Block Copolymer Modified Nanonetwork Epoxy Resin for Superior Energy Dissipation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Procedures
2.2. Preparation of Well-Ordered Nanoporous Template
2.3. Templated Polymerization
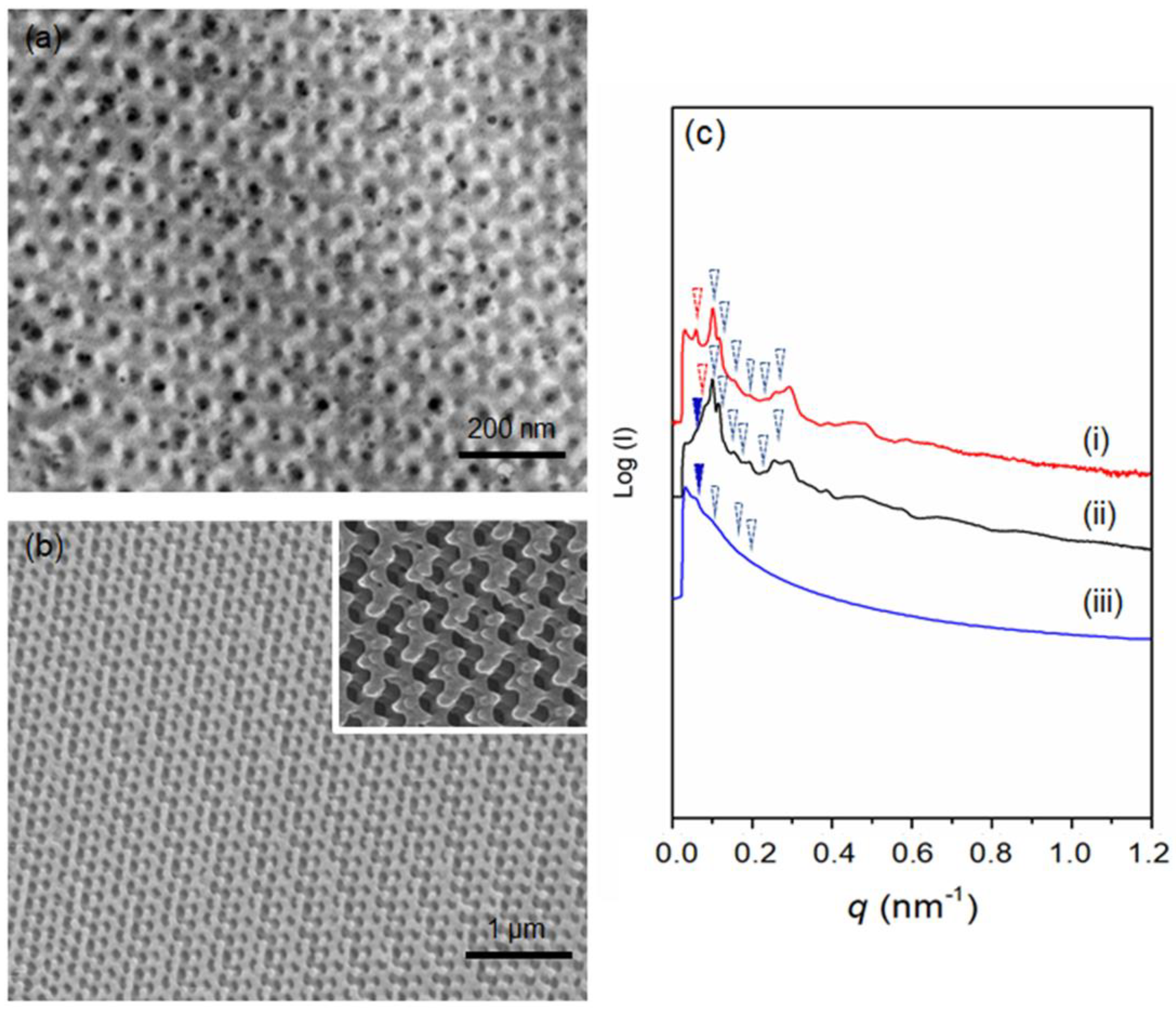
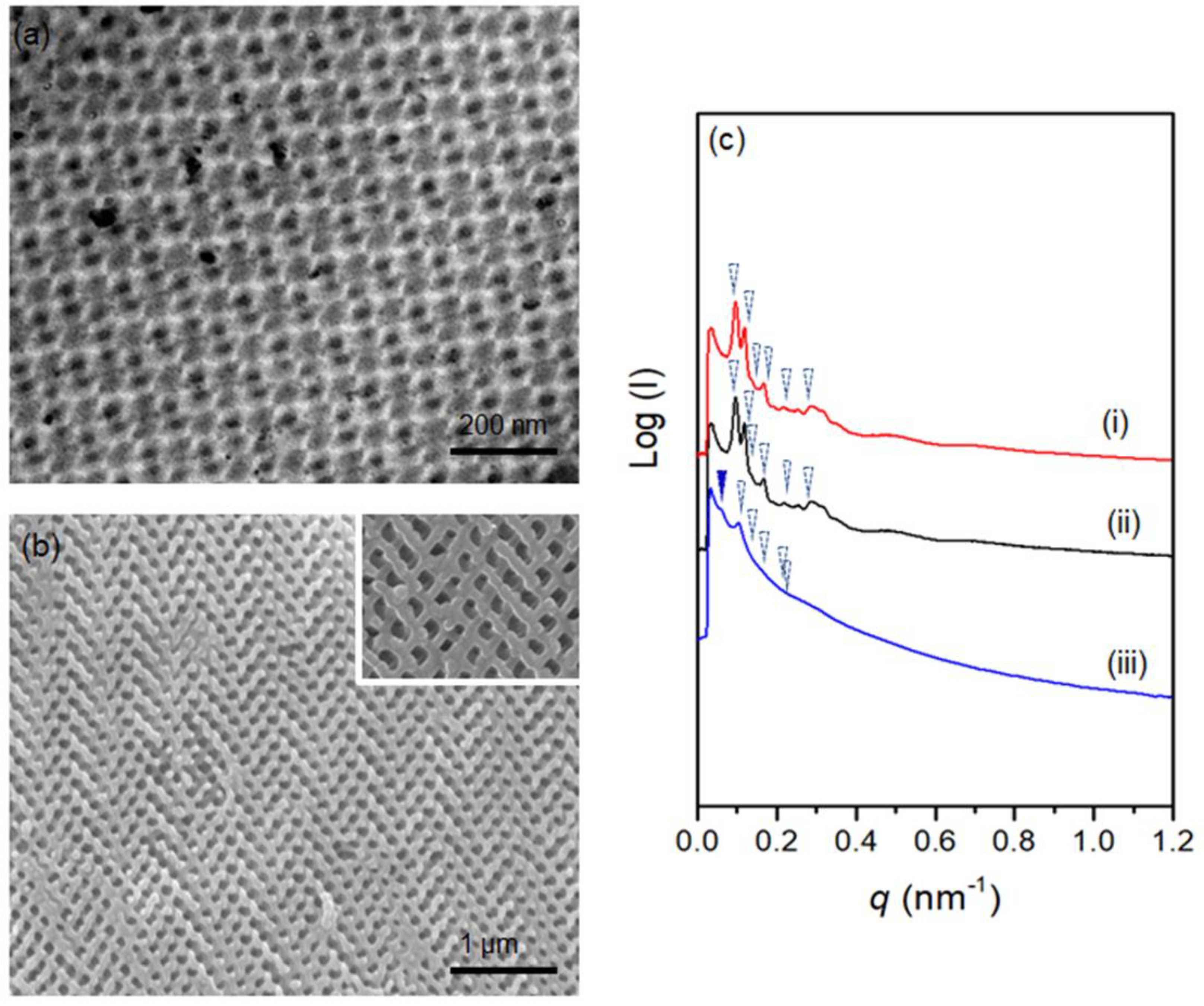
2.4. Transmission Electron Microscopy (TEM)
2.5. Field-Emission Scanning Electron Microscopy (FESEM)
2.6. Small-Angle X-ray Scattering (SAXS)
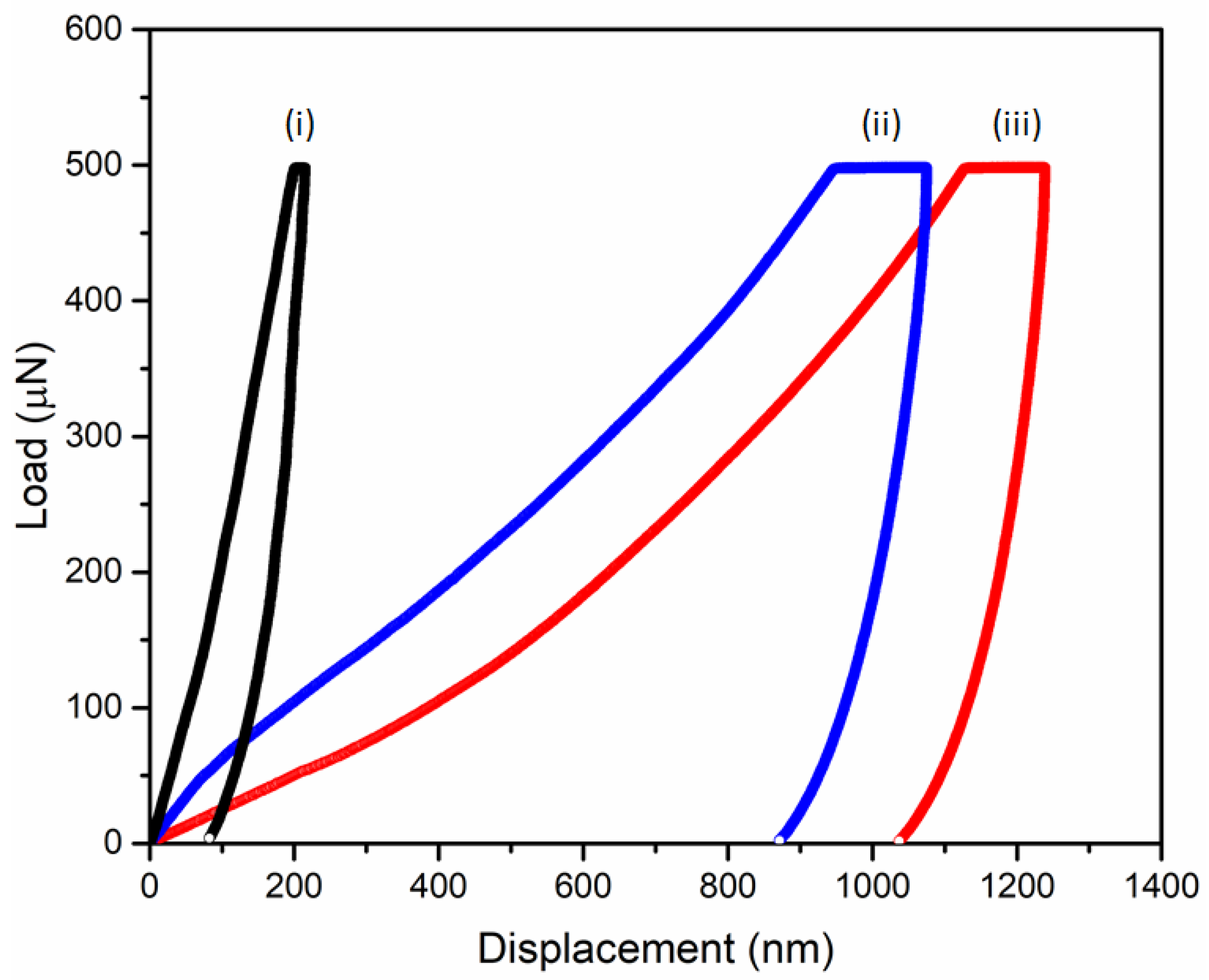
2.7. Nanoindentation Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karak, N. Modification of Epoxies. In Sustainable Epoxy Thermosets and Nanocomposites; American Chemical Society: Washington, DC, USA, 2021; Volume 1385, pp. 37–68. [Google Scholar]
- Shaw, S.J. Rubber modified epoxy resins. In Rubber Toughened Engineering Plastics; Collyer, A.A., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 165–209. [Google Scholar]
- Bucknall, C.B. Rubber toughening. In The Physics of Glassy Polymers; Haward, R.N., Young, R.J., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 363–412. [Google Scholar]
- Mimura, K.; Ito, H.; Fujioka, H. Toughening of epoxy resin modified with in situ polymerized thermoplastic polymers. Polymer 2001, 42, 9223–9233. [Google Scholar] [CrossRef]
- Choi, J.; Yee, A.F.; Laine, R.M. Toughening of Cubic Silsesquioxane Epoxy Nanocomposites Using Core−Shell Rubber Particles: A Three-Component Hybrid System. Macromolecules 2004, 37, 3267–3276. [Google Scholar] [CrossRef]
