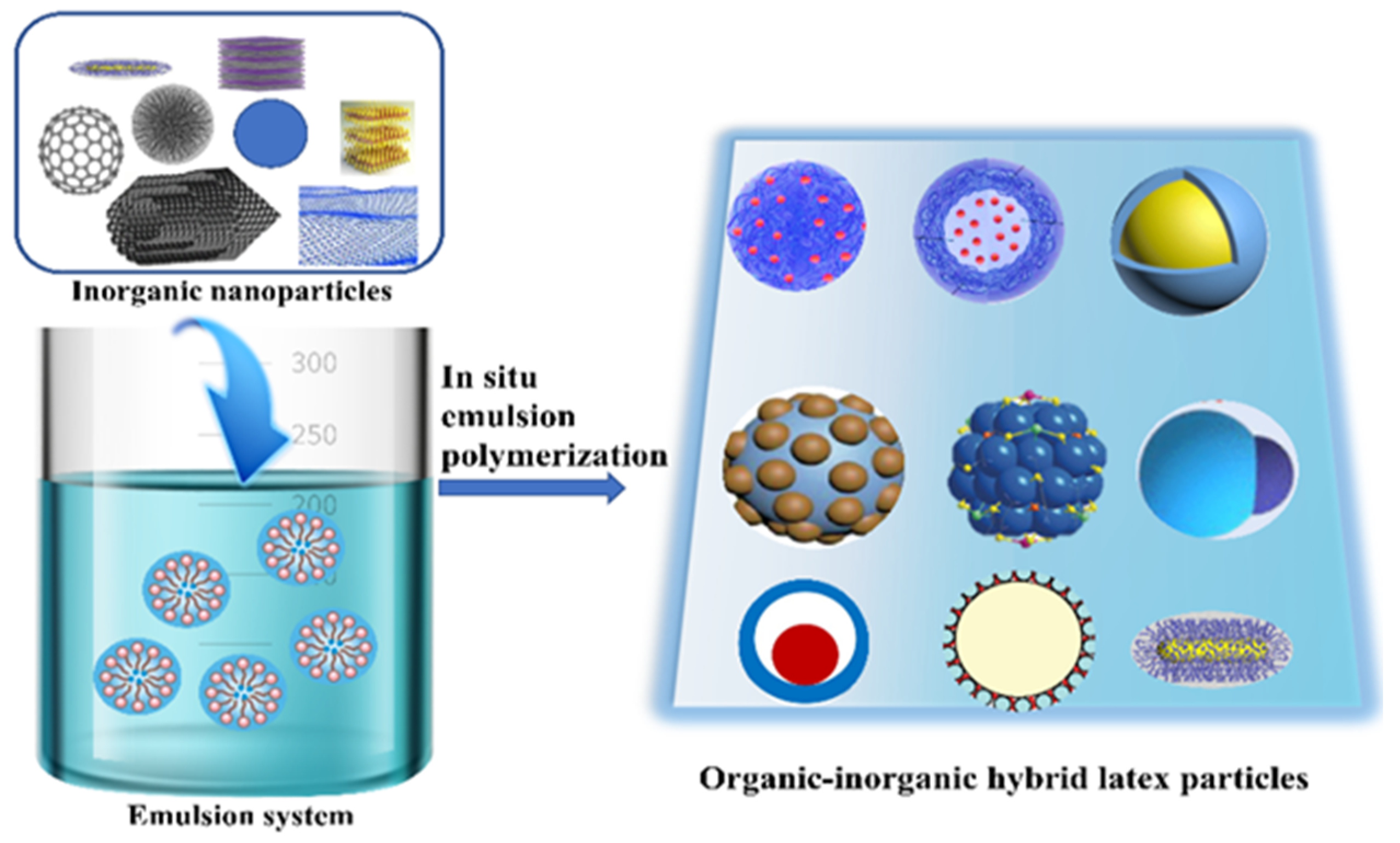
Advances in Organic–Inorganic Hybrid Latex Particles via In Situ Emulsion Polymerization
Abstract
:1. Introduction
2. Hybrid Latex Particles by In Situ Emulsion Polymerization
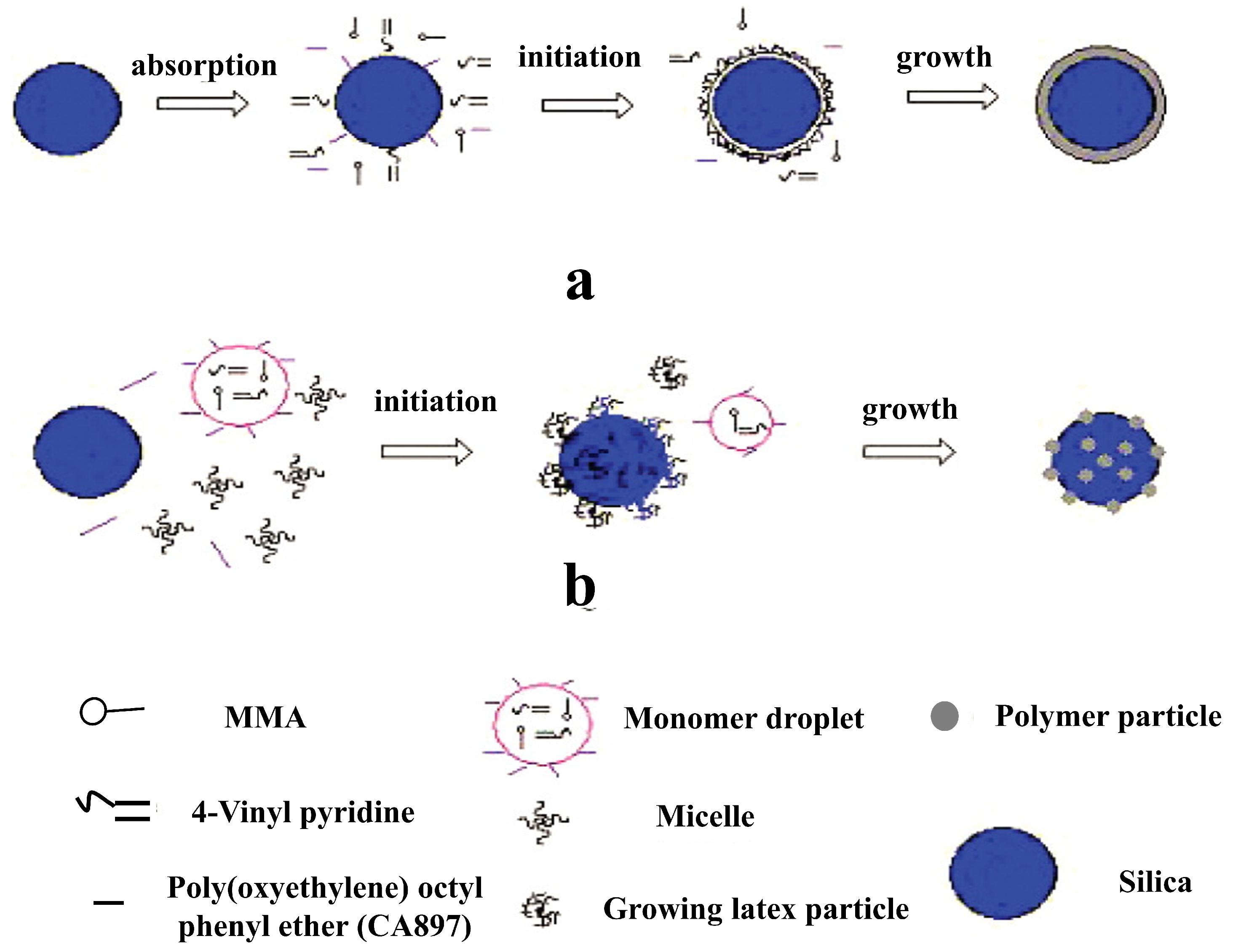
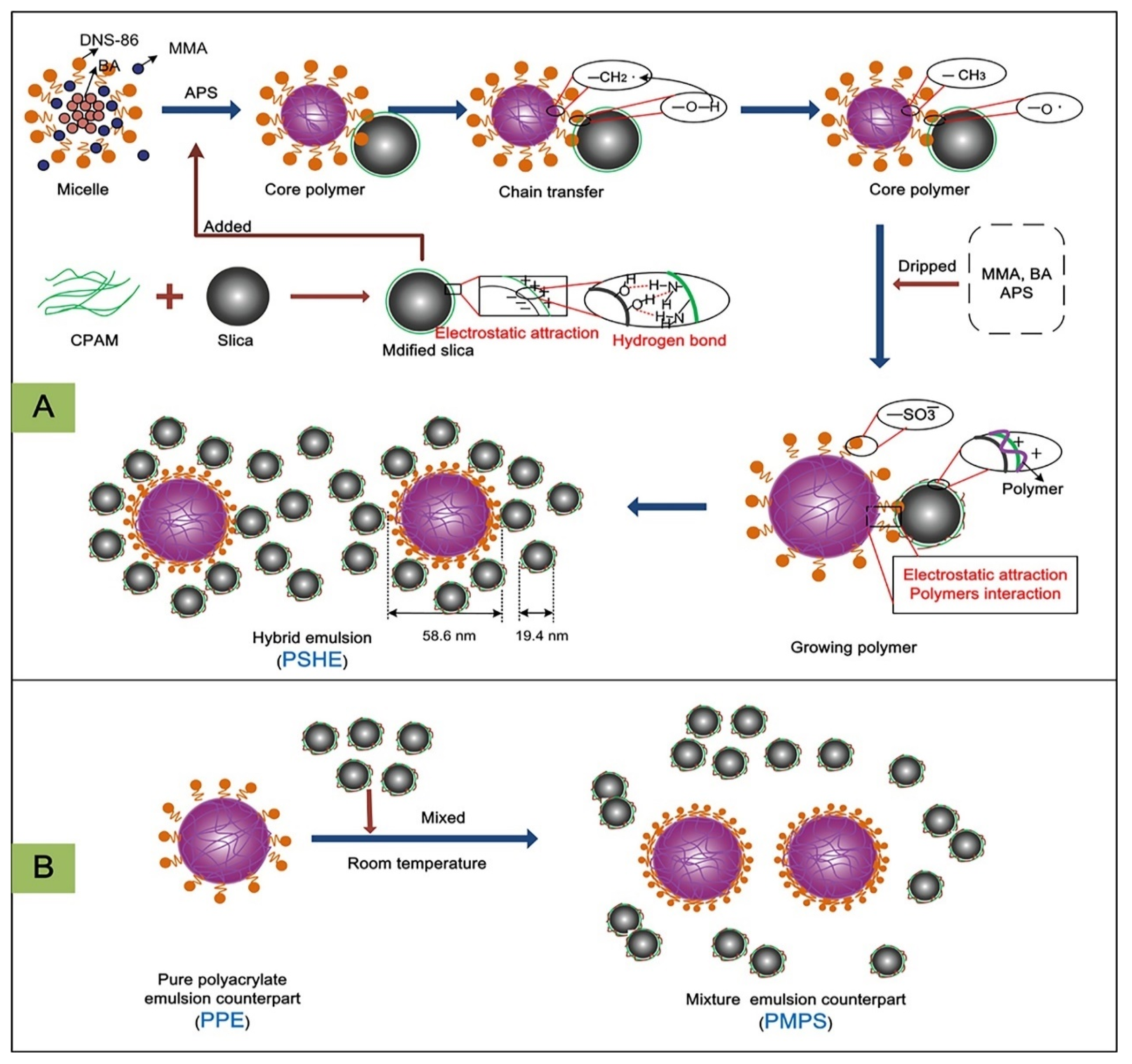
2.1. Silica-Doped Hybrid Latex Particles
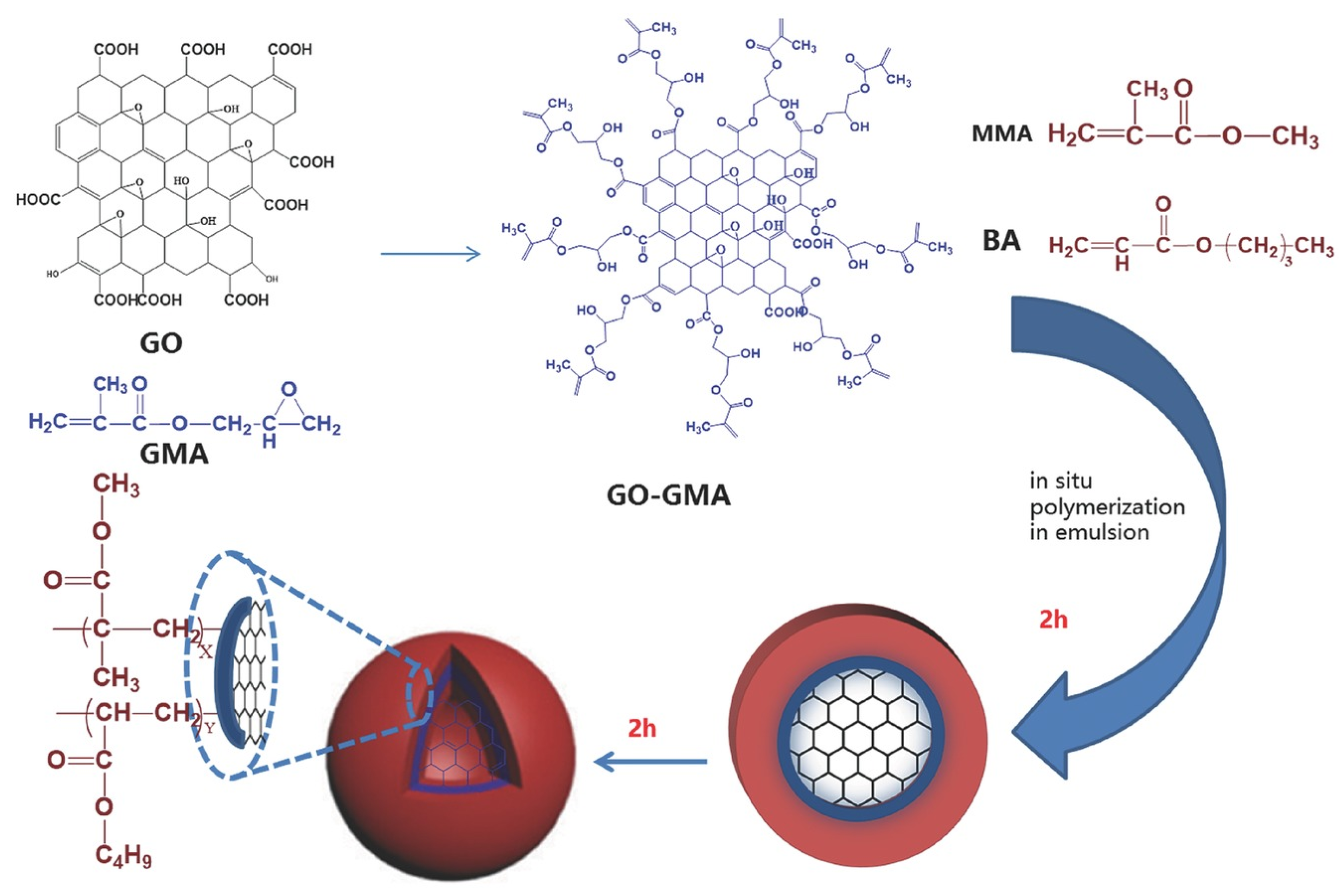
2.2. Carbon-Doped Hybrid Latex Particles
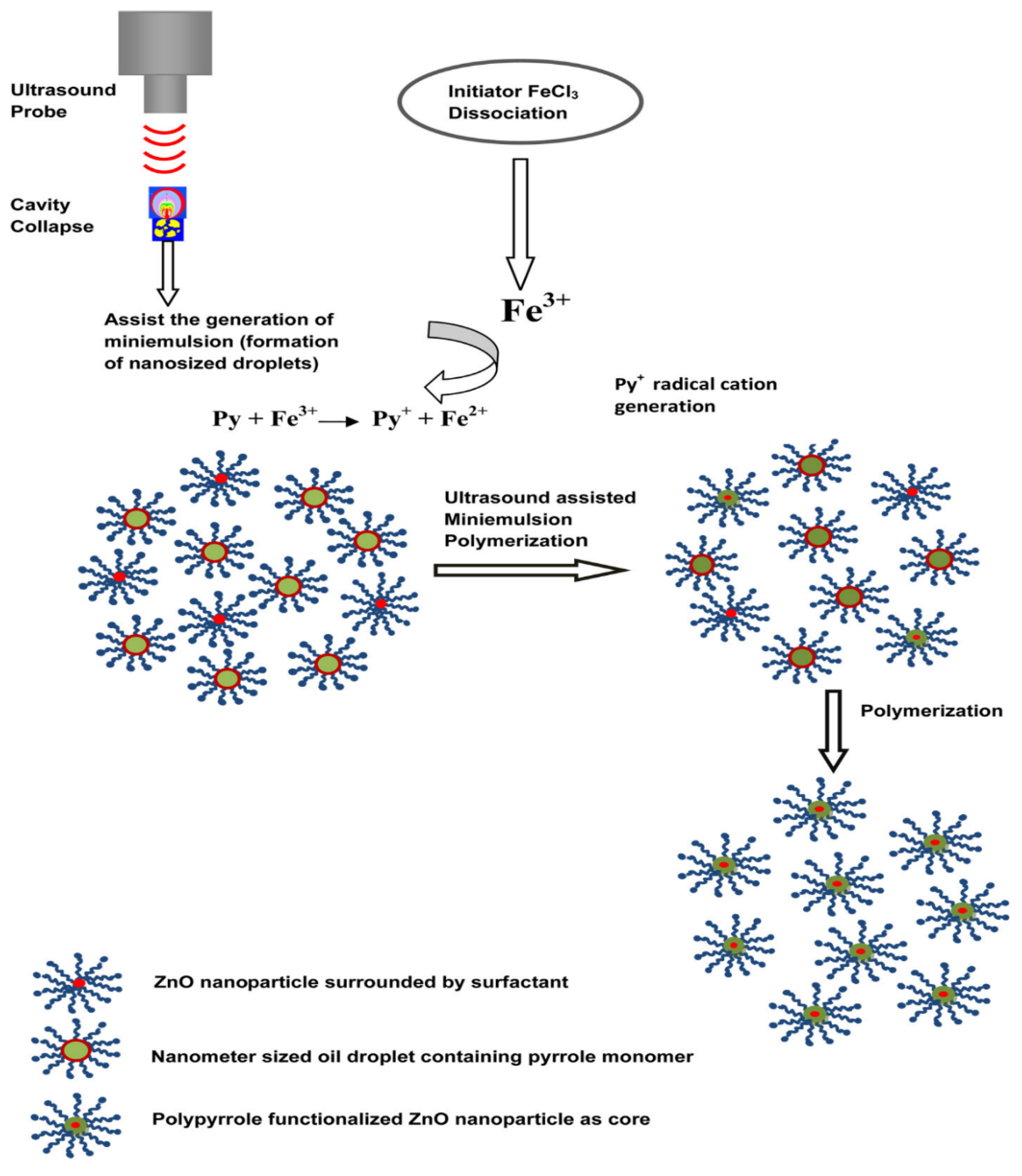
2.3. Metal-Doped Hybrid Latex Particles
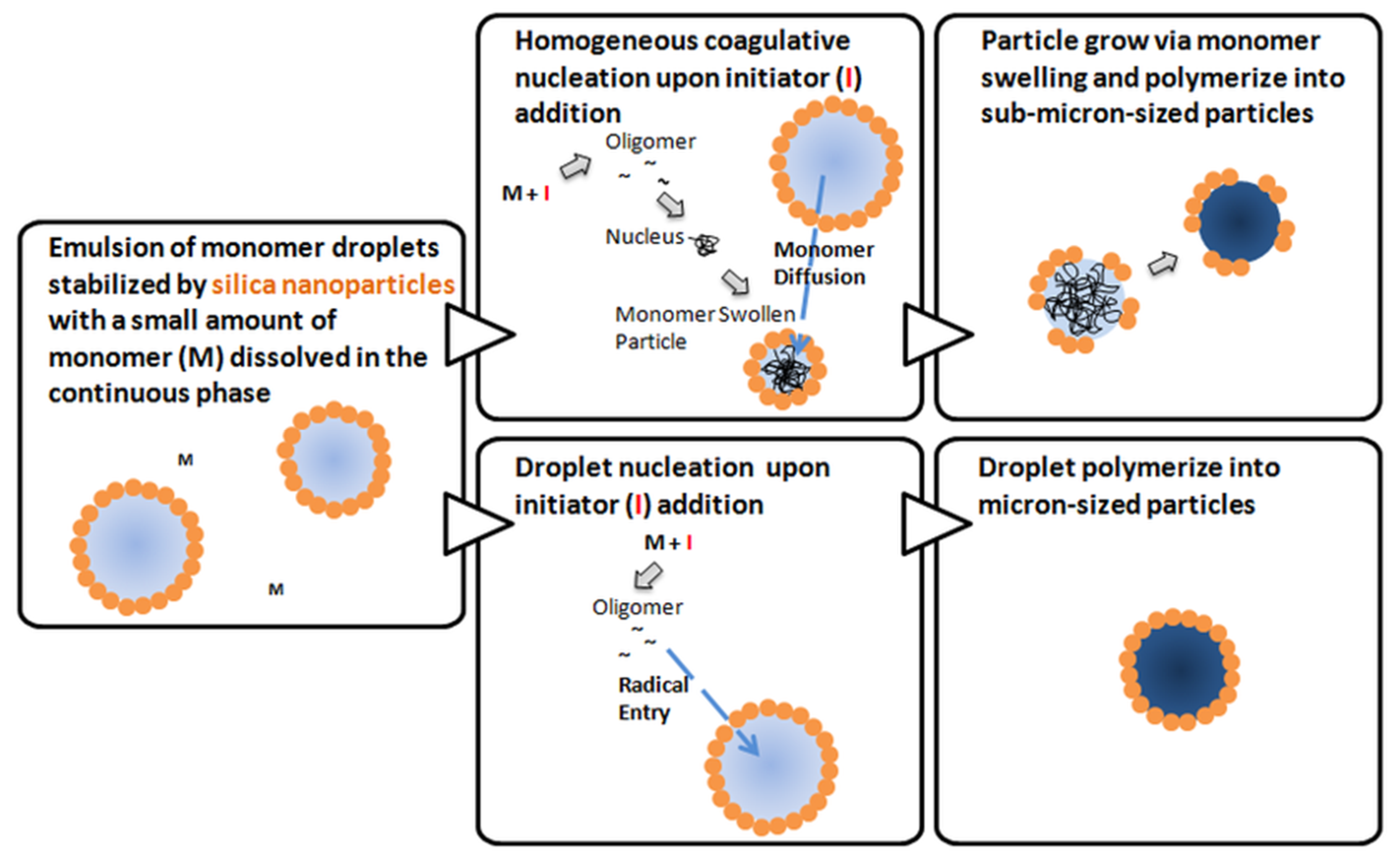
3. Hybrid Latex Particles from In Situ Pickering Emulsion Polymerization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A review of shape memory polymers and composites: Mechanisms, materials, and applications. Adv. Mater. 2020, 33, 2000713. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Lansalot, M. Organic/inorganic composite latexes: The marriage of emulsion polymerization and inorganic chemistry. Adv. Polym. Sci. 2010, 233, 53–123. [Google Scholar] [CrossRef]
- Rahman, M.M.; Elaissari, A. Organic-inorganic hybrid magnetic latex. Adv. Polym. Sci. 2010, 233, 237–281. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer composite materials: A comprehensive review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Desroches, G.J.; Macfarlane, R.J. Ordered polymer composite materials: Challenges and opportunities. Nanoscale 2021, 13, 426–443. [Google Scholar] [CrossRef]
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric composite materials for radiation shielding: A review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef]
- Mutiso, R.M.; Winey, K.I. Electrical properties of polymer nanocomposites containing rod-like nanofillers. Prog. Polym. Sci. 2015, 40, 63–84. [Google Scholar] [CrossRef]
- Shen, X.; Zheng, Q.; Kim, J.K. Rational design of two-dimensional nanofillers for polymer nanocomposites toward multifunctional applications. Prog. Mater. Sci. 2021, 115, 100708. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Desai, S. Nanofiller-reinforced polymer nanocomposites. Polym. Adv. Technol. 2008, 19, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Paquet, C.; Lin, Y.; Kumacheva, E. From hybrid microgels to photonic crystals. Adv. Funct. Mater. 2003, 13, 468–472. [Google Scholar] [CrossRef]
- Murali Mohan, Y.; Lee, K.; Premkumar, T.; Geckeler, K.E. Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications. Polymer 2006, 48, 158–164. [Google Scholar] [CrossRef]
- Suvorova, E.I.; Klechkovskaya, V.V.; Kopeikin, V.V.; Buffat, P.A. Stability of Ag nanoparticles dispersed in amphiphilic organic matrix. J. Cryst. Growth 2005, 275, 2351–2356. [Google Scholar] [CrossRef]
- Hore, M.J.A.; Composto, R.J. Functional polymer nanocomposites enhanced by nanorods. Macromolecules 2014, 47, 875–887. [Google Scholar] [CrossRef]
- Wang, T.; Keddie, J.L. Design and fabrication of colloidal polymer nanocomposites. Adv. Colloid Interface Sci. 2009, 147, 319–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, C.J.; Devon, M.J. Hollow latex particles: Synthesis and applications. Adv. Colloid Interface Sci. 2002, 99, 181–213. [Google Scholar] [CrossRef]
- van Herk, A.M. Historical overview of (mini)emulsion polymerizations and preparation of hybrid latex particles. In Hybrid Latex Particles. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 233, pp. 1–18. [Google Scholar] [CrossRef]
- Teixeira, R.F.A.; Bon, S.A.F. Physical methods for the preparation of hybrid nanocomposite polymer latex particles. Adv. Polym. Sci. 2010, 233, 19–52. [Google Scholar] [CrossRef] [Green Version]
- Bourgeat-Lami, E. Organic-inorganic nanostructured colloids. J. Nanosci. Nanotechnol. 2002, 2, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 5530, 1232–1237. [Google Scholar] [CrossRef]
- Salabat, A.; Mirhoseini, F. Polymer-based nanocomposites fabricated by microemulsion method. Polym. Compos. 2022, 43, 1282–1294. [Google Scholar] [CrossRef]
- Bai, R.; Qiu, T.; Han, F.; He, L.; Li, X. Preparation and characterization of inorganic-organic trilayer core-shell polysilsesquioxane/polyacrylate/polydimethylsiloxane hybrid latex particles. Appl. Surf. Sci. 2012, 258, 7683–7688. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, B.; Wang, P.; Yuan, N. Hybrid latex particles preparation with seeded semibatch emulsion polymerization. Colloid. Surf. A 2015, 482, 422–430. [Google Scholar] [CrossRef]
