A Comparative Investigation on Structural and Chemical Differences between the Pith and Rind of Sunflower Stalk and Their Influences on Nanofibrillation Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
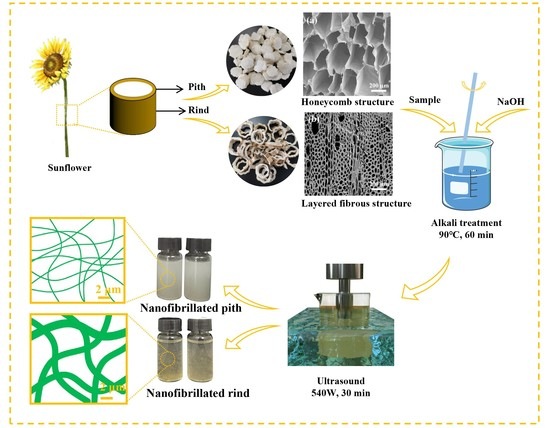
2.2. Preparation of Cellulose Nanofiber
2.3. Characterizations
3. Results and Discussion
3.1. Morphology and Chemical Composition of Pith and Rind
3.2. Alkaline Pretreatment of Pith and Rind
3.3. Nanofibrillation Behavior of Pith and Rind
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sabbagh, F.; Muhamad, I.I.; PaLe, N.; Hashim, Z. Strategies in improving properties of cellulose-based hydrogels for smart applications. In Cellulose-Based Superabsorbent Hydrogels; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 887–908. [Google Scholar] [CrossRef]
- Yadav, C.; Saini, A.; Zhang, W.; You, X.; Chauhan, I.; Mohanty, P.; Li, X. Plant-based nanocellulose: A review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3D bioprinting. Int. J. Biol. Macromol. 2021, 166, 1586–1616. [Google Scholar] [CrossRef] [PubMed]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Ikkala, O. Nanocellulose: Recent fundamental advances and emerging biological and biomimicking applications. Adv. Mater. 2020, 33, 2004349. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Muhamad, I.I. Physical and chemical characterisation of acrylamide-based hydrogels, Aam, Aam/NaCMC and Aam/NaCMC/MgO. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1439–1449. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Chan, C.H.; Chia, C.H.; Zakaria, S.; Sajab, M.S.; Chin, S.X. Cellulose nanofibrils: A rapid adsorbent for the removal of methylene blue. RSC Adv. 2015, 5, 18204–18212. [Google Scholar] [CrossRef]
- Tanpichai, S.; Biswas, S.K.; Witayakran, S.; Yano, H. Water hyacinth: A sustainable lignin-poor cellulose source for the production of cellulose nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 18884–18893. [Google Scholar] [CrossRef]
- Xu, M.; Qi, M.; Goff, H.D.; Cui, S.W. Polysaccharides from sunflower stalk pith: Chemical, structural and functional characterization. Food Hydrocoll. 2020, 100, 105082. [Google Scholar] [CrossRef]
- Grădinaru, C.M.; Serbănoiu, A.A.; Serbănoiu, B.V. Sunflower stalks versus corn cobs as raw materials for sustainable concrete. Materials 2021, 14, 5078. [Google Scholar] [CrossRef]
- Wang, L.; Ren, H.; Zhai, S.; Zhai, H. Anatomy and cell wall ultrastructure of sunflower stalk rind. J. Wood Sci. 2021, 67, 67. [Google Scholar] [CrossRef]
- Rudi, H.; Resalati, H.; Eshkiki, R.B.; Kermanian, H. Sunflower stalk neutral sulfite semi-chemical pulp: An alternative fiber source for production of fluting paper. J. Clean. Prod. 2016, 127, 562–566. [Google Scholar] [CrossRef]
- Kaymakci, A.; Ayrilmis, N.; Ozdemir, F.; Gulec, T. Utilization of sunflower stalk in manufacture of thermoplastic composite. J. Polym. Environ. 2013, 21, 1135–1142. [Google Scholar] [CrossRef]
- Martínez, M.L.; Sánchez, S.; Bravo, V. Production of xylitol and ethanol by Hansenula polymorpha from hydrolysates of sunflower stalks with phosphoric acid. Ind. Crop. Prod. 2012, 40, 160–166. [Google Scholar] [CrossRef]
