Effect of the Welan Gum Concentration on the Rheological and Structural Behaviour of Biocomposite Hydrogels with Sepiolite as Filler
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aqueous Systems
2.3. Rheological Measurements
2.4. Cryo-SEM Images
3. Results and Discussion
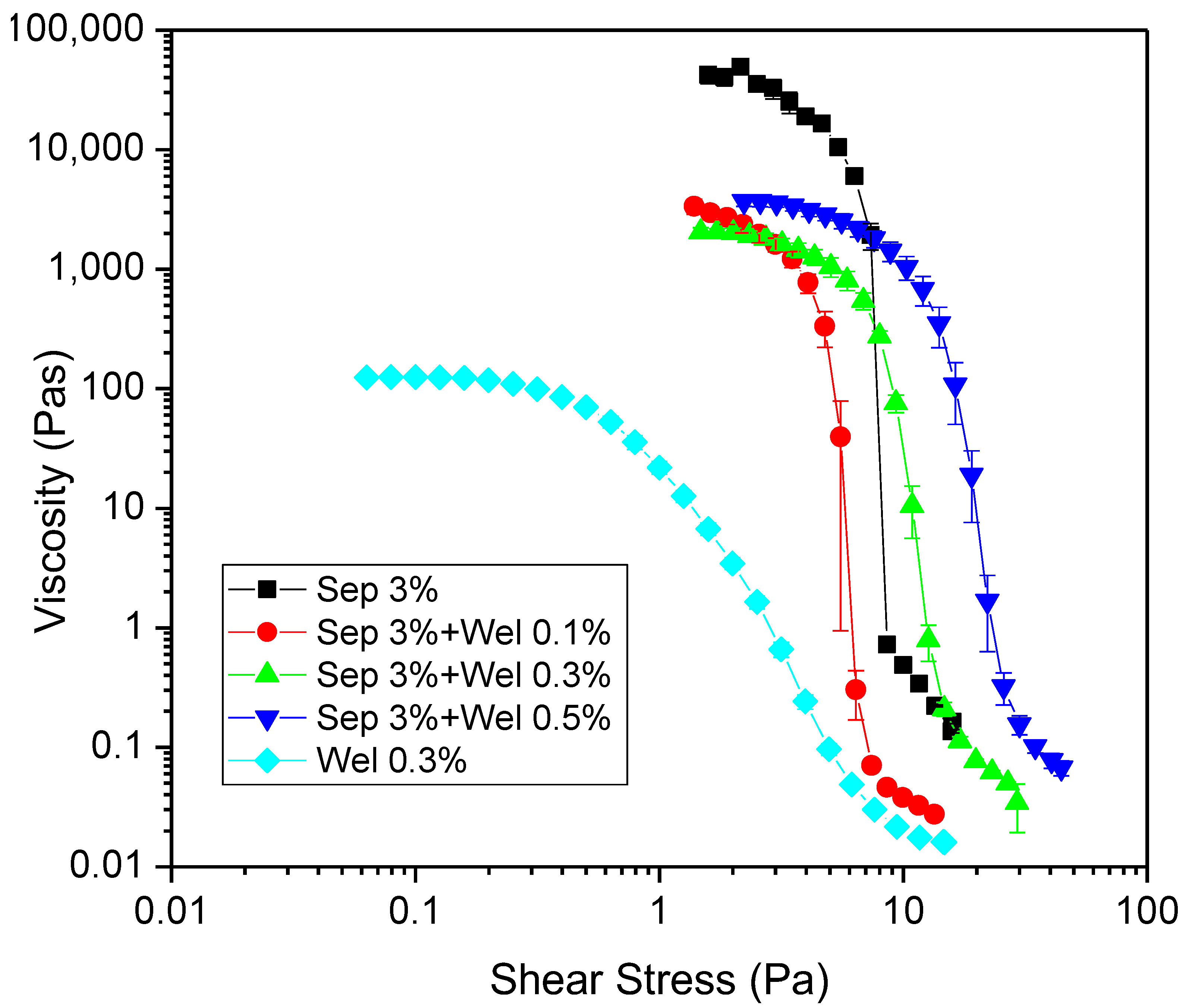
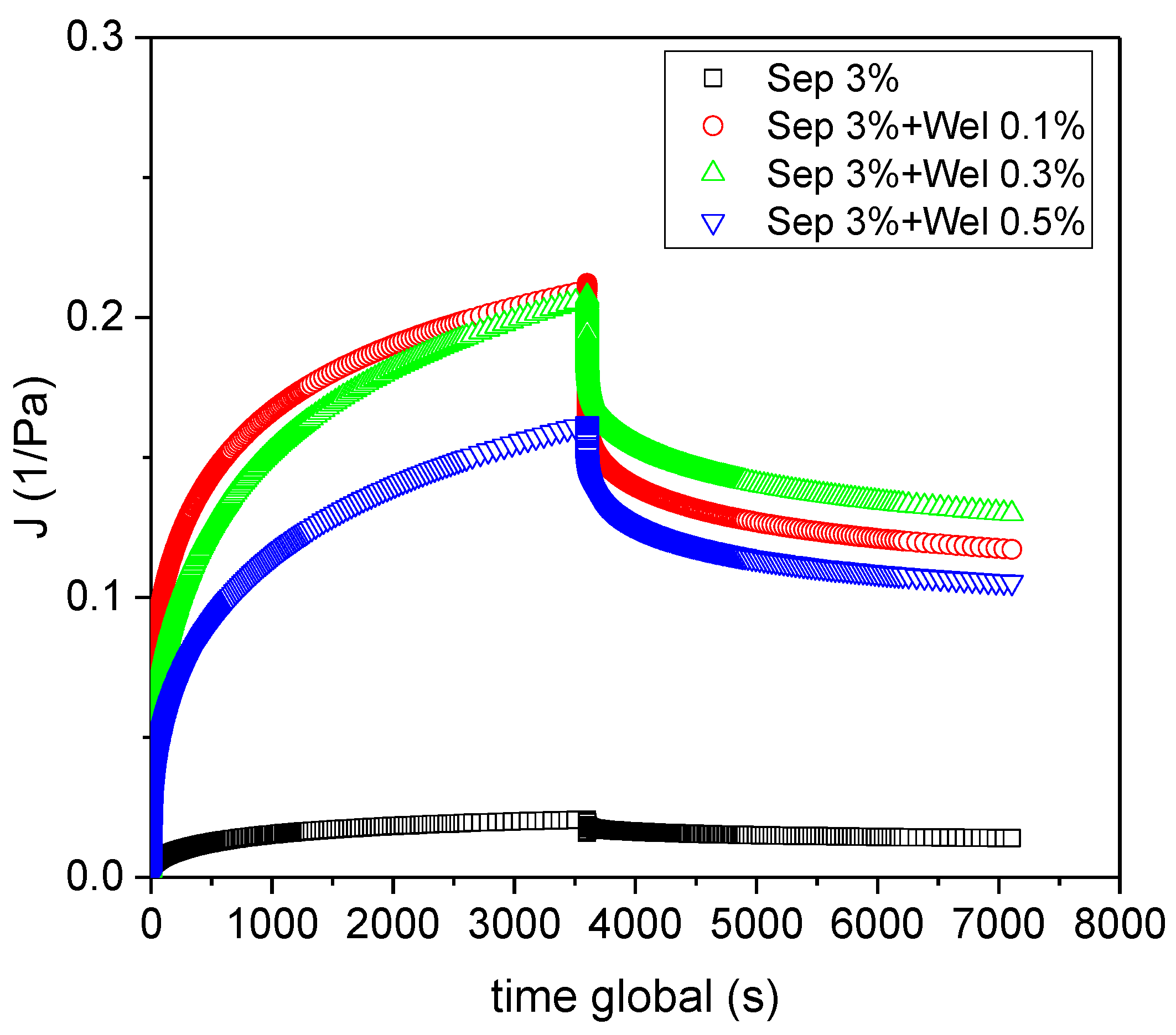
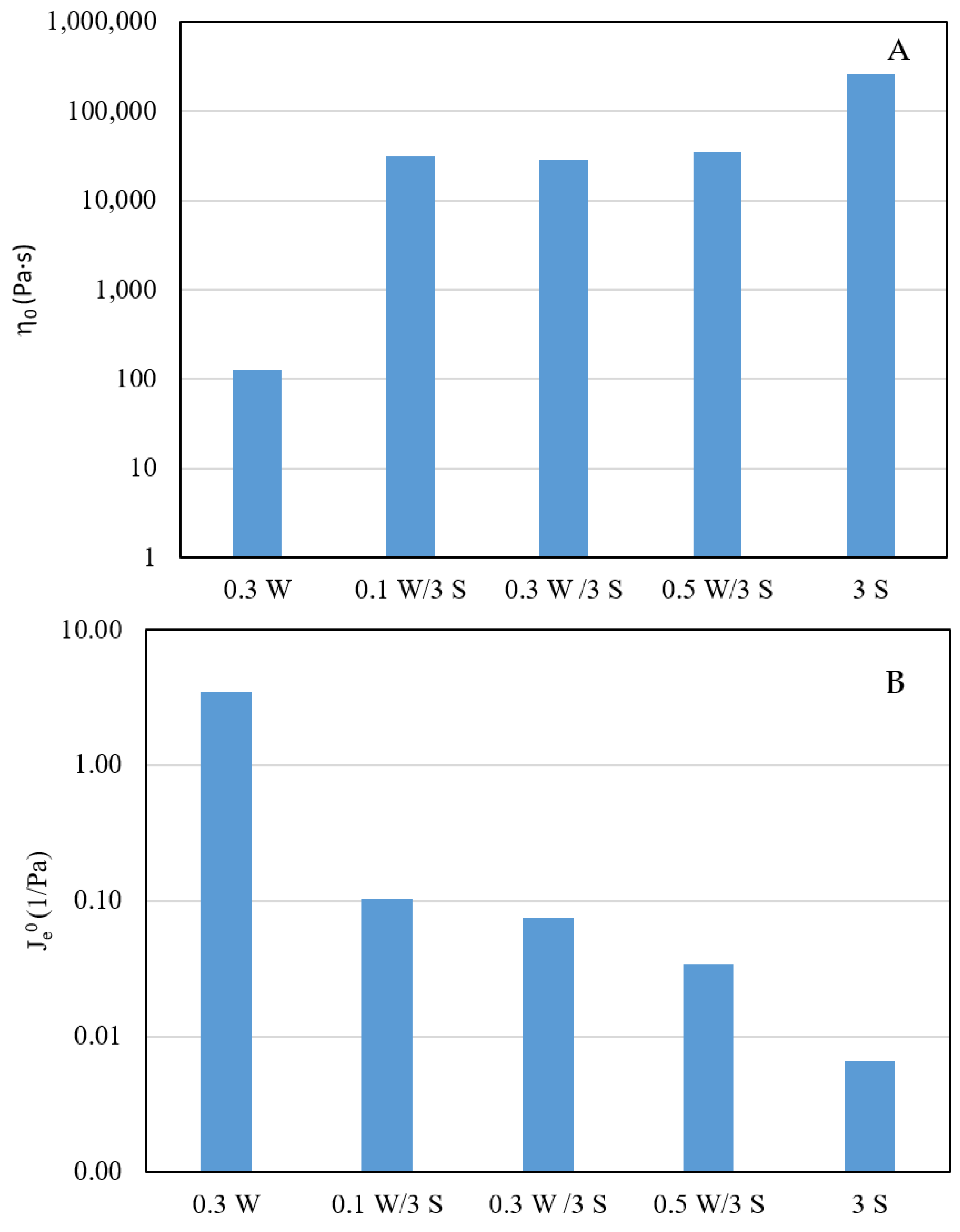
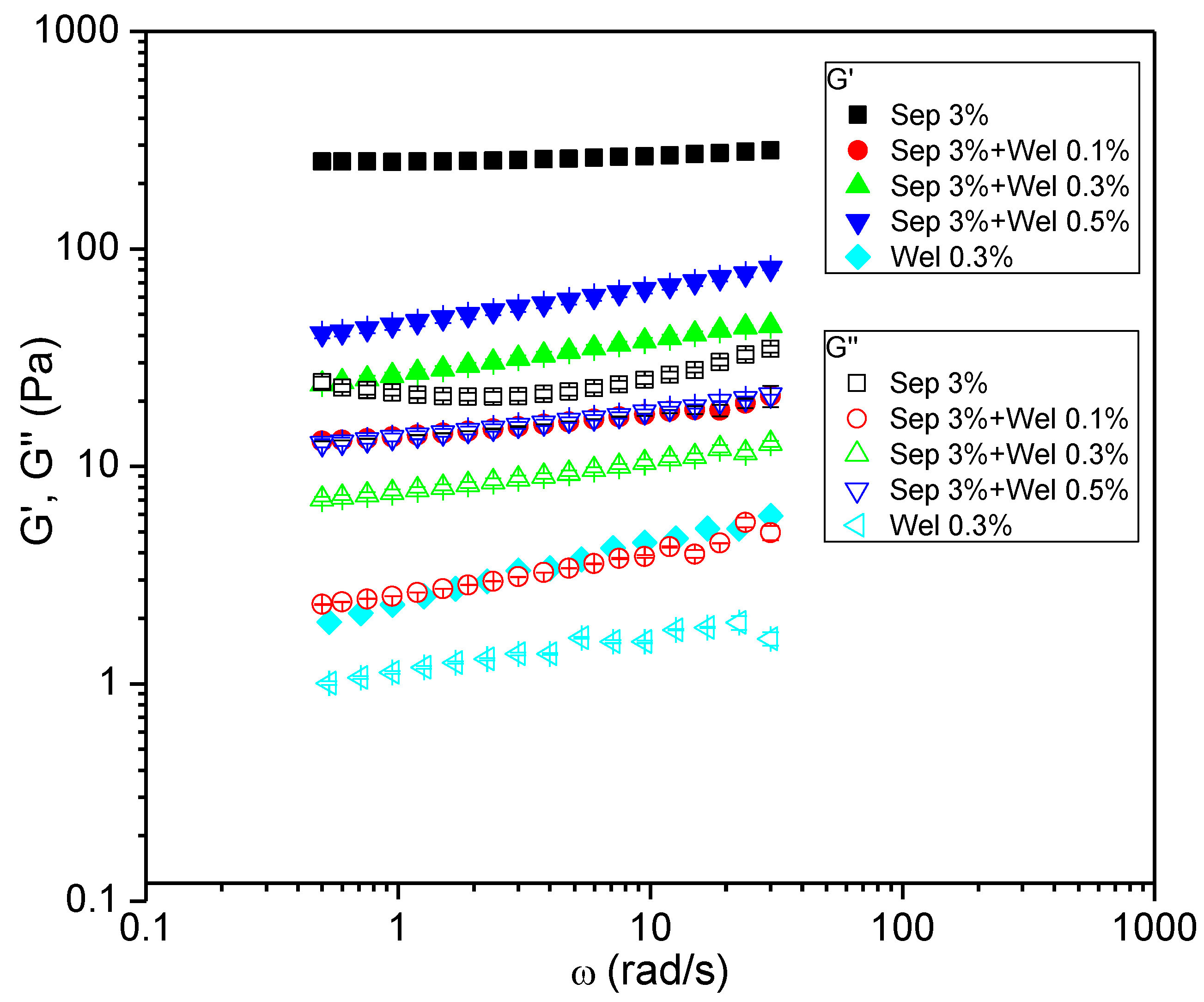
3.1. Rheological Characterization
3.2. Cryo-SEM Images
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, F.; Ullah, H.; Ali, Z.; Rahim, F.; Khan, F.; Ur Rehman, Z. Polymer-Clay Nanocomposites, Preparations and Current Applications: A Review. Curr. Nanomater. 2016, 1, 83–95. [Google Scholar] [CrossRef]
