Bioactive Films Based on Starch from White, Red, and Black Rice to Food Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
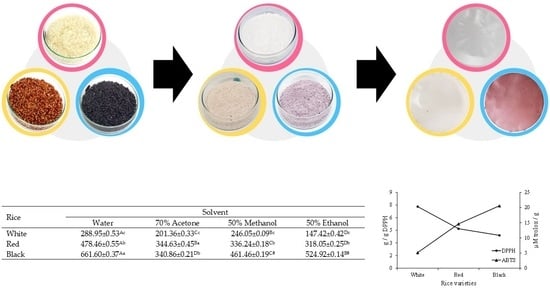
2.2.1. Starch Extraction
2.2.2. Film Production
2.2.3. Color and Opacity
2.2.4. Thickness
2.2.5. Water Solubility
2.2.6. Water Vapor Permeability
2.2.7. Bioactive Compounds and Antioxidant Activity
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Visual Aspects and Physical Analysis
3.2. Water Solubility and Water Vapor Permeability of Films
3.3. Bioactive Compounds and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gunaratne, A.; Wu, K.; Li, D.; Bentota, A.; Corke, H.; Cai, Y. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013, 138, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Ascheri, J. Extrusão de quirera de arroz para uso como ingrediente alimentar. Braz. J. Food Technol. 2009, 12, 190–199. [Google Scholar] [CrossRef]
- Wang, C.H.; Zheng, X.M.; Xu, Q.; Yuan, X.P.; Huang, L.; Zhou, H.F.; Ge, S. Genetic diversity and classification of Oryza sativa with emphasis on Chinese rice germplasm. Heredity 2014, 112, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Teng, F.; Shi, F.; Wang, L.; Chen, Z. Effects of high-temperature air fluidization (HTAF) on eating quality, digestibility, and antioxidant activity of black rice (Oryza sativa L.). Starch/Staerke 2017, 69, 1600274. [Google Scholar] [CrossRef]
- Ahuja, U.; Ahuja, S.C.; Chaudhary, N.; Thakrar, R. Red rices—Past, present and future. Asian Agrihist. 2007, 11, 291–304. [Google Scholar]
- Cha, H.M.; Han, G.; Chung, H.J. A study on the trend analysis regarding the rice consumption of Korean adults using Korean National Health and Nutrition Examination Survey data from 1998, 2001 and 2005. Nutr. Res. Pract. 2012, 6, 254–262. [Google Scholar] [CrossRef]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A.; Dall’asta, C.; Galaverna, G.; Scazzina, F.; Pellegrini, N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 2007, 51, 1006–1019. [Google Scholar] [CrossRef]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A. A study of biodiversity of flavonoid content in the rice caryopsis evidencing simultaneous accumulation of anthocyanins and proanthocyanidins in a black-grained genotype. J. Cereal Sci. 2010, 51, 28–34. [Google Scholar] [CrossRef]
- Silva, L.R.; Carvalho, C.W.P.; Velasco, J.I.; Fakhouri, F.M. Extraction and characterization of starches from pigmented rice. Int J. Biol. Macromol. 2020, 156, 485–493. [Google Scholar] [CrossRef]
- Zannini, D.; Poggetto, G.D.; Malinconico, M.; Santagata, G.; Immirzi, B. Citrus pomace biomass as a source of pectin and lignocellulose fibers: From waste to upgraded biocomposites for mulching applications. Polymers 2021, 13, 1280. [Google Scholar] [CrossRef]
