; Feng, Y.; Song, Y.; Guo, Z.; Cheng, W.; Miao, W.; Cheng, B.; Song, S. Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites. Polymers 2023, 23, 20230026. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Inoue, Y. Mechanical properties, morphologies, and crystallization behavior of plasticized poly (L-lactide)/poly (butylene succinate-co-L-lactate) blends. Polymer 2007, 48, 2768–2777. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Z.; Ju, Y.; Zhou, M.; Bai, H.; Fu, Q. Design of biodegradable PLA/PBAT blends with balanced toughness and strength via interfacial compatibilization and dynamic vulcanization. Polymer 2023, 266, 125620. [Google Scholar] [CrossRef]
- Lin, S.; Guo, W.; Chen, C.; Ma, J.; Wang, B. Mechanical properties and morphology of biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends compatibilized by transesterification. Mater. Design (1980–2015) 2012, 36, 604–608. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stabil. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Toncelli, C.; Ciardelli, F.; Bronco, S. Compatible blends of biorelated polyesters through catalytic transesterification in the melt. Polym. Degrad. Stabil. 2011, 96, 982–990. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Zhang, Y.; Wan, C.; Ma, P. Toughening modification of PLLA/PBS blends via in situ compatibilization. Polym. Eng. Sci. 2009, 49, 26–33. [Google Scholar] [CrossRef]
- Sanivada, U.K.; Mármol, G.; Brito, F.; Fangueiro, R. PLA composites reinforced with flax and jute fibers—A review of recent trends, processing parameters and mechanical properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H.; Zaidi, A.A. Natural fiber reinforced composites: Sustainable materials for emerging applications. Results Eng. 2021, 11, 100263. [Google Scholar] [CrossRef]
- Singha, A.S.; Thakur, V.K. Morphological, thermal, and physicochemical characterization of surface modified pinus fibers. Int. J. Polym. Anal. Charact. 2009, 14, 271–289. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Raw natural fiber–based polymer composites. Int. J. Polym. Anal. Charact. 2014, 19, 256–271. [Google Scholar] [CrossRef]
- Kwon, S.; Zambrano, M.C.; Pawlak, J.J.; Venditti, R.A. Effect of lignocellulosic fiber composition on the aquatic biodegradation of wood pulps and the isolated cellulose, hemicellulose and lignin components: Kinetic modelling of the biodegradation process. Cellulose 2021, 28, 2863–2877. [Google Scholar] [CrossRef]
- Amaral, H.R.; Cipriano, D.F.; Santos, M.S.; Schettino Jr, M.A.; Ferreti, J.V.; Meirelles, C.S.; Pereira, V.S.; Cunha, A.G.; Emmerich, F.G.; Freitas, J.C. Production of high-purity cellulose, cellulose acetate and cellulose-silica composite from babassu coconut shells. Carbohydr. Polym. 2019, 210, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ben Azouz, K.; Ramires, E.C.; Van den Fonteyne, W.; El Kissi, N.; Dufresne, A. Simple method for the melt extrusion of a cellulose nanocrystal reinforced hydrophobic polymer. ACS Macro Lett. 2012, 1, 236–240. [Google Scholar] [CrossRef]