- Karak, N. Vegetable Oil-Based Polymer Nanocomposites. In Vegetable Oil-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Díez-Pascual, A.M. Synthesis and Applications of Biopolymer Composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef] [Green Version]
- Gilman, J.W. Flammability and Thermal Stability Studies of ž/1 Polymer Layered-Silicate Clay Nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Mohd Zaini, N.A.; Ismail, H.; Rusli, A. Short Review on Sepiolite-Filled Polymer Nanocomposites. Polym. Plast. Technol. Eng. 2017, 56, 1665–1679. [Google Scholar] [CrossRef]
- Norkhairunnisa, M.; Azizan, A.; Mariatti, M.; Ismail, H.; Sim, L. Thermal Stability and Electrical Behavior of Polydimethylsiloxane Nanocomposites with Carbon Nanotubes and Carbon Black Fillers. J. Compos. Mater. 2012, 46, 903–910. [Google Scholar] [CrossRef]
- Wilson, R.; George, S.M.; Maria, H.J.; Plivelic, T.S.; Kumar, S.A.; Thomas, S. Clay Intercalation and Its Influence on the Morphology and Transport Properties of EVA/Clay Nanocomposites. J. Phys. Chem. C 2012, 116, 20002–20014. [Google Scholar] [CrossRef]
- Li, Y.; Ishida, H. A Study of Morphology and Intercalation Kinetics of Polystyrene−Organoclay Nanocomposites. Macromolecules 2005, 38, 6513–6519. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Xiang, W.; Liu, L.; Yang, Y.; Nugroho, R.W.N.; Fan, Y.; Rojas, O.J. Self-Assembled Networks of Short and Long Chitin Nanoparticles for Oil/Water Interfacial Superstabilization. ACS Sustain. Chem. Eng. 2019, 7, 6497–6511. [Google Scholar] [CrossRef]
- Wang, J.; Le-The, H.; Wang, Z.; Li, H.; Jin, M.; van den Berg, A.; Zhou, G.; Segerink, L.I.; Shui, L.; Eijkel, J.C.T. Microfluidics Assisted