- Freitas, T.S.M.; Garcia, V.A.S.; Filgueiras, C.T.; Velasco, J.I.; Fakhouri, F.M. Production of edible films based on pea starch with incorporation of active compounds obtained from the purple araçá (Psidium myrtoides). Polymers 2021, 12, 3134. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Benelli, P.; Spada, J.C.; Tessaro, I.C. Impact of the starch source on the physicochemical properties and biodegradability of different starch-based films. J. Appl. Polym. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Lutfi, Z.; Hasnain, A. Effect of mango kernel starch coatings on the shelf life of almond (Prunus dulcis) kernels. J. Food Process. Preserv. 2018, 42, 1–7. [Google Scholar] [CrossRef]
- Rodrigues, G.M.; Filgueiras, C.T.; Garcia, V.A.S.; Carvalho, R.A.; Velasco, J.I.; Fakhouri, F.M. Antimicrobial activity and CG-MS profile of copaíba oil for incorporation into Xanthosoma mafaffa Schott starch-based films. Polymers 2020, 12, 2883. [Google Scholar] [CrossRef]
- Song, H.G.; Choi, I.; Lee, J.S.; Chung, M.N.; Yoon, C.S.; Han, J. Comparative study on physicochemical properties of starch films prepared from five sweet potato (Ipomoea batatas) cultivars. Int. J. Biol. Macromol. 2021, 189, 758–767. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and evaluation of a novel high internal phase Pickering emulsion based on whey protein isolate nanofibrils derived by hydrothermal method. Food Hydrocol. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Characterization of emulsion films prepared from soy protein isolate at different preheating temperatures. J. Food Engin. 2020, 309, 110697. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT-Food Sci. Technol. 2021, 147, 111617. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Wang, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Chi, Y. Direct separation and purification of a-lactalbumin from cow milk whey by aqueous two-phase flotation of thermo-sensitive polymer/phosphate. J. Sci. Food Agric. 2021, 101, 4173–7182. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Molina, G.; Pelissari, F.M. Biodegradable trays based on cassava starch blended with agroindustrial residues. Compos. Part B Eng. 2020, 183, 107682. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Ferreira, R.S.B.; Lizárraga, E.; Tapia-Blácido, D.R.; Silva, N.C.C.; Angelats-Silva, L.; Siche, R. Bioactive Andean sweet potato starch-based foam incorporated with orégano or thyme essential oil. Food Packag. 2020, 23, 100457. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; Oliveira, R.A. Extraction and characterization of arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Katekhong, W.; Wongphan, P.; Klinmalai, P.; Harnkarnsujarit, N. Thermoplastic starch blow films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709. [Google Scholar] [CrossRef]
- Krochta, J.M.; Mulder-Johnston, C. Edible and biodegradable polymer films: Challenges and opportunities. Food Technol. 1997, 51, 61–74. [Google Scholar]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I.Á. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocol. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and intelligent starch-based films: A review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Greener-Donhowe, I.; Fennema, O. Edible films and coatings: Characteristics, formation, definitions, and testing methods. In Edible Films and Coatings to Improve Food Quality; Krochta, J.M., Baldwin, E., Nísperos-Carriedo, M.O., Eds.; Technomic Publishing Co. Inc.: Lancaster, PA, USA, 1994; pp. 1–24. [Google Scholar]
