Rubber-Composite-Nanoparticle-Modified Epoxy Powder Coatings with Low Curing Temperature and High Toughness
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of DMA-CNBR
2.3. Preparation of the DMA-CNBR/EP
2.4. Coating Characterization
3. Results and Discussion
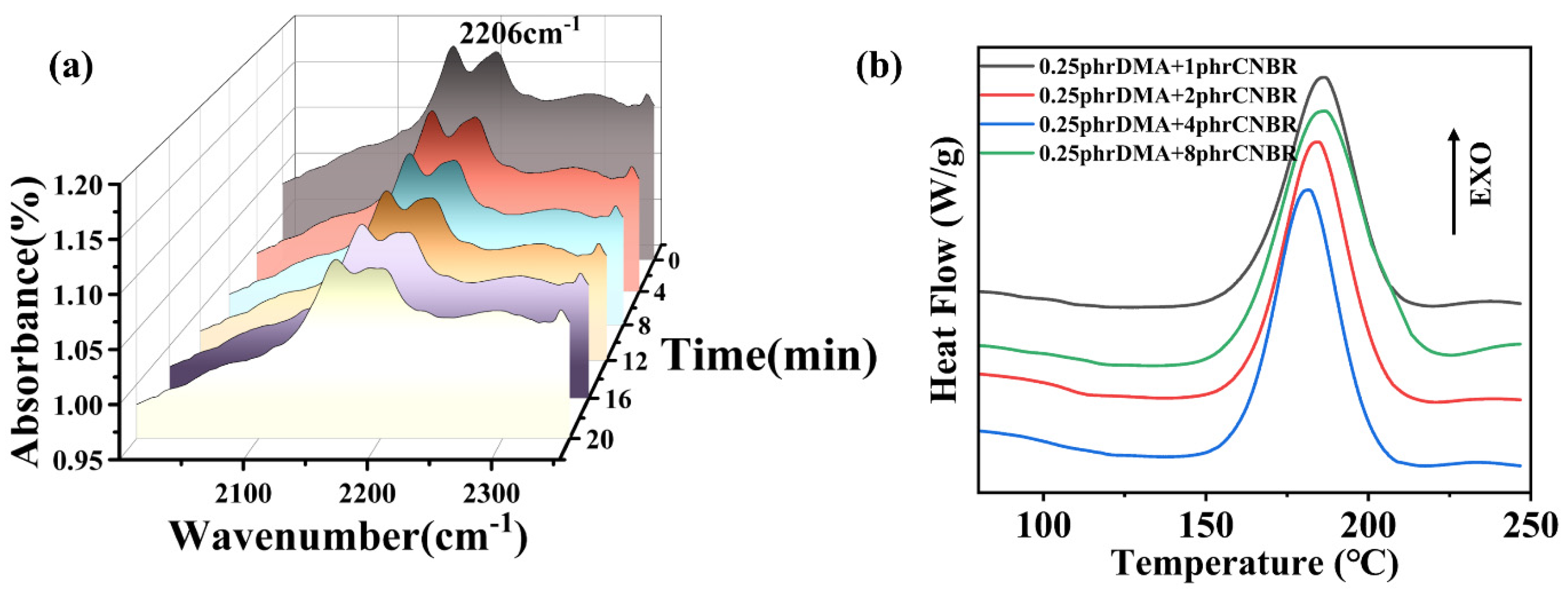
3.1. Characterization of the CNBR and DMA-CNBR/EP
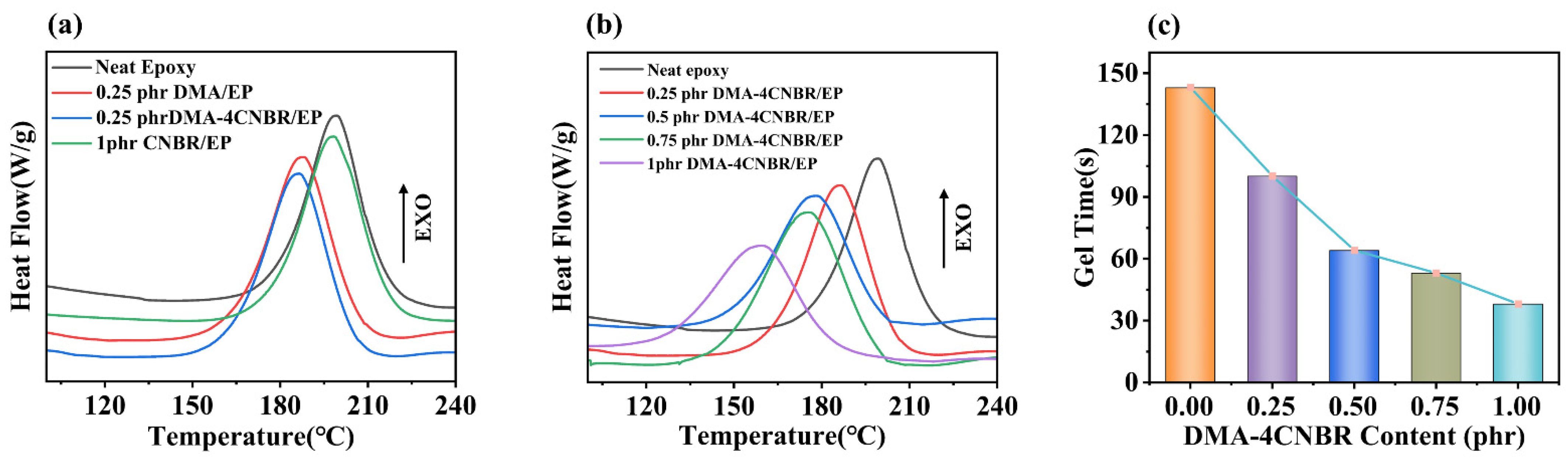
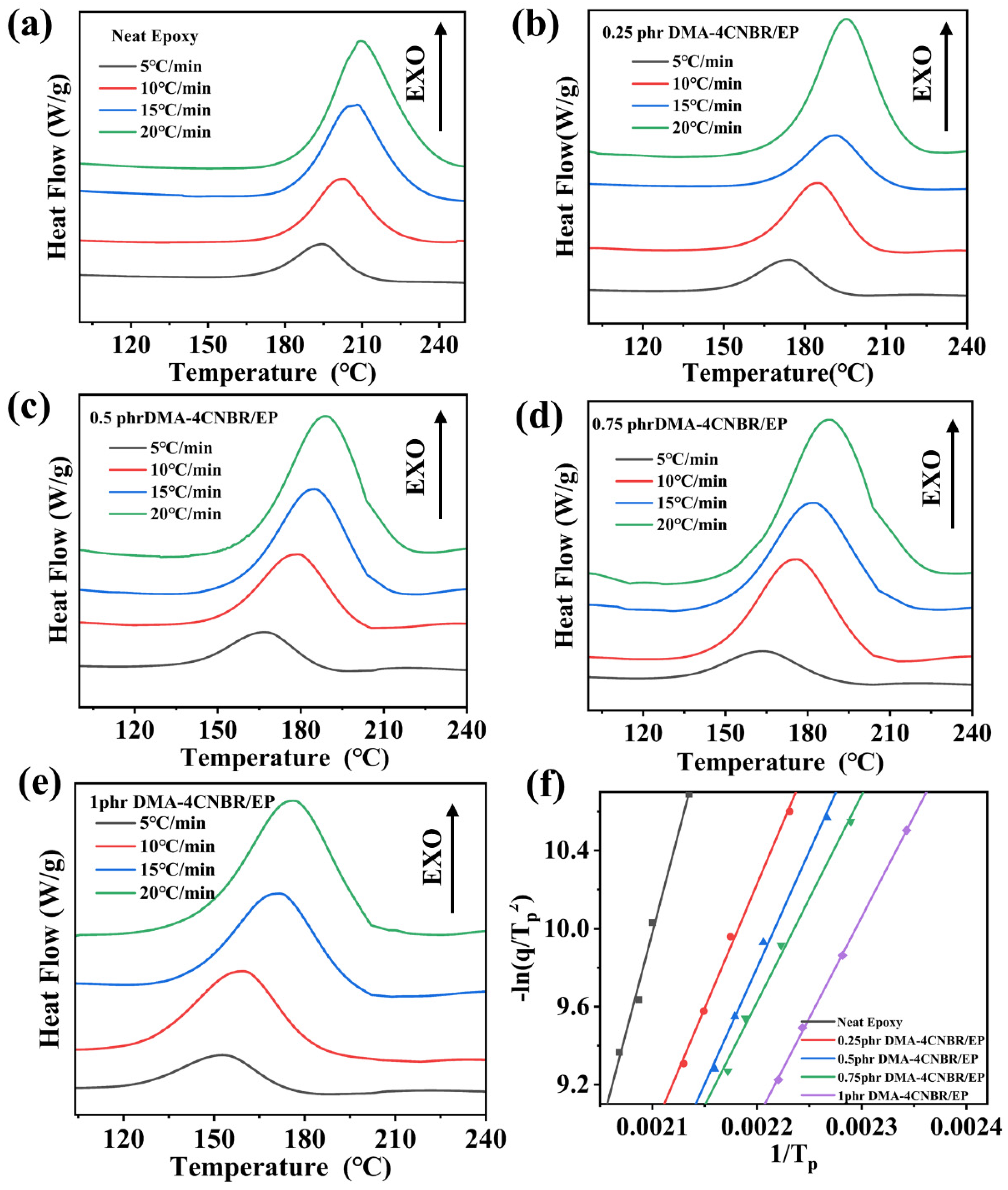
3.2. Curing Behaviors and Curing Kinetics
3.3. Mechanical and Thermal Performance
3.4. The Surfaces of Epoxy-Coating Systems Analysis
3.5. Curing Promoting Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, S.; Dang, L.; Li, P.; Zhu, J.; Lan, S.; Zhu, D. Organic-Inorganic Modification of Magnesium Borate Rod by Layered Double Hydroxide and 3-Aminopropyltriethoxysilane and Its Effect on the Properties of Epoxy Resin. Polymers 2022, 14, 3661. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, P.; Wang, Y.; Wang, G. Corrosion resistance of surface texturing epoxy resin coatings reinforced with fly ash cenospheres and multiwalled carbon nanotubes. Prog. Org. Coat. 2021, 158, 106388. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Liu, Z.; Han, Y.; Wang, C. Study on application performance of oxidized polyethylene wax in powder coatings. Prog. Org. Coat. 2019, 136, 105294. [Google Scholar] [CrossRef]
- Zhang, D.W.; Huang, Y. The bonding performances of carbon nanotube (CNT)-reinforced epoxy adhesively bonded joints on steel substrates. Prog. Org. Coat. 2021, 159, 106407. [Google Scholar] [CrossRef]
- Farshchi, N.; Gedan-Smolka, M.; Stommel, M. Preparation and Characterization of Self-Healing Polyurethane Powder Coating Using Diels-Alder Reaction. Polymers 2021, 13, 3803. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Zhu, G.; Lu, Z.; Zhang, Y.; Zhao, X.; Hou, B. Developing multi-wall carbon nanotubes/Fusion-bonded epoxy powder nanocomposite coatings with superior anti-corrosion and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127309. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Zhu, J. Flow properties of fine powders in powder coating. Particuology 2010, 8, 19–27. [Google Scholar] [CrossRef]