- Zhurka, M.; Spyridonidis, A.; Vasiliadou, I.A.; Stamatelatou, K. Biogas production from sunflower head and stalk residues: Effect of alkaline pretreatment. Molecules 2020, 25, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Xia, L.; Zhang, L.; Guo, F.; Zhang, X.; Yu, Y.; Yang, R. Sunflower-stalk-based solar-driven evaporator with a confined 2D water channel and an enclosed thermal-insulating cellular structure for stable and efficient steam generation. ACS Appl. Mater. Interfaces 2021, 13, 55299–55306. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Hao, D.; Sun, M.; Wei, T.; Xu, D.; Ai, X.; Guo, X.; Zhao, T.; Jiang, L. Nature sunflower stalk pith with zwitterionic hydrogel coating for highly efficient and sustainable solar evaporation. Adv. Funct. Mater. 2021, 32, 2108135. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, L.; Ma, X.; Zhou, X.; Xu, Y. Revalorization of sunflower stalk pith as feedstock for the coproduction of pectin and glucose using a two-step dilute acid pretreatment process. Biotechnol. Biofuels 2021, 14, 194. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Shi, J.; Zhang, W.; Wang, H. Effect of alkali treatment on microstructure and thermal stability of parenchyma cell compared with bamboo fiber. Ind. Crop. Prod. 2021, 164, 113380. [Google Scholar] [CrossRef]
- Yin, J.; Song, K.; Lu, Y. Comparison of changes in micropores and mesopores in the wood cell walls of sapwood and heartwood. Wood Sci. Technol. 2015, 49, 987–1001. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Q.; Wei, L.; Zhong, L. Simple and scalable synthesis of hierarchical for high capacitance and energy density. J. Mater. Chem. A 2020, 8, 1469. [Google Scholar] [CrossRef]
- Garemark, J.; Yang, X.; Sheng, X.; Cheung, O.; Sun, L.; Berglund, L.A.; Li, Y. Top-down approach making anisotropic cellulose aerogels as universal substrates for multifunctionalization. ACS Nano 2020, 14, 7111–7120. [Google Scholar] [CrossRef]
- Peciulyte, A.; Karlström, K.; Larsson, P.T.; Olsson, L. Impact of the supramolecular structure of cellulose on the efficiency of enzymatic hydrolysis. Biotechnol. Biofuels 2015, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, K.; Gao, X.; Han, Q.; Peng, L. Improved thermal stability of regenerated cellulose films from corn (Zea mays) stalk pith using facile preparation with low-concentration zinc chloride dissolving. Carbohydr. Polym. 2019, 217, 190–198. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, X.; Ma, Z.; Gong, J.; Wang, H.; Lv, Y. Efficient extraction and structural characterization of hemicellulose from sugarcane bagasse pith. Polymers 2020, 12, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielewicz, D.; Dybka-Stępień, K.; Surma-Ślusarska, B. Processing of Miscanthus × giganteus stalks into various soda and kraft pulps. Part I: Chemical composition, types of cells and pulping effects. Cellulose 2018, 25, 6731–6744. [Google Scholar] [CrossRef] [Green Version]
- Costa, T.H.F.; Masarin, F.; Bonifácio, T.O.; Milagres, A.M.F.; Ferraz, A. The enzymatic recalcitrance of internodes of sugar cane hybrids with contrasting lignin contents. Ind. Crop. Prod. 2013, 51, 202–211. [Google Scholar] [CrossRef]
- Hongrattanavichit, I.; Aht-Ong, D. Nanofibrillation and characterization of sugarcane bagasse agro-waste using water-based steam explosion and high-pressure homogenization. J. Clean. Prod. 2020, 277, 123471. [Google Scholar] [CrossRef]