- Nogueira, G.F.; Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of incorporating plant-derived bioactive compounds into films made with agro-based polymers for application as food packaging: A brief review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.; Famá, L.; Rojas, A.M.; Goyanes, S.; Gerschenson, L. Physical properties of tapioca-starch edible films: Influence of filmmaking and potassium sorbate. Int. Food Res. J. 2007, 40, 257–265. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Lee, N.A.; Bekhit, A.E.D.; Carne, A.; Vaskoska, R.; Vasiljevic, T. Active edible packaging based on milk proteins: A route to carry and deliver nutraceuticals. Trends Food Sci. Technol. 2021, 111, 688–705. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Thakur, N.; Kajla, P.; Kumar, M.; Trif, M. Natural antimicrobials as additives for edible food packaging applications: A review. Foods 2021, 10, 2282. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocol. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural polymers used in edible food packaging—History, function and application trends as a sustainable alternative to synthetic plastic. Polysaccharides 2022, 3, 32–58. [Google Scholar] [CrossRef]
- Lai, W.F. Design of polymeric films for antioxidant active food packaging. Int. J. Mol. Sci. 2022, 23, 12. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.W. Antioxidant pectin/pullulan edible coating incorporated with Vitis vinifera grape seed extract for extending shelf life of peanuts. Postharvest Biol. Technol. 2022, 183, 111740. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Lucas, A.A.; Tapia-Blácido, D.R. Bioactive films based on babassu mesocarp flour and starch. Food Hydrocol. 2017, 70, 383–391. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and characterization of coating based on protein nanofibers and polyphenol and application for salted duck egg yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Pascuta, M.S.; Vodnar, D.C. Nanocarriers for sustainable active packaging: An overview during and post COVID-19. Coatings 2022, 12, 102. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.L. Edible wheat gluten films: Influence of the main process variables on film properties using response surface methodology. J. Food Sci. 1992, 57, 190–199. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Test Methods for Water Vapor Transmission of Materials: E 96-95; ASTM: West Conshohocken, PA, USA, 1995; 10p. [Google Scholar]
- McHugh, T.H.; Krochta, J.M. Permeability properties of edible films. In Edible Coatings and Films to Improve Food Quality; Krochta, J.M., Baldwin, E.A., Nisperos-Carriedo, M., Eds.; Technomic Publishing: Lancaster, PA, USA, 1994; pp. 139–183. [Google Scholar]