- Dyab, A.K.F.; Al-Lohedan, H.A.; Essawy, H.A.; Abd El-Mageed, A.I.A.; Taha, F. Fabrication of core/shell hybrid organic-inorganic polymer microspheres via Pickering emulsion polymerization using laponite nanoparticles. J. Saudi Chem. Soc. 2014, 18, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Qiu, T.; Xu, C.; He, L.; Li, X. Synthesis and characterization of emulsifier-free trilayer core-shell polysilsesquioxane/polyacrylate/poly(fluorinated acrylate) hybrid latex particles. Colloid Polym. Sci. 2012, 290, 769–776. [Google Scholar] [CrossRef]
- Narongthong, J.; Nuasaen, S.; Suteewong, T.; Tangboriboonrat, P. One-pot synthesis of organic-inorganic hybrid hollow latex particles via Pickering and seeded emulsion polymerizations. Colloid Polym. Sci. 2015, 293, 1269–1274. [Google Scholar] [CrossRef]
- Tissot, I.; Novat, C.; Lefebvre, F.d.r.; Bourgeat-Lami, E. Hybrid latex particles coated with silica. Macromolecules 2001, 34, 5737–5739. [Google Scholar] [CrossRef]
- Gerasin, V.A.; Antipov, E.M.; Karbushev, V.V.; Kulichikhin, V.G.; Karpacheva, G.P.; Talroze, R.V.; Kudryavtsev, Y.V. New approaches to the development of hybrid nanocomposites: From structural materials to high-tech applications. Russ. Chem. Rev. 2013, 82, 303–332. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Lovell, P.A.; Schork, F.J. Fundamentals of emulsion polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef] [PubMed]
- Asua, J.M. Emulsion polymerization: From fundamental mechanisms to process developments. J. Polym. Sci. Pol. Chem. 2004, 42, 1025–1041. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. Ultrasound assisted in situ emulsion polymerization for polymer nanocomposite: A review. Chem. Eng. Process. 2014, 85, 86–107. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glassceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.J.; Schmidt, G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef]
- Song, W.; Markel, D.; Jin, X.; Shi, T.; Ren, W. Poly(vinyl alcohol)/collagen/hydroxyapatite hydrogel: Properties and in vitro cellular response. J. Biomed. Mater. Res. A 2012, 100, 3071–3079. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Haldorai, Y.; Long, P.Q.; Noh, S.K.; Lyoo, W.S.; Shim, J.J. Ultrasonically assisted in situ emulsion polymerization: A facile approach to prepare conducting copolymer/silica nanocomposites. Polym. Advan. Technol. 2011, 22, 781–787. [Google Scholar] [CrossRef]
- Ding, X.F.; Jiang, Y.Q.; Yu, K.F.; Hari, B.; Tao, N.N.; Zhao, J.Z.; Wang, Z.C. Silicon dioxide as coating on polystyrene nanoparticles in situ emulsion polymerization. Mater. Lett. 2004, 58, 1722–1725. [Google Scholar] [CrossRef]
- Ding, X.F.; Zhao, J.Z.; Liu, Y.H.; Zhang, H.B.; Wang, Z.C. Silica nanoparticles encapsulated by polystyrene via surface grafting and in situ emulsion polymerization. Mater. Lett. 2004, 58, 3126–3130. [Google Scholar] [CrossRef]
- Riazi, H.; Jalali-Arani, A.; Taromi, F.A. In situ synthesis of silica/polyacrylate nanocomposite particles simultaneously bearing carboxylate and sulfonate functionalities via soap-free seeded emulsion polymerization. Mater. Chem. Phys. 2018, 207, 470–478. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Pourvala, T.; Motamedi, E.; Fathi, B.; Vashaee, D.; Tayebi, L. Synthesis and characterization of encapsulated nanosilica particles with an acrylic copolymer by in situ emulsion polymerization using thermoresponsive nonionic surfactant. Materials 2013, 6, 3727–3741. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Song, C.; Bai, R.; Wei, Z.; Zhang, F. Effects of mesoporous SBA-15 contents on the properties of polystyrene composites via in-situ emulsion polymerization. J. Polym. Res. 2012, 19, 9846. [Google Scholar] [CrossRef]
- Perro, A.; Reculusa, S.; Bourgeat-Lami, E.; Duguet, E.; Ravaine, S. Synthesis of hybrid colloidal particles: From snowman-like to raspberry-like morphologies. Colloid. Surf. A 2006, 284, 78–83. [Google Scholar] [CrossRef]
- Reculusa, S.p.; Mingotaud, C.; Bourgeat-Lami, E.; Duguet, E.; Ravaine, S. Synthesis of daisy-shaped and multipod-like silica/polystyrene nanocomposites. Nano Lett. 2004, 4, 1677–1682. [Google Scholar] [CrossRef]
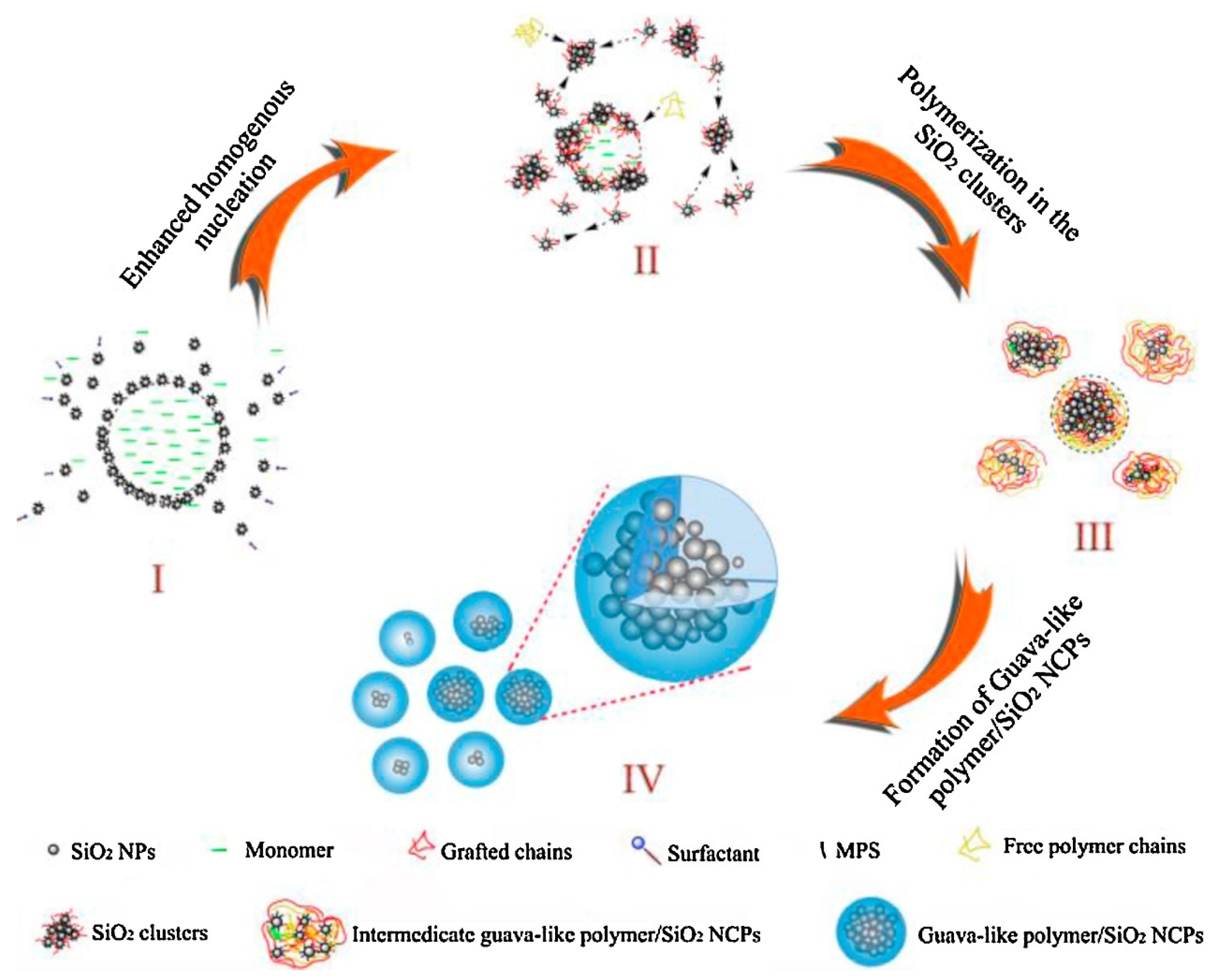
- Qi, D.; Liu, C.; Chen, Z.; Chen, H.; Huang, C.; Cao, Z. Formation mechanism of guava-like polymer/SiO2 nanocomposite particles in situ emulsion polymerization systems. Colloid. Surf. A 2016, 489, 265–274. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, L.; Zhang, X.; Chen, X.; Yang, B. Silica-PMMA core-shell and hollow nanospheres. Colloid. Surf. A 2006, 277, 145–150. [Google Scholar] [CrossRef]
- Parvole, J.; Chaduc, I.; Ako, K.; Spalla, O.; Thill, A.; Ravaine, S.; Duguet, E.; Lansalot, M.; Bourgeat-Lami, E. Efficient synthesis of snowman-and dumbbell-like silica/polymer anisotropic heterodimers through emulsion polymerization using a surface-anchored cationic initiator. Macromolecules 2012, 45, 7009–7018. [Google Scholar] [CrossRef]