Fabrication of Three-Tier Hierarchical Microparticles for Constructing Bioinspired Surfaces. ACS Nano 2019, 13, 3638–3648. [Google Scholar] [CrossRef] [Green Version]
- Yim, G.; Kang, S.; Kim, Y.-J.; Kim, Y.-K.; Min, D.-H.; Jang, H. Hydrothermal Galvanic-Replacement-Tethered Synthesis of Ir–Ag–IrO 2 Nanoplates for Computed Tomography-Guided Multiwavelength Potent Thermodynamic Cancer Therapy. ACS Nano 2019, 13, 3434–3447. [Google Scholar] [CrossRef]
- Zimpel, A.; al Danaf, N.; Steinborn, B.; Kuhn, J.; Höhn, M.; Bauer, T.; Hirschle, P.; Schrimpf, W.; Engelke, H.; Wagner, E.; et al. Coordinative Binding of Polymers to Metal–Organic Framework Nanoparticles for Control of Interactions at the Biointerface. ACS Nano 2019, 13, 3884–3895. [Google Scholar] [CrossRef] [PubMed]
- Chkirida, S.; Zari, N.; Bouhfid, R.; el kacem Qaiss, A. Insight into the Bionanocomposite Applications on Wastewater Decontamination: Review. J. Water Process Eng. 2021, 43, 102198. [Google Scholar] [CrossRef]
- Echt, T.; Plank, J. An Improved Test Protocol for High Temperature Carrying Capacity of Drilling Fluids Exemplified on a Sepiolite Mud. J. Nat. Gas. Sci. Eng. 2019, 70, 102964. [Google Scholar] [CrossRef]
- Unterman, S.; Charles, L.F.; Strecker, S.E.; Kramarenko, D.; Pivovarchik, D.; Edelman, E.R.; Artzi, N. Hydrogel Nanocomposites with Independently Tunable Rheology and Mechanics. ACS Nano 2017, 11, 2598–2610. [Google Scholar] [CrossRef] [PubMed]
- Payzant, J.; Zhou, Z. Patent WO2000072958A1, 7 December 2000. Available online: https://patents.google.com/patent/WO2000072958A1/en?oq=WO2000072958A1 (accessed on 20 December 2022).