- Chen, J.; Kinloch, A.J.; Sprenger, S.; Taylor, A.C. The mechanical properties and toughening mechanisms of an epoxy polymer modified with polysiloxane-based core-shell particles. Polymer 2013, 54, 4276–4289. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. Toughening in Graphene Ceramic Composites. ACS Nano 2011, 5, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Declet-Perez, C.; Francis, L.F.; Bates, F.S. Cavitation in Block Copolymer Modified Epoxy Revealed by In Situ Small-Angle X-Ray Scattering. ACS Macro Lett. 2013, 2, 939–943. [Google Scholar] [CrossRef]
- Dean, J.M.; Lipic, P.M.; Grubbs, R.B.; Cook, R.F.; Bates, F.S. Micellar structure and mechanical properties of block copolymer-modified epoxies. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2996–3010. [Google Scholar] [CrossRef]
- Dean, J.M.; Grubbs, R.B.; Saad, W.; Cook, R.F.; Bates, F.S. Mechanical properties of block copolymer vesicle and micelle modified epoxies. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2444–2456. [Google Scholar] [CrossRef]
- Thompson, Z.J.; Hillmyer, M.A.; Liu, J.; Sue, H.-J.; Dettloff, M.; Bates, F.S. Block Copolymer Toughened Epoxy: Role of Cross-Link Density. Macromolecules 2009, 42, 2333–2335. [Google Scholar] [CrossRef]
- Liu, J.; Sue, H.-J.; Thompson, Z.J.; Bates, F.S.; Dettloff, M.; Jacob, G.; Verghese, N.; Pham, H. Nanocavitation in Self-Assembled Amphiphilic Block Copolymer-Modified Epoxy. Macromolecules 2008, 41, 7616–7624. [Google Scholar] [CrossRef]
- Lakes, R. Materials with structural hierarchy. Nature 1993, 361, 511–515. [Google Scholar] [CrossRef]
- Cui, T.J.; Liu, R.; Smith, D.R. Introduction to Metamaterials. In Metamaterials: Theory, Design, and Applications; Cui, T.J., Smith, D., Liu, R., Eds.; Springer: Boston, MA, USA, 2010; pp. 1–19. [Google Scholar]