- Luna-Xavier, J.L.; Guyot, A.; Bourgeat-Lami, E. Synthesis and characterization of silica/poly (methyl methacrylate) nanocomposite latex particles through emulsion polymerization using a cationic azo initiator. J. Colloid Interface Sci. 2002, 250, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Luna-Xavier, J.L.; Bourgeat-Lami, E.; Guyot, A. The role of initiation in the synthesis of silica/poly(methyl methacrylate) nanocomposite latex particles through emulsion polymerization. Colloid Polym. Sci. 2001, 279, 947–958. [Google Scholar] [CrossRef]
- Han, Z.; Wang, L.; Zhu, J.; Zhang, S.; Zhou, W. Synthesis of SiO2/PS composite particles via emulsion polymerization. J. Appl. Polym. Sci. 2011, 122, 43–49. [Google Scholar] [CrossRef]
- Yang, Y.; Dan, Y. Preparation of PMMA/SiO2 composite particles via emulsion polymerization. Colloid Polym. Sci. 2003, 281, 794–799. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, M.; Zhou, S.; Wu, L. Preparation of SiO2/PMMA composite particles via conventional emulsion polymerization. J. Polym. Sci. Pol. Chem. 2006, 44, 3807–3816. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Qiao, X.; Li, X.; Wu, L. Synthesis of SiO2/poly(styrene-co-butyl acrylate) nanocomposite microspheres via miniemulsion polymerization. J. Polym. Sci. Pol. Chem. 2006, 44, 3202–3209. [Google Scholar] [CrossRef]
- Buhin, Z.; Blagojevic, S.L.; Leskovac, M. In situ emulsion polymerization and characterization of poly(butyl acrylate-co-methyl methacrylate)/silica nanosystems. Polym. Eng. Sci. 2013, 53, 2292–2298. [Google Scholar] [CrossRef]
- Jazi, M.A.; Ramezani, A.S.A.; Haddadi, S.A.; Ghaderi, S.; Azamian, F. In situ emulsion polymerization and characterization of PVAc nanocomposites including colloidal silica nanoparticles for wood specimens bonding. J. Appl. Polym. Sci. 2020, 137, 48570. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Li, X.; Zhang, Z.; Jiao, C. Preparation and characterization of a novel nanocomposite particles via in situ emulsion polymerization of vinyl functionalized silica nanoparticles and vinyl acetate. J. Sol-Gel Sci. Technol. 2013, 68, 54–59. [Google Scholar] [CrossRef]
- Ye, Y.; Zeng, X.; Li, H.; Chen, P.; Ye, C.; Zhao, F. Synthesis and characterization of nano-silica/polyacrylate composite emulsions by sol-gel method and in-situ emulsion polymerization. J. Macromol. Sci. A 2011, 48, 42–46. [Google Scholar] [CrossRef]
- Soleimani, H.; Bagheri, R.; Asadinezhad, A. Effect of silica nanoparticles on surface properties, particle size, and distribution of poly (methyl methacrylate-co-butyl acrylate-co-acrylic acid) synthesized by in situ emulsion polymerization. Prog. Org. Coat. 2019, 129, 278–284. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Arcos-Casarrubias, J.A.; Reyes-Mayer, A.; Guardian-Tapia, R. Acrylate hybrid nanocomposite coatings based on SiO2 nanoparticles. In-situ semi-batch emulsion polymerization. Eur. Polym. J. 2016, 76, 170–187. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Arcos-Casarrubias, J.A.; Hernandez-Vargas, M.L.; Reyes-Mayer, A.; Aguilar-Franco, M.; Bagdhachi, J. Acrylate hybrid nanocomposite coatings based on SiO2 nanoparticles by in-situ batch emulsion polymerization. Prog. Org. Coat. 2016, 97, 288–300. [Google Scholar] [CrossRef]
- Rubinger, C.P.L.; Costa, L.C.; Esteves, A.C.C.; Barros-Timmons, A.; Martins, J.A. Hopping conduction on PPy/SiO2 nanocomposites obtained via in situ emulsion polymerization. J. Mater. Sci. 2008, 43, 3333–3337. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Adhikari, B.; Banerjee, A. Short peptide based hydrogels: Incorporation of graphene into the hydrogel. Soft Matter 2011, 7, 9259. [Google Scholar] [CrossRef]
- Shin, S.R.; Bae, H.; Cha, J.M.; Mun, J.Y.; Chen, Y.C.; Tekin, H.; Shin, H.; Zarabi, S.; Dokmeci, M.R.; Tang, S.; et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Arslantunali, D.; Budak, G.; Hasirci, V. Multiwalled CNT-pHEMA composite conduit for peripheral nerve repair. J. Biomed. Mater. Res. A 2014, 102, 828–841. [Google Scholar] [CrossRef]
- Davaran, S.; Alimirzalu, S.; Nejati-Koshki, K.; Nasrabadi, H.T.; Akbarzadeh, A.; Khandaghi, A.A.; Abbasian, M.; Alimohammadi, S. Physicochemical Characteristics of Fe3O4 Magnetic Nanocomposites Based on Poly(N-isopropylacrylamide) for Anti-cancer Drug Delivery. Asian Pac. J. Cancer Prev. 2014, 15, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evingur, G.A.; Pekcan, O. Effect of multiwalled carbon nanotube (MWNT) on the behavior of swelling of polyacrylamide-MWNT composites. J. Reinf. Plast. Comp. 2014, 33, 1199–1206. [Google Scholar] [CrossRef] [Green Version]