- Lin, R.-H.; Chen, C.-L.; Kao, L.-H.; Yang, P.-R. Cure behavior of epoxy resins with different kinds of onium salts as latent thermal catalysts. J. Appl. Polym. Sci. 2001, 82, 3539–3551. [Google Scholar] [CrossRef]
- Yang, L.H.; Liu, F.C.; Han, E.H. Effects of P/B on the properties of anticorrosive coatings with different particle size. Prog. Org. Coat. 2005, 53, 91–98. [Google Scholar] [CrossRef]
- García, S.J.; Fischer, H.R.; White, P.A.; Mardel, J.; González-García, Y.; Mol, J.M.C.; Hughes, A.E. Self-healing anticorrosive organic coating based on an encapsulated water reactive silyl ester: Synthesis and proof of concept. Prog. Org. Coat. 2011, 70, 142–149. [Google Scholar] [CrossRef]
- Sangaj, N.S.; Malshe, V.C. Permeability of polymers in protective organic coatings. Prog. Org. Coat. 2004, 50, 28–39. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Vaganov, G.V.; Yudin, V.E.; Vuorinen, J. Characterization and corrosion protection properties of epoxy powder coatings containing nanoclays. Prog. Org. Coat. 2013, 76, 757–767. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; El Saeed, A.M.; El-Ghazawy, R.A. Chemical interaction of different sized fumed silica with epoxy via ultrasonication for improved coating. Prog. Org. Coat. 2019, 129, 1–9. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Qi, G.; Wang, X.; Zhang, J.; Han, P.; Ru, Y.; Qiao, J. A rubber-modified epoxy composite with very high toughness and heat resistance. Polym. Polym. Compos. 2019, 27, 582–586. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, X.; Li, B.; Song, Z.; Qiao, J. The study of rubber-modified plastics with higher heat resistance and higher toughness and its application. Polym. Chem. 2011, 2, 1271–1274. [Google Scholar] [CrossRef]