- Jang, D.; Greer, J.R. Transition from a strong-yet-brittle to a stronger-and-ductile state by size reduction of metallic glasses. Nat. Mater. 2010, 9, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Schaedler, T.A.; Jacobsen, A.J.; Torrents, A.; Sorensen, A.E.; Lian, J.; Greer, J.R.; Valdevit, L.; Carter, W.B. Ultralight Metallic Microlattices. Science 2011, 334, 962. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Singer, J.P.; Thomas, E.L. Micro-/Nanostructured Mechanical Metamaterials. Adv. Mater. 2012, 24, 4782–4810. [Google Scholar] [CrossRef] [PubMed]
- Schaedler, T.A.; Ro, C.J.; Sorensen, A.E.; Eckel, Z.; Yang, S.S.; Carter, W.B.; Jacobsen, A.J. Designing Metallic Microlattices for Energy Absorber Applications. Adv. Eng. Mater. 2014, 16, 276–283. [Google Scholar] [CrossRef]
- Biener, J.; Hodge, A.M.; Hamza, A.V.; Hsiung, L.M.; Satcher, J.H. Nanoporous Au: A high yield strength material. J. Appl. Phys. 2004, 97, 024301. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.L.; Anderson, D.M.; Henkee, C.S.; Hoffman, D. Periodic area-minimizing surfaces in block copolymers. Nature 1988, 334, 598–601. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymers—Designer Soft Materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Bates, F.S. Network Phases in Block Copolymer Melts. MRS Bull. 2005, 30, 525–532. [Google Scholar] [CrossRef]
- Hajduk, D.A.; Harper, P.E.; Gruner, S.M.; Honeker, C.C.; Kim, G.; Thomas, E.L.; Fetters, L.J. The Gyroid: A New Equilibrium Morphology in Weakly Segregated Diblock Copolymers. Macromolecules 1994, 27, 4063–4075. [Google Scholar] [CrossRef]