- Curcio, M.; Spizzirri, U.G.; Cirillo, G.; Vittorio, O.; Picci, N.; Nicoletta, F.P.; Iemma, F.; Hampel, S. On demand delivery of ionic drugs from electro-responsive CNT hybrid films. RSC Adv. 2015, 5, 44902–44911. [Google Scholar] [CrossRef]
- Toumia, Y.; Orlanducci, S.; Basoli, F.; Licoccia, S.; Paradossi, G. “Soft” confinement of graphene in hydrogel matrixes. J. Phys. Chem. B 2015, 119, 2051–2061. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ramazani, S.A.A.; Mashayekhan, S. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int. J. Biol. Macromol. 2015, 74, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Pei, X.; Zeng, Y.; He, R.; Li, Z.; Wang, J.; Wan, Q.; Li, X. Functionalized nanoscale graphene oxide for high efficient drug delivery of cisplatin. J. Nanopart. Res. 2014, 16, 2709. [Google Scholar] [CrossRef]
- Guo, Q.F.; Cao, H.; Li, X.H.; Liu, S.W. Thermosensitive hydrogel drug delivery system containing doxorubicin loaded CS-GO nanocarriers for controlled release drugin situ. Mater. Technol. 2015, 30, 294–300. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Gun’ko, Y.K. Mechanical Reinforcement of Polymers Using Carbon Nanotubes. Adv. Mater. 2006, 18, 689–706. [Google Scholar] [CrossRef]
- Byrne, M.T.; Guńko, Y.K. Recent advances in research on carbonnanotube-polymer composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Chen, H.; Xu, J.; Gao, Y.; Li, H. Phenylboronate-diol crosslinked polymer/SWCNT hybrid gels with reversible sol-gel transition. Polym. Adv. Technol. 2014, 25, 233–239. [Google Scholar] [CrossRef]
- Li, C.; Cheng, W.; Yan, Z.; Ge, S.; Shao, Q.; Naik, N.; Pan, D.; Guo, Z. Soap-free styrene-acrylic/carbon nanotubes composite latex by in situ emulsion polymerization: Preparation, properties and characterizations. Surf. Interfaces 2021, 25, 101204. [Google Scholar] [CrossRef]
- Xia, H.S.; Wang, Q.; Qiu, G.H. Polymer-encapsulated carbon nanotubes prepared through ultrasonically initiated in situ emulsion polymerization. Chem. Mater. 2003, 15, 3879–3886. [Google Scholar] [CrossRef]
- Oh, M.; Kim, S. Effect of dodecyl benzene sulfonic acid on the preparation of polyaniline/activated carbon composites by in situ emulsion polymerization. Electrochim. Acta 2012, 59, 196–201. [Google Scholar] [CrossRef]
- Khan, M.U.; Reddy, K.R.; Snguanwongchai, T.; Haque, E.; Gomes, V.G. Polymer brush synthesis on surface modified carbon nanotubes via in situ emulsion polymerization. Colloid Polym. Sci. 2016, 294, 1599–1610. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
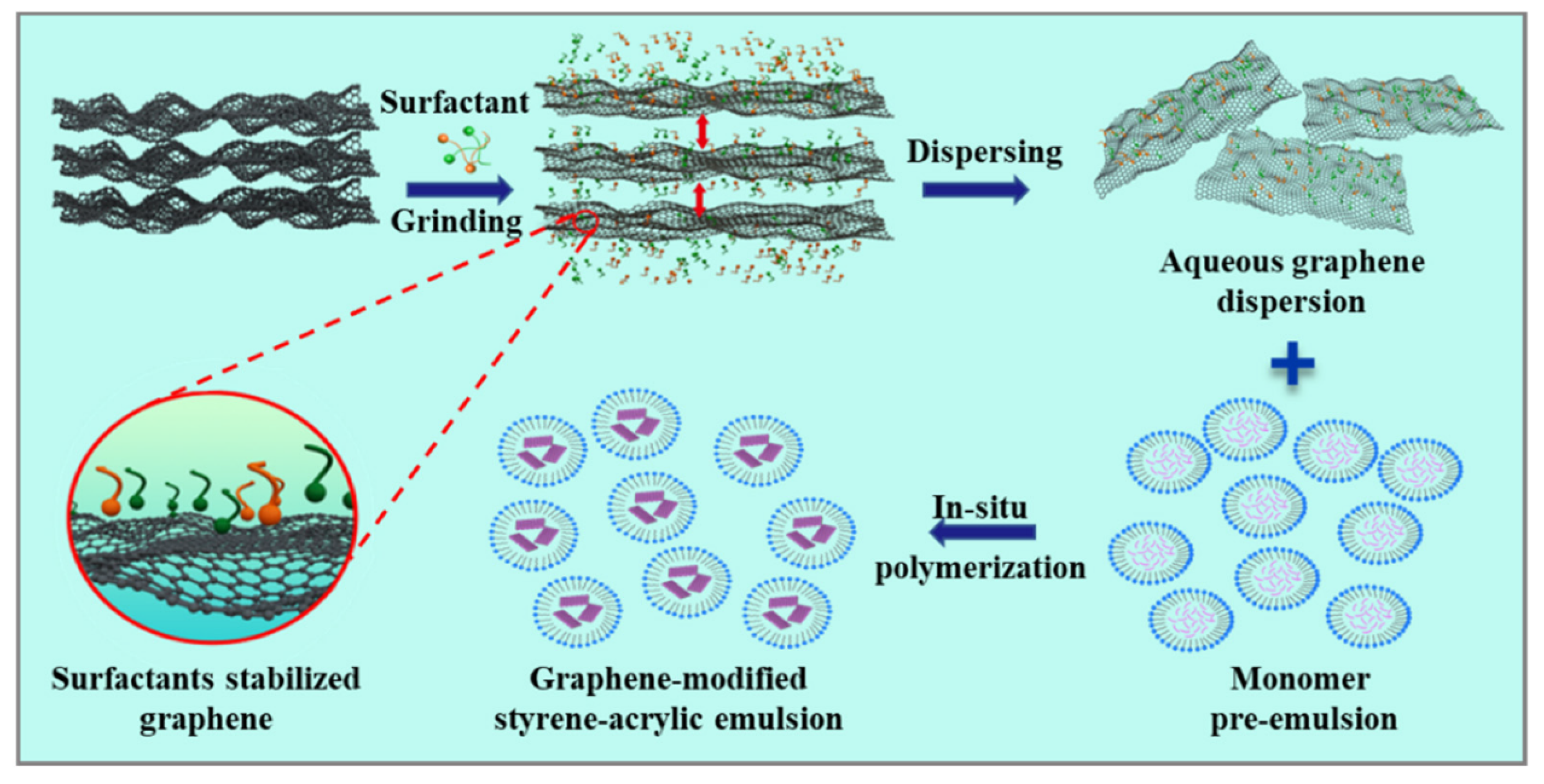
- Li, Y.; Luo, J.; Huang, B.; Jin, H.; Sun, X.; Cao, C.; Chen, Q.; Qian, Q. Fabrication of graphene-modified styrene-acrylic emulsion by in situ aqueous polymerization. Polymers 2022, 14, 3763. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Wang, J.; Wan, L.; Liu, F.; Zheng, H.; Chen, R.; Xu, C. Preparation and properties of graphene nanosheets-polystyrene nanocomposites via in situ emulsion polymerization. Chem. Phys. Lett. 2010, 484, 247–253. [Google Scholar] [CrossRef]
- Imran, S.M.; Salman, A.; Shao, G.N.; Haider, M.S.; Abbas, N.; Park, S.; Hussain, M.; Kim, H.T. Study of the electroconductive properties of conductive polymers-graphene/graphene oxide nanocomposites synthesized via in situ emulsion polymerization. Polym. Compos. 2018, 39, 2142–2150. [Google Scholar] [CrossRef]
- Imran, S.M.; Kim, Y.; Shao, G.N.; Hussain, M.; Choa, Y.-h.; Kim, H.T. Enhancement of electroconductivity of polyaniline/graphene oxide nanocomposites through in situ emulsion polymerization. J. Mater. Sci. 2014, 49, 1328–1335. [Google Scholar] [CrossRef]
- Imran, S.M.; Shao, G.N.; Haider, M.S.; Abbas, N.; Hussain, M.; Kim, H.T. Electroconductive performance of polypyrrole/graphene nanocomposites synthesized through in situ emulsion polymerization. J. Appl. Polym. Sci. 2015, 132, 41800. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Yang, L.; Yi, K.; Li, Z.; Yao, J. Facilely prepared polypyrrole-graphene oxide-sodium dodecylbenzene sulfonate nanocomposites by in situ emulsion polymerization for high-performance supercapacitor electrodes. J. Solid State Electr. 2014, 18, 2139–2147. [Google Scholar] [CrossRef]
- Pazat, A.; Beyou, E.; Barres, C.; Bruno, F.; Janin, C. In situ emulsion cationic polymerization of isoprene onto the surface of graphite oxide sheets. Appl. Surf. Sci. 2017, 396, 902–911. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, P.; Zhao, X.; Xu, J. Preparation of poly(vinyl alcohol)-grafted graphene oxide/poly(vinyl alcohol) nanocomposites via in-situ low-temperature emulsion polymerization and their thermal and mechanical characterization. Appl. Surf. Sci. 2017, 396, 1098–1107. [Google Scholar] [CrossRef]