- Ratna, D.; Simon, G.P. Mechanical characterization and morphology of carboxyl randomized poly(2-ethyl hexyl acrylate) liquid rubber toughened epoxy resins. Polymer 2001, 42, 7739–7747. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Start, P.R.; Raghavan, D.; Hudson, S.D. The influence of clay and elastomer concentration on the morphology and fracture energy of preformed acrylic rubber dispersed clay filled epoxy nanocomposites. Polymer 2005, 46, 11255–11262. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Y.; Ning, R. Effects of nanoparticles SiO2 on the performance of nanocomposites. Mater. Lett. 2003, 57, 2940–2944. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Velasco, F.; Bautista, A.; Galiana, B. Functionalizing organic powder coatings with nanoparticles through ball milling for wear applications. Appl. Surf. Sci. 2020, 513, 145834. [Google Scholar] [CrossRef]
- Yu, H.J.; Wang, L.; Shi, Q.; Jiang, G.H.; Zhao, Z.R.; Dong, X.C. Study on nano-CaCO3 modified epoxy powder coatings. Prog. Org. Coat. 2006, 55, 296–300. [Google Scholar] [CrossRef]
- Misra, A.; Shukla, M.; Shukla, M.K.; Srivastava, D.; Nagpal, A.K. Nano CaCO3 modified multifunctional epoxy nanocomposites: A study on flexural and structural properties. Mater. Today Proc. 2021, 47, 3295–3300. [Google Scholar] [CrossRef]
- Piazza, D.; Lorandi, N.P.; Pasqual, C.I.; Scienza, L.C.; Zattera, A.J. Influence of a microcomposite and a nanocomposite on the properties of an epoxy-based powder coating. Mater. Sci. Eng. A 2011, 528, 6769–6775. [Google Scholar] [CrossRef]
- Cai, H.; Li, P.; Sui, G.; Yu, Y.; Li, G.; Yang, X.; Ryu, S. Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal DSC. Thermochim. Acta 2008, 473, 101–105. [Google Scholar] [CrossRef]
- Wise, C.W.; Cook, W.D.; Goodwin, A.A. CTBN rubber phase precipitation in model epoxy resins. Polymer 2000, 41, 4625–4633. [Google Scholar] [CrossRef]