- Kalla, A.M.; Franklin, M.E.E.; Pushpadassa, H.A.; MH, S.K.; Battula, S.N. Isolation and characterization of cellulose from coconut shell powder and its reinforcement in casein films. Pharma Innov. J. 2022, SP-11, 1043–1053. [Google Scholar]
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and characterization of cellulose nanofibrils from Helicteres isora plant. Ind. Crop. Prod. 2014, 59, 27–34. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Assis, O.B.; Silva, N.C.; Esposto, B.S.; Martins, M.A.; Tapia-Blácido, D.R. Chemical treatment and characterization of soybean straw and soybean protein isolate/straw composite films. Carbohydr. Polym. 2017, 157, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Kwak, J.H.; Zhang, Z.C.; Brown, H.M.; Arey, B.W.; Holladay, J.E. Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis. Carbohydr. Polym. 2007, 68, 235–241. [Google Scholar] [CrossRef]
- He, X.; Luzi, F.; Hao, X.; Yang, W.; Torre, L.; Xiao, Z.; Xie, Y.; Puglia, D. Thermal, antioxidant and swelling behaviour of transparent polyvinyl (alcohol) films in presence of hydrophobic citric acid-modified lignin nanoparticles. Int. J. Biol. Macromol. 2019, 127, 665–676. [Google Scholar] [CrossRef]
- Sucinda, E.; Majid, M.A.; Ridzuan, M.; Cheng, E.; Alshahrani, H.; Mamat, N. Development and characterisation of packaging film from Napier cellulose nanowhisker reinforced polylactic acid (PLA) bionanocomposites. Int. J. Biol. Macromol. 2021, 187, 43–53. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin Jr, A.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Phua, Y.J.; Lau, N.S.; Sudesh, K.; Chow, W.S.; Mohd Ishak, Z.A. Biodegradability studies of poly(butylene succinate)/organo-montmorillonite nanocomposites under controlled compost soil conditions: Effects of clay loading and compatibiliser. Polym. Degrad. Stabil. 2012, 97, 1345–1354. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Boix, A.C. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydr. Polym. 2018, 191, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Qazanfarzadeh, Z.; Kadivar, M. Properties of whey protein isolate nanocomposite films reinforced with nanocellulose isolated from oat husk. Int. J. Biol. Macromol. 2016, 91, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Mansora, A.M.; Lima, J.S.; Anib, F.N.; Hashima, H.; Hoa, W.S. Characteristics of cellulose, hemicellulose and lignin of MD2 pineapple biomass. Chem. Eng. 2019, 72, 79–84. [Google Scholar]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef]
- Banu Jamaldheen, S.; Kurade, M.B.; Basak, B.; Yoo, C.G.; Oh, K.K.; Jeon, B.-H.; Kim, T.H. A review on physico-chemical delignification as a pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2022, 346, 126591. [Google Scholar] [CrossRef]