- Spasevska, D.; Leal, G.P.; Fernandez, M.; Gilev, J.B.; Paulis, M.; Tomovska, R. Crosslinked reduced graphene oxide/polymer composites via in situ synthesis by semicontinuous emulsion polymerization. RSC Adv. 2015, 5, 16414–16421. [Google Scholar] [CrossRef] [Green Version]
- Arzac, A.; Patricia Leal, G.; Fajgar, R.; Tomovska, R. Comparison of the emulsion mixing and in situ polymerization techniques for synthesis of water-borne reduced graphene oxide/polymer composites: Advantages and drawbacks. Part. Part. Syst. Charact. 2014, 31, 143–151. [Google Scholar] [CrossRef]
- Yue, L.; Li, W.; Cao, Y.; Bai, Y. Core-shell composite synthesized through in situ polymerization in emulsion with high electrical conductivity sensitive to humidity. Part. Part. Syst. Charact. 2017, 34, 1600423. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Thomas, V.; Namdeo, M.; Mohan, Y.M.; Bajpai, S.K.; Bajpai, M. Review on polymer, hydrogel and microgel metal nanocomposites: A facile nanotechnological approach. J. Macromol. Sci. A 2008, 45, 107–119. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle-hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Schexnailder, P.; Schmidt, G. Nanocomposite polymer hydrogels. Colloid Polym. Sci. 2009, 287, 1–11. [Google Scholar] [CrossRef]
- Wang, C.; Flynn, N.T.; Langer, R. Controlled structure and properties of thermoresponsive nanoparticle-hydrogel composites. Adv. Mater. 2004, 16, 1074–1079. [Google Scholar] [CrossRef]
- Skardal, A.; Zhang, J.; McCoard, L.; Oottamasathien, S.; Prestwich, G.D. Dynamically crosslinked gold nanoparticle—Hyaluronan hydrogels. Adv. Mater. 2010, 22, 4736–4740. [Google Scholar] [CrossRef] [PubMed]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Dey, M.; Swain, S.K. Emulsifier-free emulsion polymerization of acrylonitrile: Effect of in situ developed Cu(II)/glycine chelate complex initiated by monopersulfate. J. Appl. Polym. Sci. 1999, 74, 2785–2790. [Google Scholar] [CrossRef]
- Xu, G.C.; Xiong, J.Y.; Ji, X.L.; Wang, Y.L. Synthesis of Nanosilver/PMMA composites via ultrasonically Bi-In situ emulsion polymerization. J. Thermoplast. Compos. 2007, 20, 523–533. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef]
- Pinto, D.; Bernardo, L.; Amaro, A.; Lopes, S. Mechanical properties of epoxy nanocomposites using titanium dioxide as reinforcement—A review. Constr. Build. Mater. 2015, 95, 506–524. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, J. Using ultrasound-assisted dispersion and in situ emulsion polymerization to synthesize TiO2/ASA (acrylonitrile-styrene-acrylate) nanocomposites. Compos. Part B-Eng. 2016, 99, 196–202. [Google Scholar] [CrossRef]
- Che, X.C.; Jin, Y.Z.; Lee, Y.S. Preparation of nano-TiO2/polyurethane emulsions via in situ RAFT polymerization. Prog. Org. Coat. 2010, 69, 534–538. [Google Scholar] [CrossRef]
- Ai, Z.; Sun, G.; Zhou, Q.; Xie, C. Polyacrylate-core/TiO2-shell nanocomposite particles prepared by in situ emulsion polymerization. J. Appl. Polym. Sci. 2006, 102, 1466–1470. [Google Scholar] [CrossRef]
- Yang, M.; Dan, Y. Preparation of poly(methyl methacrylate)/titanium oxide composite particles via in-situ emulsion polymerization. J. Appl. Polym. Sci. 2006, 101, 4056–4063. [Google Scholar] [CrossRef]
- Zou, J.; Zhao, Y.; Yang, M.; Dan, Y. Preparation and characterization of polystyrene/titanium dioxide composite particles containing organic ultraviolet-stabilizer groups. J. Appl. Polym. Sci. 2007, 104, 2792–2798. [Google Scholar] [CrossRef]
- Ye, C.; Li, H.; Cai, A.; Gao, Q.; Zeng, X. Preparation and characterization of organic nano-titanium dioxide/acrylate composite emulsions by in-situ emulsion polymerization. J. Macromol. Sci. A 2011, 48, 309–314. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.E.; Labena, A.; Khamis, E.A. Core/shell (ZnO/polyacrylamide) nanocomposite: In-situ emulsion polymerization, corrosion inhibition, anti-microbial and anti-biofilm characteristics. J. Taiwan Inst. Chem. E 2016, 63, 512–522. [Google Scholar] [CrossRef]
- Barkade, S.S.; Pinjari, D.V.; Singh, A.K.; Gogate, P.R.; Naik, J.B.; Sonawane, S.H.; Ashokkumar, M.; Pandit, A.B. Ultrasound assisted miniemulsion polymerization for preparation of polypyrrole-zinc oxide (PPy/ZnO) functional latex for liquefied petroleum gas sensing. Ind. Eng. Chem. Res. 2013, 52, 7704–7712. [Google Scholar] [CrossRef]