- Gennadios, A.; Weller, C.L.; Testin, R.F. Property modification of edible wheat, gluten-based films. Trans. ASABE 1993, 36, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. Comun. Técnico EMBRAPA 2007, 127, 1–4. [Google Scholar]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre ABTS. Comun. Técnico EMBRAPA 2007, 128, 1–4. [Google Scholar]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocol. 2019, 89, 143–152. [Google Scholar] [CrossRef]
- Homez-Jara, A.; Daza, L.D.; Aguirre, D.M.; Muñoz, J.A.; Solanilla, J.F.; Váquiro, H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch/Starke 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Cavasini, R.; Fakhouri, F.M.; Oliveira, R.A. Bioactive films of arrowroot starch and blackberry pulp: Physical. mechanical and barrier properties and stability to pH and sterilization. Food Chem. 2019, 275, 417–425. [Google Scholar] [CrossRef]
- Alrimawi, B.H.; Chan, M.Y.; Ooi, X.Y.; Chan, S.Y.; Goh, C.F. The interplay between drug and sorbitol contents determines the mechanical and swelling properties of potential rice starch films for buccal drug delivery. Polymers 2021, 13, 578. [Google Scholar] [CrossRef]
- Ploypetchara, T.; Gohtani, S. Characteristics of rice starch film blended with sugar (trehalose/allose) and oil (canola oil/coconut oil): Part I—Filmogenic solution behavior and mechanical properties. J. Food Sci. 2020, 85, 3372–3379. [Google Scholar] [CrossRef]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Rodríguez, M.; Osés, J.; Ziani, K.; Maté, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Int. Food Res. J. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Gómez-Luría, D.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Films from corn, wheat, and rice starch ghost phase fractions display overall superior performance than whole starch films. Starch/Staerke 2017, 69, 1700059. [Google Scholar] [CrossRef]


| Code | Starch Quantity (g) * | Plasticizer Type | Plasticizer Quantity (%) ** | ||
|---|---|---|---|---|---|
| White | Red | Black | |||
| W1 | R1 | B1 | 3 | Sorbitol | 25 |
| W2 | R2 | B2 | 3 | Glycerol | 25 |
| W3 | R3 | B3 | 3 | Sorbitol + Glycerol (1:1) | 25 |
| W4 | R4 | B4 | 3 | Sorbitol | 30 |
| W5 | R5 | B5 | 3 | Glycerol | 30 |
| W6 | R6 | B6 | 3 | Sorbitol + Glycerol (1:1) | 30 |
| W7 | R7 | B7 | 5 | Sorbitol | 25 |
| W8 | R8 | B8 | 5 | Glycerol | 25 |
| W9 | R9 | B9 | 5 | Sorbitol + Glycerol (1:1) | 25 |
| W10 | R10 | B10 | 5 | Sorbitol | 30 |
| W11 | R11 | B11 | 5 | Glycerol | 30 |
| W12 | R12 | B12 | 5 | Sorbitol + Glycerol (1:1) | 30 |
| Code | L* | a* | b* | C* | Opacity (%) | Thickness (mm) |
|---|---|---|---|---|---|---|
| W1 | 94.16 ± 0.70 a | −0.34 ± 0.02 ab | 4.45 ± 0.12 bc | 4.39 ± 0.12 bc | 45.42 ± 0.53 ac | 0.068 ± 0.006 b |
| W2 | 93.20 ± 0.45 bc | −0.37 ± 0.02 abc | 4.23 ± 0.04 e | 4.27 ± 0.04 d | 45.76 ± 0.70 a | 0.075 ± 0.005 b |
| W3 | 92.52 ± 0.45 c | −0.33 ± 0.03 a | 4.31 ± 0.06 ce | 4.32 ± 0.06 cd | 45.68 ± 0.40 ab | 0.078 ± 0.007 b |
| W4 | 92.95 ± 0.51 b | −0.38 ± 0.03 bcd | 4.29 ± 0.04 de | 4.31 ± 0.04 de | 45.95 ± 0.45 a | 0.075 ± 0.006 b |