- Dilamian, M.; Noroozi, B. A combined homogenization-high intensity ultrasonication process for individualizaion of cellulose micro-nano fibers from rice straw. Cellulose 2019, 26, 5831–5849. [Google Scholar] [CrossRef]
- Fonseca, A.S.; Panthapulakkal, S.; Konar, S.K.; Sain, M.; Bufalino, L.; Raabe, J.; Miranda, I.P.A.; Martins, M.A.; Tonoli, G.H.D. Improving cellulose nanofibrillation of non-wood fiber using alkaline and bleaching pre-treatments. Ind. Crop. Prod. 2019, 131, 203–212. [Google Scholar] [CrossRef]
- Fung, W.Y.; Yuen, K.; Liong, M.T. Characterization of fibrous residues from agrowastes and the production of nanofibers. J. Agric. Food Chem. 2010, 58, 8077–8084. [Google Scholar] [CrossRef]
- Xiao, C.; Anderson, C.T. Roles of pectin in biomass yield and processing for biofuels. Front. Plant Sci. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alila, S.; Besbes, I.; Rei, M.; Mutjé, P.; Boufi, S. Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): A comparative study. Ind. Crop. Prod. 2013, 41, 250–259. [Google Scholar] [CrossRef]
- Han, X.; Bi, R.; Khatri, V.; Oguzlu, H.; Takada, M.; Jiang, J.; Jiang, F.; Bao, J.; Saddler, J.N. Use of endoglucanase and accessory enzymes to facilitate mechanical pulp nanofibrillation. ACS Sustain. Chem. Eng. 2021, 9, 1406–1413. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Li, P.; Sirviö, J.A.; Haapala, A.; Liimatainen, H. Cellulose nanofibrils from nonderivatizing urea-based deep eutectic solvent pretreatments. ACS Appl. Mater. Interfaces 2017, 9, 2846–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehaqui, H.; Kulasinski, K.; Pfenninger, N.; Zimmermann, T.; Tingaut, P. Highly carboxylated cellulose nanofibers via succinic anhydride esterification of wheat fibers and facile mechanical disintegration. Biomacromolecules 2017, 18, 242–248. [Google Scholar] [CrossRef] [PubMed]











| Materials | Density (mg/cm3) | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|
| Pith | 27 ± 3.22 | 1.77 | 0.006 |
| Rind | 318 ± 45.98 | 0.73 | 0.003 |
| Materials | Tinitial (°C) | T30 (°C) | Tmax (°C) |
|---|---|---|---|
| Pith | 221 | 281 | 311 |
| P-1 | 255 | 313 | 338 |
| P-4 | 261 | 318 | 344 |
| Rind | 246 | 304 | 331 |
| R-1 | 270 | 329 | 351 |
| R-4 | 275 | 331 | 349 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ren, W.; Liu, F.; Xia, L.; Wu, X.; Yang, R.; Yu, Y.; Zhang, X. A Comparative Investigation on Structural and Chemical Differences between the Pith and Rind of Sunflower Stalk and Their Influences on Nanofibrillation Efficiency. Polymers 2022, 14, 930. https://doi.org/10.3390/polym14050930
Zhang L, Ren W, Liu F, Xia L, Wu X, Yang R, Yu Y, Zhang X. A Comparative Investigation on Structural and Chemical Differences between the Pith and Rind of Sunflower Stalk and Their Influences on Nanofibrillation Efficiency. Polymers. 2022; 14(5):930. https://doi.org/10.3390/polym14050930
Chicago/Turabian StyleZhang, Lingyan, Wenting Ren, Fangqingxin Liu, Linmin Xia, Xiaomei Wu, Rilong Yang, Yan Yu, and Xuexia Zhang. 2022. "A Comparative Investigation on Structural and Chemical Differences between the Pith and Rind of Sunflower Stalk and Their Influences on Nanofibrillation Efficiency" Polymers 14, no. 5: 930. https://doi.org/10.3390/polym14050930