- Ma, Z.; Mohapatra, J.; Wei, K.; Liu, J.P.; Sun, S. Magnetic nanoparticles: Synthesis, anisotropy, and applications. Chem. Rev. 2021, 123, 3904–3943. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Kostopoulou, A.; LaGrow, A.P. Magnetic nanopartNicle composites: Synergistic effects and applications. Adv. Sci. 2021, 8, 2004951. [Google Scholar] [CrossRef]
- Behrens, S.; Appel, I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016, 39, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kloust, H.; Schmidtke, C.; Feld, A.; Schotten, T.; Eggers, R.; Fittschen, U.E.A.; Schulz, F.; Poeselt, E.; Ostermann, J.; Bastus, N.G.; et al. In situ functionalization and PEO coating of iron oxide nanocrystals using seeded emulsion polymerization. Langmuir 2013, 29, 4915–4921. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, F.; Li, L.; Wang, Y.; Qu, H. A novel CoFe2O4/polyacrylate nanocomposite prepared via an in situ polymerization in emulsion system. React. Funct. Polym. 2009, 69, 43–47. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Cui, X.; Cong, D.; Wang, H. Preparation and characterization of magnetic hollow PMMA nanospheres via in situ emulsion polymerization. Colloid. Surf. A 2010, 363, 71–77. [Google Scholar] [CrossRef]
- Yoon, H.; Rhym, Y.M.; Shim, S.E. Optical properties of core/shell typed PMMA/CdS nanoparticles prepared by in situ and ex situ surfactant-free emulsion polymerization. Colloid Polym. Sci. 2011, 289, 1185–1189. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.; Guan, Y.; He, Y.; Li, S.; Liu, H.; Zhou, L.; Wei, C.; Yu, C.; Chen, Y. Transparent and strong polymer nanocomposites generated from Pickering emulsion gels stabilized by cellulose nanofibrils. Carbohydr. Polym. 2019, 224, 115202. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current trends in pickering emulsions: Particle morphology and applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Moreno, A.; Morsali, M.; Liu, J.; Sipponen, M.H. Access to tough and transparent nanocomposites via Pickering emulsion polymerization using biocatalytic hybrid lignin nanoparticles as functional surfactants. Green Chem. 2021, 23, 3001–3014. [Google Scholar] [CrossRef]
- Piao, S.H.; Gao, C.Y.; Choi, H.J. Pickering emulsion-polymerized conducting polymer nanocomposites and their applications. Chem. Pap. 2016, 71, 179–188. [Google Scholar] [CrossRef]
- Fujisawa, S. Material design of nanocellulose/polymer composites via Pickering emulsion templating. Polym. J. 2020, 53, 103–109. [Google Scholar] [CrossRef]
- Tiarks, F.; Landfester, K.; Antonietti, M. Silica nanoparticles as surfactants and fillers for latexes made by miniemulsion polymerization. Langmuir 2001, 17, 5775–5780. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Cardenas, S. Advanced polymeric solids containing nano- and micro-particles prepared via emulsion-based polymerization approaches. A review. Anal. Chim. Acta 2022, 1208, 339669. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Luo, M.; Sanyal, S.; Rege, K.; Dai, L. The one-step Pickering emulsion polymerization route for synthesizing organic-inorganic nanocomposite particles. Materials 2010, 3, 1186–1202. [Google Scholar] [CrossRef] [Green Version]
- Lotierzo, A.; Bon, S.A.F. A mechanistic investigation of Pickering emulsion polymerization. Polym. Chem. 2017, 8, 5100–5111. [Google Scholar] [CrossRef] [Green Version]
- Balmer, J.A.; Armes, S.P.; Fowler, P.W.; Tarnai, T.; Gaspar, Z.; Murray, K.A.; Williams, N.S.J. Packing efficiency of small silica particles on large latex particles: A facile route to colloidal nanocomposites. Langmuir 2009, 25, 5339–5347. [Google Scholar] [CrossRef]
- Fielding, L.A.; Tonnar, J.; Armes, S.P. All-acrylic film-forming colloidal polymer/silica nanocomposite particles prepared by aqueous emulsion polymerization. Langmuir 2011, 27, 11129–11144. [Google Scholar] [CrossRef] [Green Version]
- Balmer, J.A.; Le Cunff, E.C.; Armes, S.P.; Murray, M.W.; Murray, K.A.; Williams, N.S. When does silica exchange occur between vinyl polymer-silica nanocomposite particles and sterically stabilized latexes? Langmuir 2010, 26, 13662–13671. [Google Scholar] [CrossRef]
- Schmid, A.; Tonnar, J.; Armes, S.P. A new highly efficient route to polymer-silica colloidal nanocomposite particles. Adv. Mater. 2008, 20, 3331–3336. [Google Scholar] [CrossRef]
- Balmer, J.A.; Mykhaylyk, O.O.; Fairclough, J.P.A.; Ryan, A.J.; Armes, S.P.; Murray, M.W.; Murray, K.A.; Williams, N.S.J. Unexpected facile redistribution of adsorbed silica nanoparticles between latexes. J. Am. Chem. Soc. 2010, 132, 2166–2168. [Google Scholar] [CrossRef] [PubMed]