- Meng, L.; Watson, B.W.; Qin, Y. Hybrid conjugated polymer/magnetic nanoparticle composite nanofibers through cooperative non-covalent interactions. Nanoscale Adv. 2020, 2, 2462–2470. [Google Scholar] [CrossRef]
- Darling, S.B. Directing the self-assembly of block copolymers. Prog. Polym. Sci. 2007, 32, 1152–1204. [Google Scholar] [CrossRef]
- Hsueh, H.-Y.; Yao, C.-T.; Ho, R.-M. Well-ordered nanohybrids and nanoporous materials from gyroid block copolymer templates. Chem. Soc. Rev. 2015, 44, 1974–2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, Y.; Berger, A.; Yau, E.; He, C.; Zhang, L.; Gösele, U.; Knez, M.; Steinhart, M. Nanoscopic Morphologies in Block Copolymer Nanorods as Templates for Atomic-Layer Deposition of Semiconductors. Adv. Mater. 2009, 21, 2763–2766. [Google Scholar] [CrossRef]
- Hsueh, H.-Y.; Huang, Y.-C.; Ho, R.-M.; Lai, C.-H.; Makida, T.; Hasegawa, H. Nanoporous Gyroid Nickel from Block Copolymer Templates via Electroless Plating. Adv. Mater. 2011, 23, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.-Y.; Chen, H.-Y.; She, M.-S.; Chen, C.-K.; Ho, R.-M.; Gwo, S.; Hasegawa, H.; Thomas, E.L. Inorganic Gyroid with Exceptionally Low Refractive Index from Block Copolymer Templating. Nano Lett. 2010, 10, 4994–5000. [Google Scholar] [CrossRef]