| W5 | 93.37 ± 0.35 ab | −0.36 ± 0.02 abc | 4.83 ± 0.08 a | 4.85 ± 0.08 a | 44.87 ± 0.44 bc | 0.078 ± 0.005 b |
| W6 | 94.15 ± 0.41 a | −0.38 ± 0.01 bcd | 4.53 ± 0.06 b | 4.56 ± 0.06 b | 44.72 ± 0.16 c | 0.071 ± 0.007 b |
| W7 | 92.64 ± 0.18 bc | −0.40 ± 0.01 cde | 4.38 ± 0.09 cd | 4.43 ± 0.09 ce | 37.61 ± 0.31 d | 0.104 ± 0.008 a |
| W8 | 93.03 ± 0.31 bc | −0.43 ± 0.01 efg | 4.44 ± 0.04 bc | 4.45 ± 0.04 bc | 37.38 ± 0.37 d | 0.107 ± 0.006 a |
| W9 | 92.61 ± 0.23 bc | −0.43 ± 0.02 efg | 4.52 ± 0.05 b | 4.55 ± 0.05 b | 37.66 ± 0.18 d | 0.104 ± 0.005 a |
| W10 | 93.05 ± 0.15 bc | −0.42 ± 0.01 df | 4.44 ± 0.02 bc | 4.45 ± 0.02 bc | 37.46 ± 0.18 d | 0.101 ± 0.004 a |
| W11 | 93.15 ± 0.21 bc | −0.46 ± 0.02 efg | 4.56 ± 0.05 b | 4.58 ± 0.05 b | 37.94 ± 0.37 d | 0.095 ± 0.005 a |
| W12 | 93.07 ± 0.24 bc | −0.46 ± 0.01 g | 4.52 ± 0.02 b | 4.54 ± 0.02 b | 37.83 ± 0.10 d | 0.096 ± 0.003 a |
| R1 | 87.10 ± 0.79 a | 2.91 ± 0.35 ce | 8.70 ± 0.40 de | 2.98 ± 0.34 cde | 39.06 ± 0.60 cde | 0.071 ± 0.005 e |
| R2 | 86.45 ± 1.23 ab | 2.75 ± 0.34 ce | 8.94 ± 0.24 cd | 2.79 ± 0.33 df | 38.38 ± 0.65 de | 0.079 ± 0.009 ce |
| R3 | 87.04 ± 0.87 a | 2.29 ± 0.19 e | 8.10 ± 0.27 e | 2.24 ± 0.19 ef | 39.18 ± 0.42 cd | 0.080 ± 0.009 be |
| R4 | 87.82 ± 0.95 a | 2.13 ± 0.24 e | 7.97 ± 0.33 e | 2.09 ± 0.24 f | 37.92 ± 0.53 e | 0.070 ± 0.004 e |
| R5 | 87.23 ± 0.84 a | 2.24 ± 0.36 e | 8.30 ± 0.45 de | 2.30 ± 0.37 ef | 36.75 ± 0.97 f | 0.073 ± 0.008 de |
| R6 | 86.28 ± 0.42 ab | 2.57 ± 0.20 de | 8.54 ± 0.30 de | 2.68 ± 0.19 def | 38.84 ± 0.17 cde | 0.073 ± 0.009 de |
| R7 | 84.92 ± 0.30 b | 3.72 ± 0.14 ab | 10.21 ± 0.23 b | 3.83 ± 0.13 ab | 40.49 ± 0.17 ab | 0.099 ± 0.007 abc |
| R8 | 84.96 ± 0.64 b | 3.95 ± 0.57 ab | 10.23 ± 0.49 b | 3.73 ± 0.56 ab | 39.65 ± 0.37 bc | 0.093 ± 0.009 bcd |
| R9 | 82.63 ± 0.27 c | 4.47 ± 0.21 a | 11.15 ± 0.30 a | 4.54 ± 0.20 a | 40.98 ± 0.18 a | 0.087 ± 0.005 be |
| R10 | 85.30 ± 0.53 b | 3.34 ± 0.24 bcd | 9.65 ± 0.33 bc | 3.39 ± 0.24 bcd | 39.65 ± 0.30 bc | 0.101 ± 0.007 ab |
| R11 | 85.29 ± 0.78 b | 3.42 ± 0.27 bc | 9.86 ± 0.34 b | 3.29 ± 0.27 bcd | 39.27 ± 0.90 cd | 0.101 ± 0.007 ab |
| R12 | 86.37 ± 0.35 ab | 3.54 ± 0.76 bc | 9.73 ± 0.62 bc | 3.11 ± 0.75 bc | 38.86 ± 0.22 cde | 0.118 ± 0.005 a |
| B1 | 66.92 ± 0.61 a | 11.83 ± 0.67 f | 5.34 ± 0.10 d | 12.74 ± 0.62 f | 44.40 ± 0.58 ef | 0.062 ± 0.008 e |
| B2 | 60.97 ± 0.25 c | 13.13 ± 0.68 def | 6.45 ± 0.43 ab | 14.57 ± 0.46 de | 47.17 ± 0.64 d | 0.062 ± 0.006 e |
| B3 | 59.40 ± 0.62 d | 13.99 ± 0.36 de | 6.44 ± 0.57 ab | 15.32 ± 0.23 cd | 46.87 ± 0.62 d | 0.071 ± 0.008 ce |
| B4 | 65.87 ± 0.95 ab | 12.72 ± 0.58 ef | 5.64 ± 0.19 cd | 14.10 ± 0.56 ef | 45.09 ± 0.59 e | 0.069 ± 0.006 de |
| B5 | 61.22 ± 0.89 e | 13.37 ± 0.69 de | 6.07 ± 0.31 bc | 14.71 ± 0.56 de | 46.89 ± 0.72 d | 0.076 ± 0.007 bce |
| B6 | 57.28 ± 0.67 ef | 14.45 ± 0.30 cd | 5.64 ± 0.41 cd | 15.66 ± 0.69 cd | 44.34 ± 0.69 ef | 0.070 ± 0.008 ce |
| B7 | 65.33 ± 0.39 b | 15.68 ± 0.89 bc | 5.65 ± 0.19 cd | 16.43 ± 0.53 bc | 43.32 ± 0.53 f | 0.086 ± 0.009 acd |