- Bijarimi, M.; Ahmad, S.; Rasid, R. Mechanical, thermal and morphological properties of poly(lactic acid)/natural rubber nanocomposites. J. Reinf. Plast. Compos. 2013, 32, 1656–1667. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly (L-lactide) with poly (propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef]
- Messin, T.; Follain, N.; Guinault, A.; Sollogoub, C.; Gaucher, V.; Delpouve, N.; Marais, S. Structure and Barrier Properties of Multinanolayered Biodegradable PLA/PBSA Films: Confinement Effect via Forced Assembly Coextrusion. ACS Appl. Mater. Interfaces 2017, 9, 29101–29112. [Google Scholar] [CrossRef] [Green Version]
- Wan Ishak, W.H.; Rosli, N.A.; Ahmad, I. Influence of amorphous cellulose on mechanical, thermal, and hydrolytic degradation of poly(lactic acid) biocomposites. Sci. Rep. 2020, 10, 11342. [Google Scholar] [CrossRef]
- Basak, S.; Patil, P.G.; Shaikh, A.J.; Samanta, K.K. Green coconut shell extract and boric acid: New formulation for making thermally stable cellulosic paper. J. Chem. Technol. Biotechnol. 2016, 91, 2871–2881. [Google Scholar] [CrossRef]
- Iwatake, A.; Nogi, M.; Yano, H. Cellulose nanofiber-reinforced polylactic acid. Compos. Sci. Technol. 2008, 68, 2103–2106. [Google Scholar] [CrossRef]




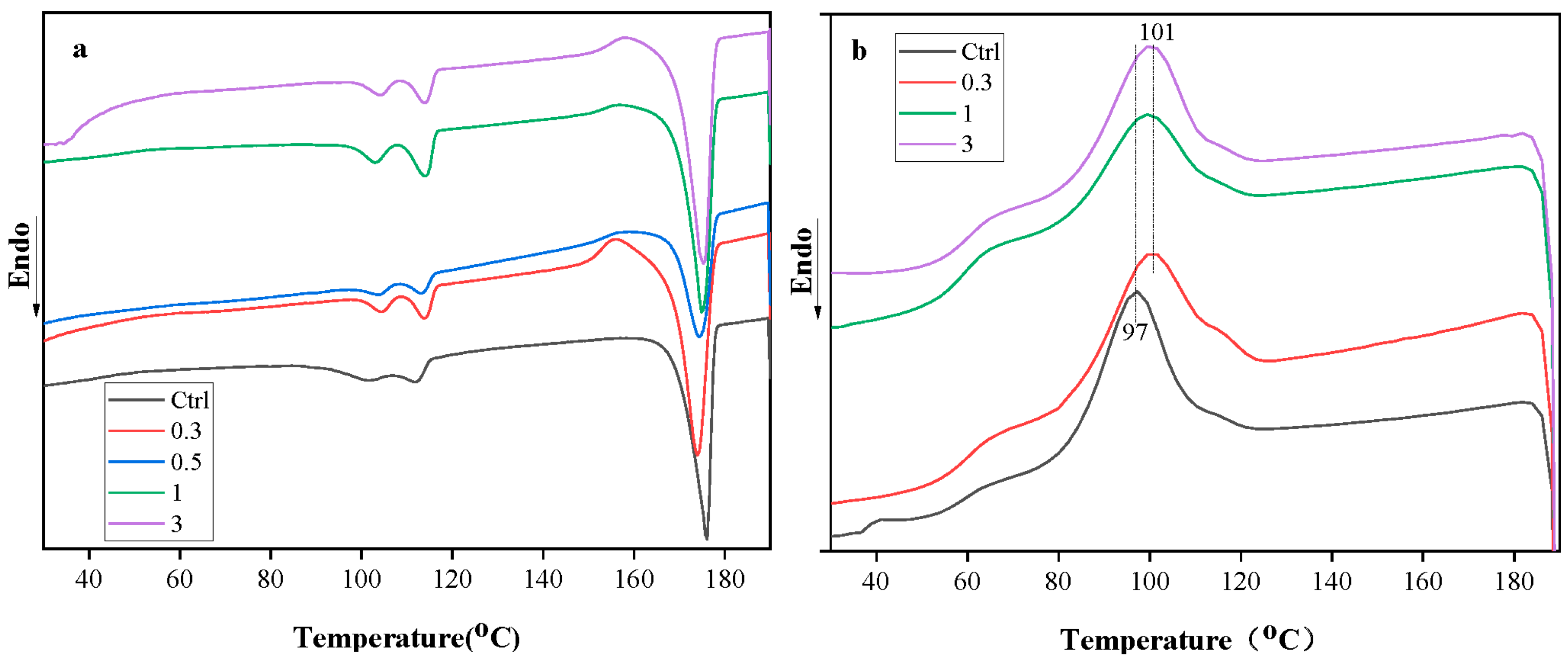
| Formulation | ∆HmPBS (J/g) | ∆HmPLA (J/g) | ∆Hcc (J/g) | Xc (%) |
|---|---|---|---|---|
| Ctrl | 4.4 | 37.42 | 15.77 | 27.34 |
| 0.3 | 5.38 | 37.49 | 23.53 | 20.36 |
| 0.5 | 3.46 | 26.63 | 11.19 | 19.93 |
| 1 | 8.47 | 38.75 | 19.58 | 29.34 |
| 3 | 5.61 | 38.7 | 20.14 | 29.12 |
| Formulation | Tonset (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|
| Ctrl | 339.6 | 363.6 | 0.13 |
| 0.3 | 341 | 365 | 0.05 |
| 0.5 | 339.6 | 363.6 | 0 |
| 1 | 329 | 359.6 | 0.15 |
| 3 | 327.6 | 357 | 5.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Tang, L.; Zheng, J.; Jin, Y.; Chang, R.; Yu, X.; Song, Y.; Huang, R. A Novel UV Barrier Poly(lactic acid)/Poly(butylene succinate) Composite Biodegradable Film Enhanced by Cellulose Extracted from Coconut Shell. Polymers 2023, 15, 3000. https://doi.org/10.3390/polym15143000
He X, Tang L, Zheng J, Jin Y, Chang R, Yu X, Song Y, Huang R. A Novel UV Barrier Poly(lactic acid)/Poly(butylene succinate) Composite Biodegradable Film Enhanced by Cellulose Extracted from Coconut Shell. Polymers. 2023; 15(14):3000. https://doi.org/10.3390/polym15143000
Chicago/Turabian StyleHe, Xiaoyan, Lisheng Tang, Jun Zheng, Yuanyuan Jin, Ruobin Chang, Xiaoquan Yu, Yihu Song, and Ran Huang. 2023. "A Novel UV Barrier Poly(lactic acid)/Poly(butylene succinate) Composite Biodegradable Film Enhanced by Cellulose Extracted from Coconut Shell" Polymers 15, no. 14: 3000. https://doi.org/10.3390/polym15143000