- Blank, W.J.; He, Z.A.; Picci, M. Catalysis of the epoxy-carboxyl reaction. J. Coat. Technol. 2002, 74, 33–41. [Google Scholar] [CrossRef]
- Merfeld, G.; Molaison, C.; Koeniger, R.; Acar, A.E.; Mordhorst, S.; Suriano, J.; Irwin, P.; Warner, R.S.; Gray, K.; Smith, M.; et al. Acid/epoxy reaction catalyst screening for low temperature (120 °C) powder coatings. Prog. Org. Coat. 2005, 52, 98–109. [Google Scholar] [CrossRef]
- Hesabi, M.; Salimi, A.; Beheshty, M.H. Effect of tertiary amine accelerators with different substituents on curing kinetics and reactivity of epoxy/dicyandiamide system. Polym. Test. 2017, 59, 344–354. [Google Scholar] [CrossRef]
- Sawicz-Kryniger, K.; Popielarz, R. Comparison of the effectiveness of epoxy cure accelerators using a fluorescent molecular probe. Polym. Test. 2013, 32, 1558–1564. [Google Scholar] [CrossRef]
- Dong, W.; Liu, Y.; Zhang, X.; Gao, J.; Huang, F. Preparation of High Barrier and Exfoliated-Type Nylon-6/Ultrafine Full-Vulcanized Powdered Rubber/Clay Nanocomposites. Macromolecules 2005, 38, 4551–4553. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Liu, S.; Gui, H.; Lai, J.; Liu, Y.; Gao, J.; Huang, F.; Song, Z.; Tan, B.; et al. Ultrafine full-vulcanized powdered rubbers/PVC compounds with higher toughness and higher heat resistance. Polymer 2005, 46, 10614–10617. [Google Scholar] [CrossRef]
- Alessi, S.; Caponetti, E.; Güven, O.; Akbulut, M.; Spadaro, G.; Spinella, A. Study of the Curing Process of DGEBA Epoxy Resin Through Structural Investigation. Macromol. Chem. Phys. 2015, 216, 538–546. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, J.; Song, X.; Liang, C.; Xu, S. Curing kinetics of nanostructured epoxy blends toughened with epoxidized carboxyl-terminated liquid rubber. Thermochim. Acta 2015, 605, 8–15. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, Q.; Wang, J.; Huo, Z.; Sun, H. Curing kinetics and properties of epoxy resin–fluorenyl diamine systems. Polymer 2008, 49, 4399–4405. [Google Scholar] [CrossRef]