- Crossland, E.J.W.; Kamperman, M.; Nedelcu, M.; Ducati, C.; Wiesner, U.; Smilgies, D.M.; Toombes, G.E.S.; Hillmyer, M.A.; Ludwigs, S.; Steiner, U.; et al. A Bicontinuous Double Gyroid Hybrid Solar Cell. Nano Lett. 2009, 9, 2807–2812. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Lin, T.-C.; Hsueh, H.-Y.; Lin, S.-C.; He, X.-D.; Ho, R.-M. Nanoporous Gyroid-Structured Epoxy from Block Copolymer Templates for High Protein Adsorbability. Langmuir 2016, 32, 6419–6428. [Google Scholar] [CrossRef]
- Siddique, S.K.; Lin, T.-C.; Chang, C.-Y.; Chang, Y.-H.; Lee, C.-C.; Chang, S.-Y.; Tsai, P.-C.; Jeng, Y.-R.; Thomas, E.L.; Ho, R.-M. Nanonetwork Thermosets from Templated Polymerization for Enhanced Energy Dissipation. Nano Lett. 2021, 21, 3355–3363. [Google Scholar] [CrossRef]
- Lo, T.-Y.; Chao, C.-C.; Ho, R.-M.; Georgopanos, P.; Avgeropoulos, A.; Thomas, E.L. Phase Transitions of Polystyrene-b-poly(dimethylsiloxane) in Solvents of Varying Selectivity. Macromolecules 2013, 46, 7513–7524. [Google Scholar] [CrossRef]
- Georgopanos, P.; Lo, T.-Y.; Ho, R.-M.; Avgeropoulos, A. Synthesis, molecular characterization and self-assembly of (PS-b-PDMS)n type linear (n = 1, 2) and star (n = 3, 4) block copolymers. Polym. Chem. 2017, 8, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-Y.; Manesi, G.-M.; Yang, C.-Y.; Hung, Y.-C.; Yang, K.-C.; Chiu, P.-T.; Avgeropoulos, A.; Ho, R.-M. Mesoscale networks and corresponding transitions from self-assembly of block copolymers. Proc. Natl. Acad. Sci. USA 2021, 118, e2022275118. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-H.; Higuchi, T.; Chen, H.-L.; Tsai, J.-C.; Jinnai, H.; Hashimoto, T. Stabilizing the Ordered Bicontinuous Double Diamond Structure of Diblock Copolymer by Configurational Regularity. Macromolecules 2018, 51, 4049–4058. [Google Scholar] [CrossRef]