| B8 | 57.76 ± 0.56 e | 16.15 ± 0.14 ab | 6.64 ± 0.02 ab | 17.49 ± 0.52 ab | 49.02 ± 0.52 c | 0.085 ± 0.004 acd |
| B9 | 56.03 ± 0.50 fg | 16.88 ± 0.66 ab | 6.54 ± 0.11 ab | 17.98 ± 0.70 a | 51.21 ± 0.70 b | 0.090 ± 0.009 ac |
| B10 | 53.10 ± 0.59 h | 17.42 ± 0.79 a | 6.17 ± 0.08 ac | 18.82 ± 0.75 a | 53.29 ± 0.75 a | 0.095 ± 0.007 ab |
| B11 | 55.39 ± 0.29 g | 16.62 ± 0.71 ab | 6.76 ± 0.09 a | 18.26 ± 0.31 ab | 50.66 ± 0.31 b | 0.099 ± 0.003 a |
| B12 | 55.48 ± 0.22 g | 17.22 ± 0.81 a | 6.49 ± 0.07 ab | 18.41 ± 0.97 a | 49.93 ± 0.97 bc | 0.086 ± 0.005 acd |
| White Starch (W) | Red Starch (R) | Black Starch (B) | ||||
|---|---|---|---|---|---|---|
| Code | Water Solubility (%) | WVP 1 (g mm/m2 dkPa) | Water Solubility (%) | WVP 1 (g mm/m2 dkPa) | Water Solubility (%) | WVP 1 (g mm/m2 dkPa) |
| 1 | 11.10 ± 0.68 cd | 1.73 ± 0.36 ce | 11.72 ± 0.69 de | 1.48 ± 0.21 gh | 11.53 ± 0.83 f | 8.20 ± 0.12 b |
| 2 | 6.73 ± 0.91 e | 7.85 ± 0.91 a | 4.43 ± 0.38 g | 2.91 ± 0.56 fg | 2.12 ± 0.17 i | 15.72 ± 0.48 a |
| 3 | 9.60 ± 0.27 d | 6.33 ± 0.46 b | 8.63 ± 0.57 f | 1.88 ± 0.47 gh | 4.11 ± 0.03 gh | 9.32 ± 0.12 b |
| 4 | 12.04 ± 0.51 c | 2.57 ± 0.52 c | 12.70 ± 0.32 d | 4.06 ± 0.18 ef | 10.50 ± 0.24 f | 2.83 ± 0.64 e |
| 5 | 5.89 ± 0.83 e | 6.15 ± 0.32 b | 3.88 ±0.20 g | 12.47 ± 0.47 b | 3.34 ± 0.06 hi | 3.59 ± 0.35 e |
| 6 | 9.89 ± 0.40 d | 5.20 ± 0.32 b | 10.57 ± 0.59 e | 6.38 ± 0.38 d | 5.05 ± 0.81 g | 3.16 ± 0.81 e |
| 7 | 19.52 ± 0.23 a | 0.90 ± 0.01 defg | 17.27 ± 0.76 b | 5.55 ± 0.23 de | 28.14 ± 0.82 a | 5.00 ± 0.61 d |
| 8 | 15.57 ± 0.67 b | 2.51 ± 0.46 c | 13.00 ± 0.19 d | 15.55 ± 0.79 a | 18.87 ± 0.77 d | 6.59 ± 0.27 c |
| 9 | 18.54 ± 0.31 a | 1.58 ± 0.45 cf | 14.89 ± 0.45 c | 10.32 ± 0.74 c | 21.69 ± 0.19 c | 5.58 ± 0.51 cd |
| 10 | 20.09 ± 0.98 a | 0.32 ± 0.01 g | 20.66 ± 0.68 a | 0.94 ± 0.27 h | 23.53 ± 0.27 b | 0.60 ± 0.14 f |
| 11 | 14.66 ± 0.53 b | 1.94 ± 0.17 cd | 15.85 ± 0.76 bc | 12.83 ± 0.90 b | 17.15 ± 0.49 e | 3.63 ± 0.23 e |
| 12 | 15.84 ± 0.52 b | 0.76 ± 0.16 defg | 15.96 ± 0.48 bc | 11.71 ± 0.25 bc | 20.33 ± 0.30 cd | 2.41 ± 0.42 e |
| Rice Starch | Phenolic Compounds (mg EAG/100 g) | Antioxidant Activity | |
|---|---|---|---|
| DPPH (g/g DPPH) | ABTS (µM trolox/g) | ||
| White | 0.58 ± 0.02 c | 7.31 ± 0.09 a | 5.16 ± 0.10 c |
| Red | 25.26 ± 0.87 b | 4.69 ± 0.23 b | 14.63 ± 0.14 b |
| Black | 164.96 ± 0.25 a | 3.90 ± 0.11 c | 20.59 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos da Silva, L.; Velasco, J.I.; Fakhouri, F.M. Bioactive Films Based on Starch from White, Red, and Black Rice to Food Application. Polymers 2022, 14, 835. https://doi.org/10.3390/polym14040835
Ramos da Silva L, Velasco JI, Fakhouri FM. Bioactive Films Based on Starch from White, Red, and Black Rice to Food Application. Polymers. 2022; 14(4):835. https://doi.org/10.3390/polym14040835
Chicago/Turabian StyleRamos da Silva, Luan, José Ignacio Velasco, and Farayde Matta Fakhouri. 2022. "Bioactive Films Based on Starch from White, Red, and Black Rice to Food Application" Polymers 14, no. 4: 835. https://doi.org/10.3390/polym14040835