- Ma, H.; Wei, G.; Liu, Y.; Zhang, X.; Gao, J.; Huang, F.; Tan, B.; Song, Z.; Qiao, J. Effect of elastomeric nanoparticles on properties of phenolic resin. Polymer 2005, 46, 10568–10573. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Y.; Zhang, X.; Wei, G.; Gao, J.; Song, Z.; Zhang, M.; Qiao, J. Effect of Elastomeric Nanoparticles on Toughness and Heat Resistance of Epoxy Resins. Macromol. Rapid Commun. 2002, 23, 786–790. [Google Scholar] [CrossRef]



| Ingredients (g) | DMA-4CNBR Content Relative to Epoxy Resin (wt%) | ||||
| 0 | 0.25 | 0.5 | 0.75 | 1 | |
| Epoxy resin (604) | 100 | 100 | 100 | 100 | 100 |
| Curing agent (dicyandiamide) | 6 | 6 | 6 | 6 | 6 |
| Defoaming agent (benzoin) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Flatting agent (588) | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| DMA | 0 | 0.25 | 0.5 | 0.75 | 1 |
| CNBR | 0 | 1 | 2 | 3 | 4 |
| Samples | DMA-4CNBR Content Relative to Epoxy Resin (wt%) | ||||
| 0 | 0.25 | 0.5 | 0.75 | 1 | |
| Ea (kJ/mol) | 165.4 | 105.5 | 99.1 | 88.2 | 86.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, H.; Wang, X.; Guan, J.; Li, M.; Chen, Y. Rubber-Composite-Nanoparticle-Modified Epoxy Powder Coatings with Low Curing Temperature and High Toughness. Polymers 2023, 15, 195. https://doi.org/10.3390/polym15010195
Zhang R, Wang H, Wang X, Guan J, Li M, Chen Y. Rubber-Composite-Nanoparticle-Modified Epoxy Powder Coatings with Low Curing Temperature and High Toughness. Polymers. 2023; 15(1):195. https://doi.org/10.3390/polym15010195
Chicago/Turabian StyleZhang, Run, Haosheng Wang, Xiaoze Wang, Jian Guan, Meiqi Li, and Yunfa Chen. 2023. "Rubber-Composite-Nanoparticle-Modified Epoxy Powder Coatings with Low Curing Temperature and High Toughness" Polymers 15, no. 1: 195. https://doi.org/10.3390/polym15010195