- Hsueh, H.-Y.; Ling, Y.-C.; Wang, H.-F.; Chien, L.-Y.C.; Hung, Y.-C.; Thomas, E.L.; Ho, R.-M. Shifting Networks to Achieve Subgroup Symmetry Properties. Adv. Mater. 2014, 26, 3225–3229. [Google Scholar] [CrossRef]
- Feng, X.; Burke, C.J.; Zhuo, M.; Guo, H.; Yang, K.; Reddy, A.; Prasad, I.; Ho, R.-M.; Avgeropoulos, A.; Grason, G.M.; et al. Seeing mesoatomic distortions in soft-matter crystals of a double-gyroid block copolymer. Nature 2019, 575, 175–179. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]


| Sample | (kg mol−1) a | (kg mol−1) a | (kg mol−1) a | Đb | fPDMSvc |
|---|---|---|---|---|---|
| PS precursor | 51 | 1.03 | |||
| PS-b-PDMS | 51 | 35 | 86 | 1.05 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, S.K.; Sadek, H.; Lee, T.-L.; Tsai, C.-Y.; Chang, S.-Y.; Tsai, H.-H.; Lin, T.-S.; Manesi, G.-M.; Avgeropoulos, A.; Ho, R.-M. Block Copolymer Modified Nanonetwork Epoxy Resin for Superior Energy Dissipation. Polymers 2022, 14, 1891. https://doi.org/10.3390/polym14091891
Siddique SK, Sadek H, Lee T-L, Tsai C-Y, Chang S-Y, Tsai H-H, Lin T-S, Manesi G-M, Avgeropoulos A, Ho R-M. Block Copolymer Modified Nanonetwork Epoxy Resin for Superior Energy Dissipation. Polymers. 2022; 14(9):1891. https://doi.org/10.3390/polym14091891
Chicago/Turabian StyleSiddique, Suhail K., Hassan Sadek, Tsung-Lun Lee, Cheng-Yuan Tsai, Shou-Yi Chang, Hsin-Hsien Tsai, Te-Shun Lin, Gkreti-Maria Manesi, Apostolos Avgeropoulos, and Rong-Ming Ho. 2022. "Block Copolymer Modified Nanonetwork Epoxy Resin for Superior Energy Dissipation" Polymers 14, no. 9: 1891. https://doi.org/10.3390/polym14091891