- Percy, M.J.; Barthet, C.; Lobb, J.C.; Khan, M.A.; Lascelles, S.F.; Vamvakaki, M.; Armes, S.P. Synthesis and characterization of vinyl polymer-silica colloidal nanocomposites. Langmuir 2000, 16, 6913–6920. [Google Scholar] [CrossRef]
- Percy, M.J.; Armes, S.P. Surfactant-free synthesis of colloidal poly(methyl methacrylate)/silica nanocomposites in the absence of auxiliary comonomers. Langmuir 2002, 18, 4562–4565. [Google Scholar] [CrossRef]
- Percy, M.J.; Amalvy, J.I.; Randall, D.P.; Armes, S.P. Synthesis of vinyl polymer-silica colloidal nanocomposites prepared using commercial alcoholic silica sols. Langmuir 2004, 20, 2184–2190. [Google Scholar] [CrossRef]
- Schmid, A.; Fujii, S.; Armes, S.P. Synthesis of micrometer-sized silica-stabilized polystyrene latex particles. Langmuir 2005, 21, 8103–8105. [Google Scholar] [CrossRef]
- Dupin, D.; Schmid, A.; Balmer, J.A.; Armes, S.P. Efficient synthesis of poly(2-vinylpyridine)-silica colloidal nanocomposite particles using a cationic azo initiator. Langmuir 2007, 23, 11812–11818. [Google Scholar] [CrossRef]
- Schmid, A.; Armes, S.P.; Leite, C.A.P.; Galembeck, F. Efficient preparation of polystyrene/silica colloidal nanocomposite particles by emulsion polymerization using a glycerol-functionalized silica sol. Langmuir 2009, 25, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Scherl, P.; Armes, S.P.; Leite, C.A.P.; Galembeck, F. Synthesis and characterization of film-forming colloidal nanocomposite particles prepared via surfactant-free aqueous emulsion copolymerization. Macromolecules 2009, 42, 3721–3728. [Google Scholar] [CrossRef]
- Barthet, C.; Hickey, A.J.; Cairns, D.B.; Armes, S.P. Synthesis of novel polymer-silica colloidal nanocomposites via free-radical polymerization of vinyl monomers. Adv. Mater. 1999, 11, 408–410. [Google Scholar] [CrossRef]
- Fujii, S.; Armes, S.P.; Binks, B.P.; Murakami, R. Stimulus-responsive particulate emulsifiers based on lightly cross-linked poly(4-vinylpyridine)-silica nanocomposite microgels. Langmuir 2006, 22, 6818–6825. [Google Scholar] [CrossRef] [PubMed]
- Amalvy, J.I.; Percy, M.J.; Armes, S.P.; Wiese, H. Synthesis and characterization of novel film-forming vinyl polymer/silica colloidal nanocomposites. Langmuir 2001, 17, 4770–4778. [Google Scholar] [CrossRef]
- Qi, D.M.; Bao, Y.Z.; Huang, Z.M.; Weng, Z.X. Anchoring of polyacrylate onto silica and formation of polyacrylate/silica nanocomposite particles via in situ emulsion polymerization. Colloid Polym. Sci. 2008, 286, 233–241. [Google Scholar] [CrossRef]
- Qi, D.M.; Bao, Y.Z.; Weng, Z.X.; Huang, Z.M. Preparation of acrylate polymer/silica nanocomposite particles with high silica encapsulation efficiency via miniemulsion polymerization. Polymer 2006, 47, 4622–4629. [Google Scholar] [CrossRef]
- Bao, Y.; Zhao, W.; Huang, Z. Preparation of mesoporous carbons from acrylonitrile-methyl methacrylate copolymer/silica nanocomposites synthesized by in-situ emulsion polymerization. Chin. J. Chem. Eng. 2013, 21, 691–697. [Google Scholar] [CrossRef]
- Colver, P.J.; Colard, C.A.L.; Bon, S.A.F. Multilayered nanocomposite polymer colloids using emulsion polymerization stabilized by solid particles. J. Am. Chem. Soc. 2008, 130, 16850–16851. [Google Scholar] [CrossRef] [PubMed]
- Cauvin, S.; Colver, P.J.; Bon, S.A.F. Pickering stabilized miniemulsion polymerization: Preparation of clay armored latexes. Macromolecules 2005, 38, 7887–7889. [Google Scholar] [CrossRef]
- Bon, S.A.F.; Colver, P.J. Pickering miniemulsion polymerization using laponite clay as a stabilizer. Langmuir 2007, 23, 8316–8322. [Google Scholar] [CrossRef]
- Pakdel, A.; Pourmahdian, S.; Eslami, H. One-pot preparation of core-shell, organic-inorganic, hybrid latexes by in situ nanoparticle precipitation in pickering emulsion polymerization. Macromol. Chem. Phys. 2012, 213, 1944–1952. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; He, X.; Kong, D.; Zhou, C.; Wu, H.; Yang, Z.; Yang, Z.; Hu, Y. Preparation of cinnamon oil-loaded antibacterial composite microcapsules by in situ polymerization of pickering emulsion templates. Macromol. Mater. Eng. 2020, 305, 1900851. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Zhou, S.; You, B. Synthesis of raspberry-like PMMA/SiO2 nanocomposite particles via a surfactant-free method. Macromolecules 2004, 37, 9613–9619. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.; You, B.; Wu, L. A novel preparation method of raspberry-like PMMA/SiO2 hybrid microspheres. Macromolecules 2005, 38, 6411–6417. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, S.; Pi, P.; Cheng, J.; Wang, L.; Yang, J.; Wen, X. Polyacrylate/silica nanoparticles hybrid emulsion coating with high silica content for high hardness and dry-wear-resistant. Prog. Org. Coat. 2018, 121, 30–37. [Google Scholar] [CrossRef]