- O’Neill, M.A.; Selvendran, R.R.; Morris, V.J.; Eagles, J. Structure of the Extracellular Polysaccharide Produced by the Bacterium Alcaligenes (ATCC 31555) Species. Carbohydr. Res. 1986, 147, 295–313. [Google Scholar] [CrossRef]
- Kaur, V.; Bera, M.B.; Panesar, P.S.; Kumar, H.; Kennedy, J.F. Welan Gum: Microbial Production, Characterization, and Applications. Int. J. Biol. Macromol. 2014, 65, 454–461. [Google Scholar] [CrossRef]
- Tian, G.; Han, G.; Wang, F.; Liang, J. Sepiolite Nanomaterials: Structure, Properties and Functional Applications. In Nanomaterials from Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–201. [Google Scholar]
- Khayat, K.H. Effect of Welan Gum-Superplasticizer Combinations on Properties of Cement Grouts Use of Internal Curing for HSC and UHPC View Project Design and Performance of Roller-Compacted Concrete for Pavement Applications View Project. Spec. Publ. 2000, 195, 249–268. [Google Scholar]
- Gao, C. Potential Applications of Welan Gum in Upstream Petroleum Industry. Int. J. Oil Gas Coal Eng. 2016, 4, 16. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Liu, C.; Zeng, L.; Chen, S. The Competitive Adsorption Characteristics of Welan Gum and Superplasticizer in Cement Mortar. J. Wuhan Univ. Technol. -Mater. Sci. Ed. 2016, 31, 131–138. [Google Scholar] [CrossRef]
- Asubiaro, A.; Shah, S.N. Rheological and Hydraulic Properties of Welan Gum Fluids in Straight and Coiled Tubings. J. Fluids Eng. 2008, 130, 0815061–08150616. [Google Scholar] [CrossRef]
- Plank, J.; Ng, S.; Foraita, S. Intercalation of the Microbial Biopolymers Welan Gum and EPS I into Layered Double Hydroxides. Z. Nat. B 2012, 67, 479–487. [Google Scholar] [CrossRef]
- Roy, N.; Bhowmick, A.K. Novel in Situ Polydimethylsiloxane-Sepiolite Nanocomposites: Structure-Property Relationship. Polymer 2010, 51, 5172–5185. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romero, E. Advances in the Crystal Chemistry of Sepiolite and Palygorskite. In Developments in Clay Science; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 3, pp. 33–65. ISBN 9780444536075. [Google Scholar]
- Murray, H.H.; Pozo, M.; Galán, E. Chapter 4—An Introduction to Palygorskite and Sepiolite Deposits—Location, Geology and Uses. In Developments in Palygorskite-Sepiolite Research; Galàn, E., Singer, A., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 85–99. [Google Scholar]
- Sipos, P.; Németh, T.; Kis, V.K.; Mohai, I. Sorption of Copper, Zinc and Lead on Soil Mineral Phases. Chemosphere 2008, 73, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Santarén, J.; Esteban-Cubillo, A.; Aparicio, P. Current Industrial Applications of Palygorskite and Sepiolite. In Developments in Clay Science; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 3, pp. 281–298. ISBN 9780444536075. [Google Scholar]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Liu, L.; Ulhassan, Z.; He, Z.; Yang, X. Sepiolite Clay: A Review of Its Applications to Immobilize Toxic Metals in Contaminated Soils and Its Implications in Soil–Plant System. Environ. Technol. Innov. 2021, 23, 101598. [Google Scholar] [CrossRef]