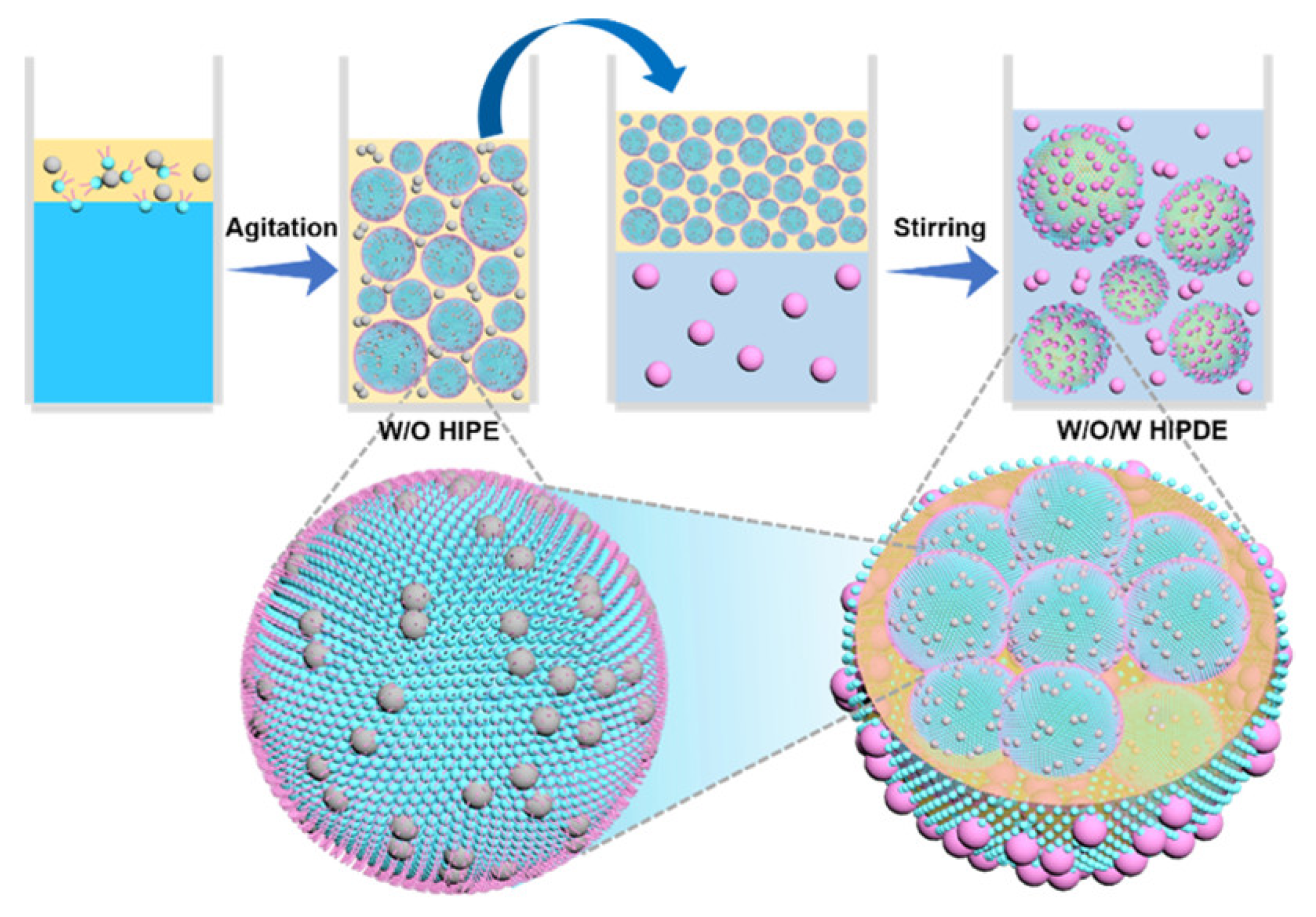
- Guan, X.; Ngai, T. pH-sensitive W/O Pickering high internal phase emulsions and W/O/W high internal water-phase double emulsions with tailored microstructures costabilized by lecithin and silica inorganic particles. Langmuir 2021, 37, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
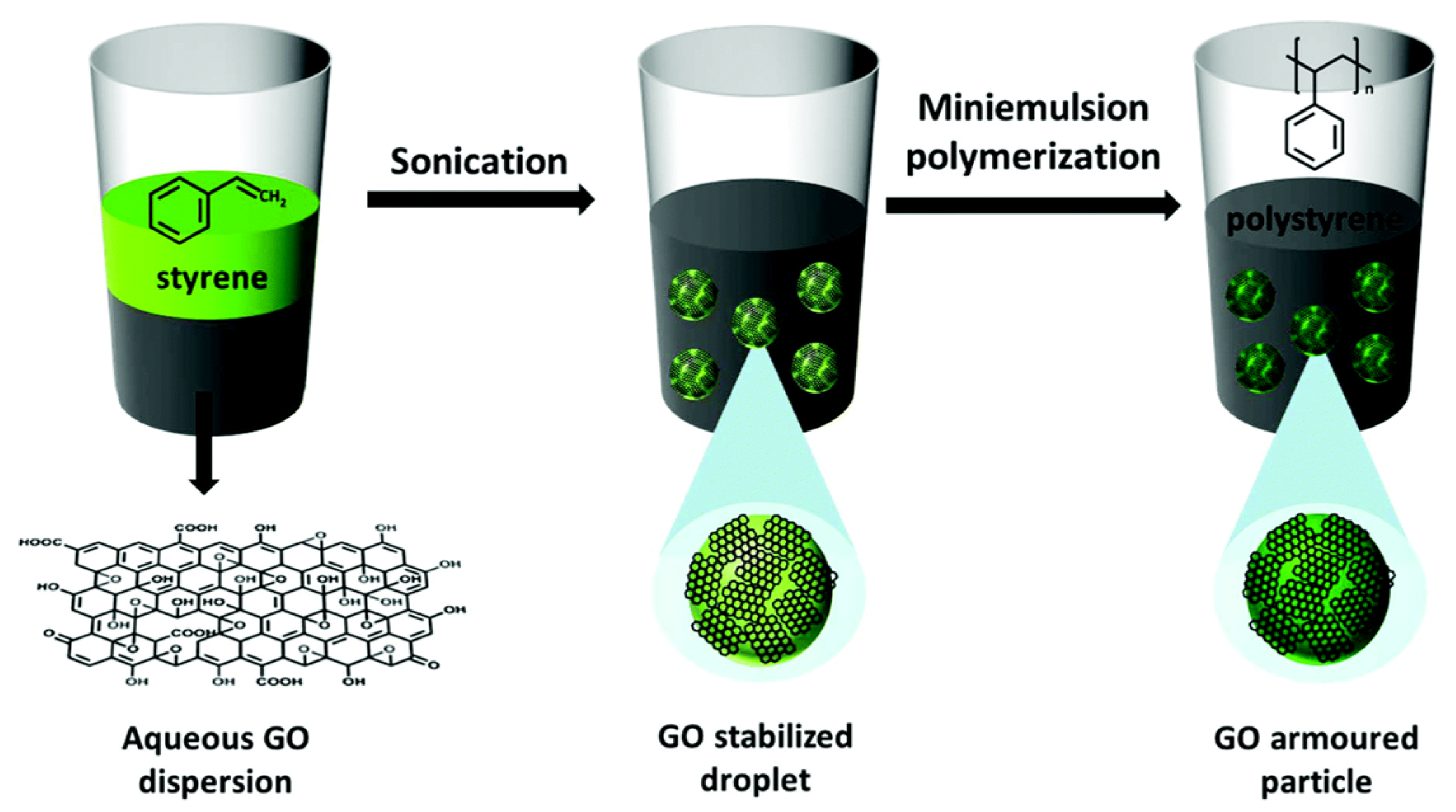
- Che Man, S.H.; Thickett, S.C.; Whittaker, M.R.; Zetterlund, P.B. Synthesis of polystyrene nanoparticles “armoured” with nanodimensional graphene oxide sheets by miniemulsion polymerization. J. Polym. Sci. Pol. Chem. 2013, 51, 47–58. [Google Scholar] [CrossRef]
- Che Man, S.H.; Mohd Yusof, N.Y.; Whittaker, M.R.; Thickett, S.C.; Zetterlund, P.B. Influence of monomer type on miniemulsion polymerization systems stabilized by graphene oxide as sole surfactant. J. Polym. Sci. Pol. Chem. 2013, 51, 5153–5162. [Google Scholar] [CrossRef]
- Fadil, Y.; Jasinski, F.; Wing Guok, T.; Thickett, S.C.; Minami, H.; Zetterlund, P.B. Pickering miniemulsion polymerization using graphene oxide: Effect of addition of a conventional surfactant. Polym. Chem. 2018, 9, 3368–3378. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Duan, L.; Zhang, W.; Su, M.; Sun, Z.; He, P. Polystyrene/graphene oxide nanocomposites synthesized via Pickering polymerization. Prog. Org. Coat. 2016, 99, 23–31. [Google Scholar] [CrossRef]
- Xie, P.; Ge, X.; Fang, B.; Li, Z.; Liang, Y.; Yang, C. Pickering emulsion polymerization of graphene oxide-stabilized styrene. Colloid Polym. Sci. 2013, 291, 1631–1639. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Jin, X.; Wang, T. Study on the PMMA/GO nanocomposites with good thermal stability prepared by in situ Pickering emulsion polymerization. J. Therm. Anal. Calorim. 2014, 117, 755–763. [Google Scholar] [CrossRef]
- Gudarzi, M.M.; Sharif, F. Self assembly of graphene oxide at the liquid-liquid interface: A new route to the fabrication of graphene based composites. Soft Matter 2011, 7, 3432–3440. [Google Scholar] [CrossRef]
- He, Y.; Wu, F.; Sun, X.; Li, R.; Guo, Y.; Li, C.; Zhang, L.; Xing, F.; Wang, W.; Gao, J. Factors that affect Pickering emulsions stabilized by graphene oxide. ACS Appl. Mater. Inter. 2013, 5, 4843–4855. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Song, X.; Du, Q. Fabrication of two kinds of polymer microspheres stabilized by modified titania during Pickering emulsion polymerization. Macromol. Chem. Phys. 2010, 211, 2517–2529. [Google Scholar] [CrossRef]
- Song, X.; Yin, G.; Zhao, Y.; Wang, H.; Du, Q. Effect of an anionic monomer on the pickering emulsion polymerization stabilized by titania hydrosol. J. Polym. Sci. Pol. Chem. 2009, 47, 5728–5736. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Y.; Wang, H.; Du, Q. Fabrication of polymer microspheres using titania as a photocatalyst and pickering stabilizer. Langmuir 2009, 25, 4443–4449. [Google Scholar] [CrossRef] [PubMed]
- Chou, I.C.; Lee, C.F.; Chiu, W.Y. Preparation of novel suspensions of ZnO/living block copolymer latex nanoparticles via pickering emulsion polymerization and their long term stability. J. Polym. Sci. Pol. Chem. 2011, 49, 3524–3535. [Google Scholar] [CrossRef]
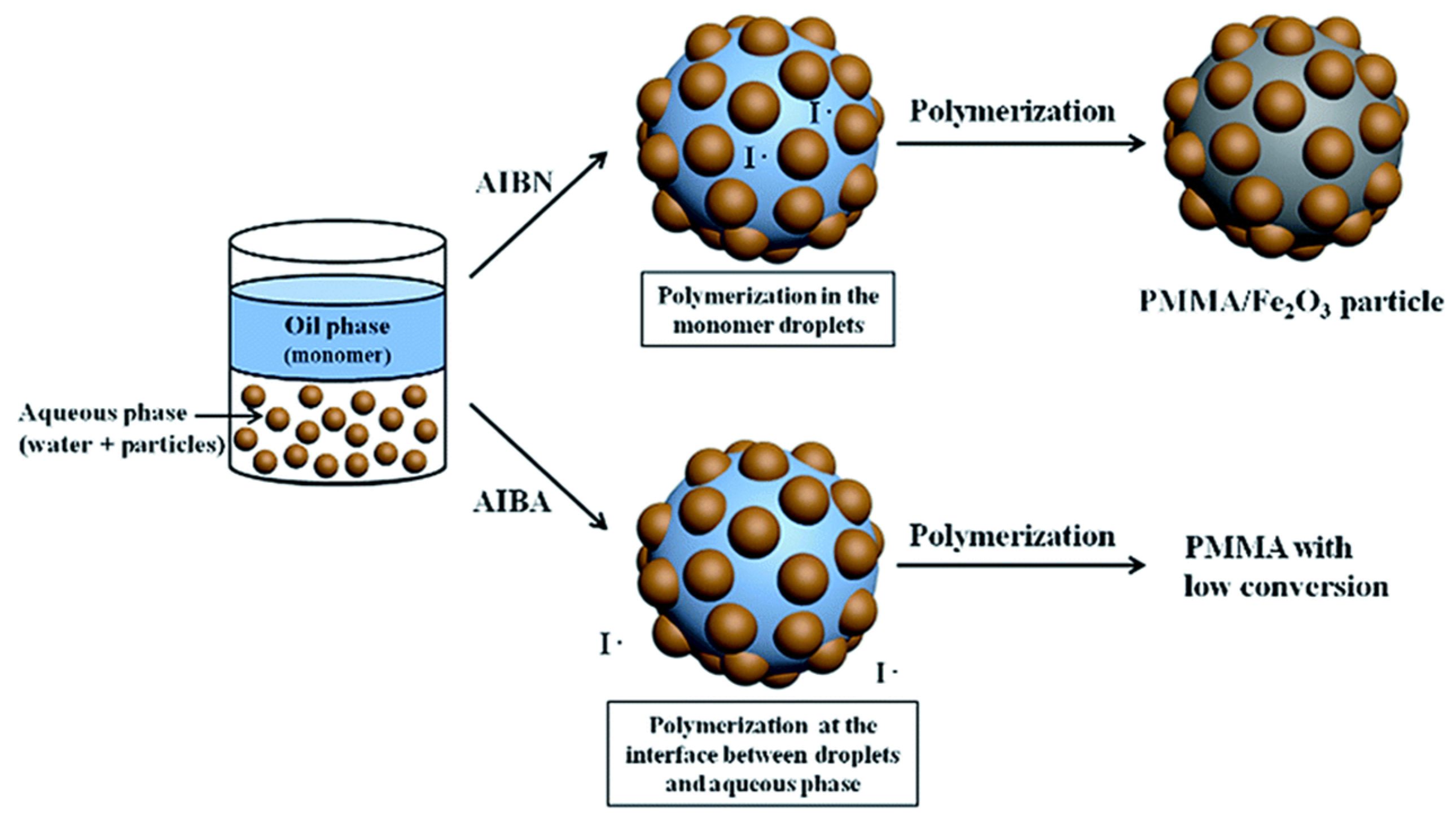
- Woo, J.C.; Hyo, S.J.; Hyoung, J.C. Pickering emulsion polymerized smart magnetic poly(methyl methacrylate)/Fe2O3 composite particles and their stimulus-response. RSC Adv. 2015, 5, 23094–23100. [Google Scholar] [CrossRef]
- Kim, Y.J.; Liu, Y.D.; Seo, Y.; Choi, H.J. Pickering-emulsion-polymerized polystyrene/Fe2O3 composite particles and their magnetoresponsive characteristics. Langmuir 2013, 29, 4959–4965. [Google Scholar] [CrossRef]
- Zhou, M.J.; Zhou, S.Z.; Pang, X.C.; Li, K.R.; Qiao, X.G. Preparation of superparamagnetic γ-Fe2O3@LS@PS composite latex particles through pickering miniemulsion polymerization. Colloid. Surf. A 2020, 585, 124040. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sun, B.; Hao, Z.; Zhang, J. Advances in Organic–Inorganic Hybrid Latex Particles via In Situ Emulsion Polymerization. Polymers 2023, 15, 2995. https://doi.org/10.3390/polym15142995
Wang Y, Sun B, Hao Z, Zhang J. Advances in Organic–Inorganic Hybrid Latex Particles via In Situ Emulsion Polymerization. Polymers. 2023; 15(14):2995. https://doi.org/10.3390/polym15142995
Chicago/Turabian StyleWang, Yubin, Baojiang Sun, Zhiwei Hao, and Jianhua Zhang. 2023. "Advances in Organic–Inorganic Hybrid Latex Particles via In Situ Emulsion Polymerization" Polymers 15, no. 14: 2995. https://doi.org/10.3390/polym15142995