- Chandran, N.; Sarathchandran, C.; Thomas, S. Rheology of Polymer-Clay Nanocomposites. In Rheology of Polymer Blends and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Maqueda, C.; Partal, P.; Villaverde, J.; Perez-Rodriguez, J.L. Characterization of Sepiolite-Gel-Based Formulations for Controlled Release of Pesticides. Appl. Clay Sci. 2009, 46, 289–295. [Google Scholar] [CrossRef]
- Carmona, J.A.; Ramírez, P.; Trujillo-Cayado, L.A.; Caro, A.; Muñoz, J. Rheological and Microstructural Properties of Sepiolite Gels. Influence of the Addition of Ionic Surfactants. J. Ind. Eng. Chem. 2018, 59, 1–7. [Google Scholar] [CrossRef]
- Alvarez, A. Sepiolite: Properties and Uses. In Palygorskite—Sepiolite: Occurrences, Genesis and Uses; Singer, A., Galan, E., Eds.; Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 37, pp. 253–287. [Google Scholar]
- Carmona, J.A.; Ramírez, P.; García, M.C.; Santos, J.; Muñoz, J. Linear and Non-Linear Flow Behavior of Welan Gum Solutions. Rheol Acta 2019, 58, 1–8. [Google Scholar] [CrossRef]
- Philippe, A.M.; Baravian, C.; Imperor-Clerc, M.; de Silva, J.; Paineau, E.; Bihannic, I.; Davidson, P.; Meneau, F.; Levitz, P.; Michot, L.J. Rheo-SAXS Investigation of Shear-Thinning Behaviour of Very Anisometric Repulsive Disc-like Clay Suspensions. J. Phys. Condens. Matter 2011, 23, 194112. [Google Scholar] [CrossRef]
- Kiani, H.; Mousavi, M.E.; Mousavi, Z.E. Particle Stability in Dilute Fermented Dairy Drinks: Formation of Fluid Gel and Impact on Rheological Properties. Food Sci. Technol. Int. 2010, 16, 543–551. [Google Scholar] [CrossRef]
- García, M.C.; Alfaro, M.C.; Muñoz, J. Yield Stress and Onset of Nonlinear Time-Dependent Rheological Behaviour of Gellan Fluid Gels. J. Food Eng. 2015, 159, 42–47. [Google Scholar] [CrossRef]
- Calero, N.; Santos, J.; Muñoz, J. Methodology to Estimate the Yield Stress Applied to Ultraconcentrated Detergents as Model Systems. Chem. Eng. Sci. 2017, 166, 115–121. [Google Scholar] [CrossRef]
- Iqbal Khan, Z.; Habib, U.; Binti Mohamad, Z.; Razak Bin Rahmat, A.; Amira Sahirah Binti Abdullah, N. Mechanical and Thermal Properties of Sepiolite Strengthened Thermoplastic Polymer Nanocomposites: A Comprehensive Review. Alex. Eng. J. 2022, 61, 975–990. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; University of Wales, Institute of Non-Newtonian Fluid Mechanics: Cardiff, UK, 2000; ISBN 0953803201. [Google Scholar]
- Coussot, P.; Tabuteau, H.; Chateau, X.; Tocquer, L.; Ovarlez, G. Aging and Solid or Liquid Behavior in Pastes. J. Rheol. 2006, 50, 975–994. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona, J.A.; Ramírez, P.; Calero, N.; Muñoz, J. Effect of the Welan Gum Concentration on the Rheological and Structural Behaviour of Biocomposite Hydrogels with Sepiolite as Filler. Polymers 2023, 15, 33. https://doi.org/10.3390/polym15010033
Carmona JA, Ramírez P, Calero N, Muñoz J. Effect of the Welan Gum Concentration on the Rheological and Structural Behaviour of Biocomposite Hydrogels with Sepiolite as Filler. Polymers. 2023; 15(1):33. https://doi.org/10.3390/polym15010033
Chicago/Turabian StyleCarmona, José A., Pablo Ramírez, Nuria Calero, and José Muñoz. 2023. "Effect of the Welan Gum Concentration on the Rheological and Structural Behaviour of Biocomposite Hydrogels with Sepiolite as Filler" Polymers 15, no. 1: 33. https://doi.org/10.3390/polym15010033






