Separation and Characterization of Cellulose Fibers from Cannabis Bast Using Foamed Nickel by Cathodic Electro-Fenton Oxidation Strategy
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Chemicals
2.3. Degumming with Biological Enzyme
2.4. Oxidation Degumming in Alkaline Condition
2.5. Oxidative Degumming with Electro-Fenton Reagent
2.6. Constituent Content Test
2.7. Residual Glue Rate Test
2.8. Mechanical and Physical Test
2.9. FT-IR Analysis
2.10. XPS Analysis
2.11. XRD Analysis
2.12. SEM Analysis
2.13. TGA Analysis
2.14. Antibacterial Test
2.15. Degumming Waste Liquid Test
3. Results and Discussion
3.1. Chemical Composition of Different Degumming Methods of Cannabis Fiber
3.2. Mechanical and Physical Test
3.3. FT-IR Analysis
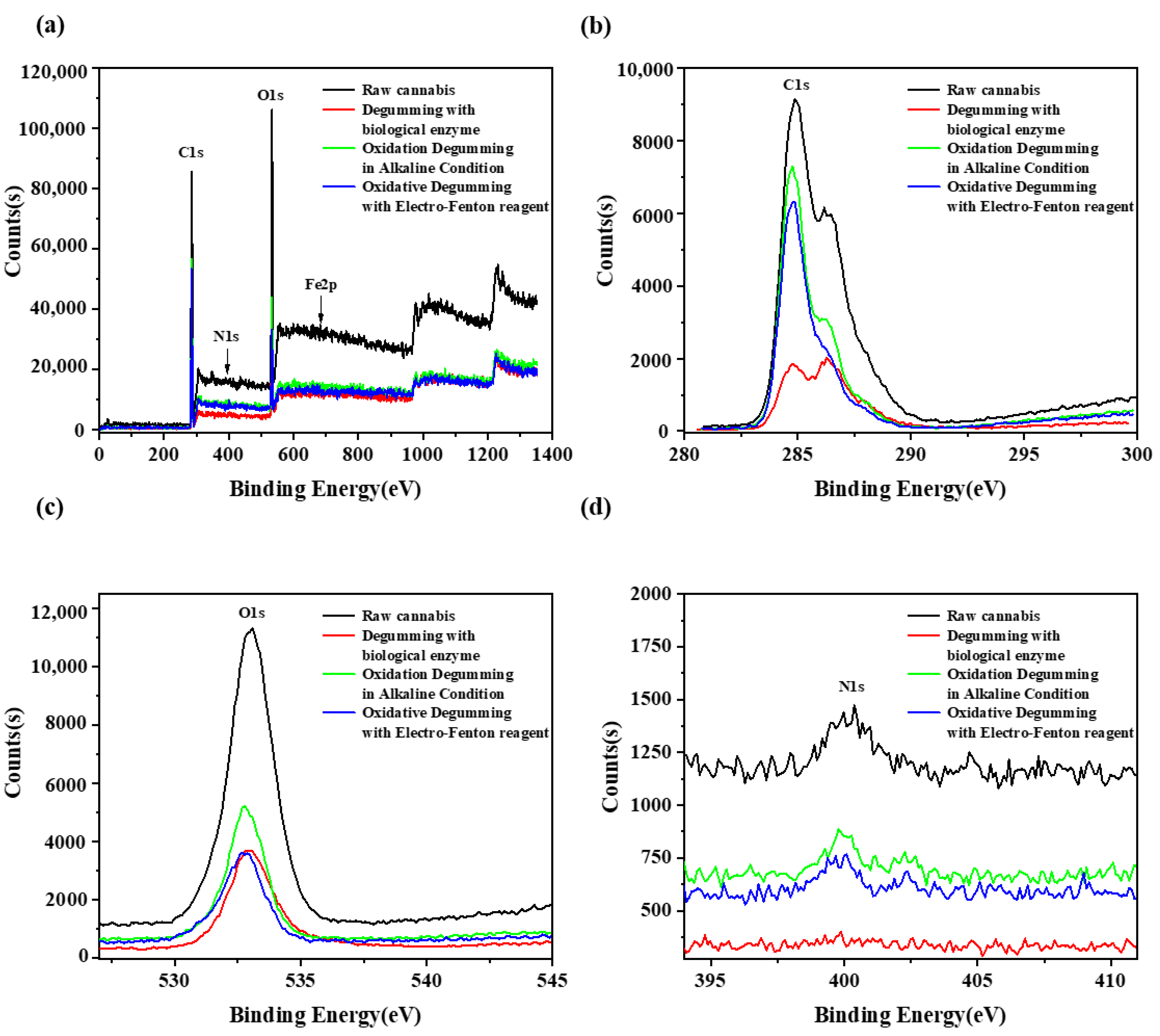
3.4. XPS Analysis
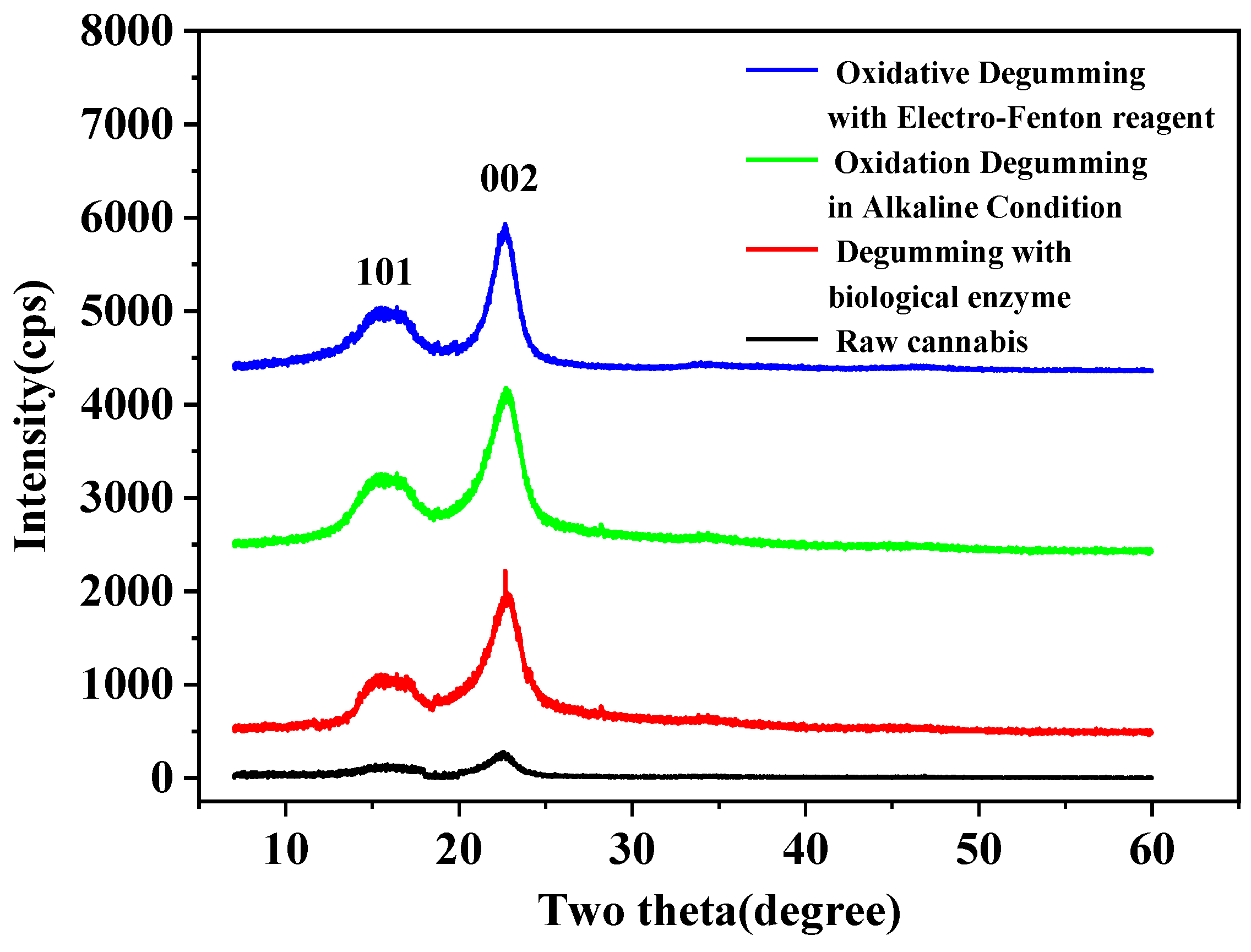
3.5. XRD Analysis
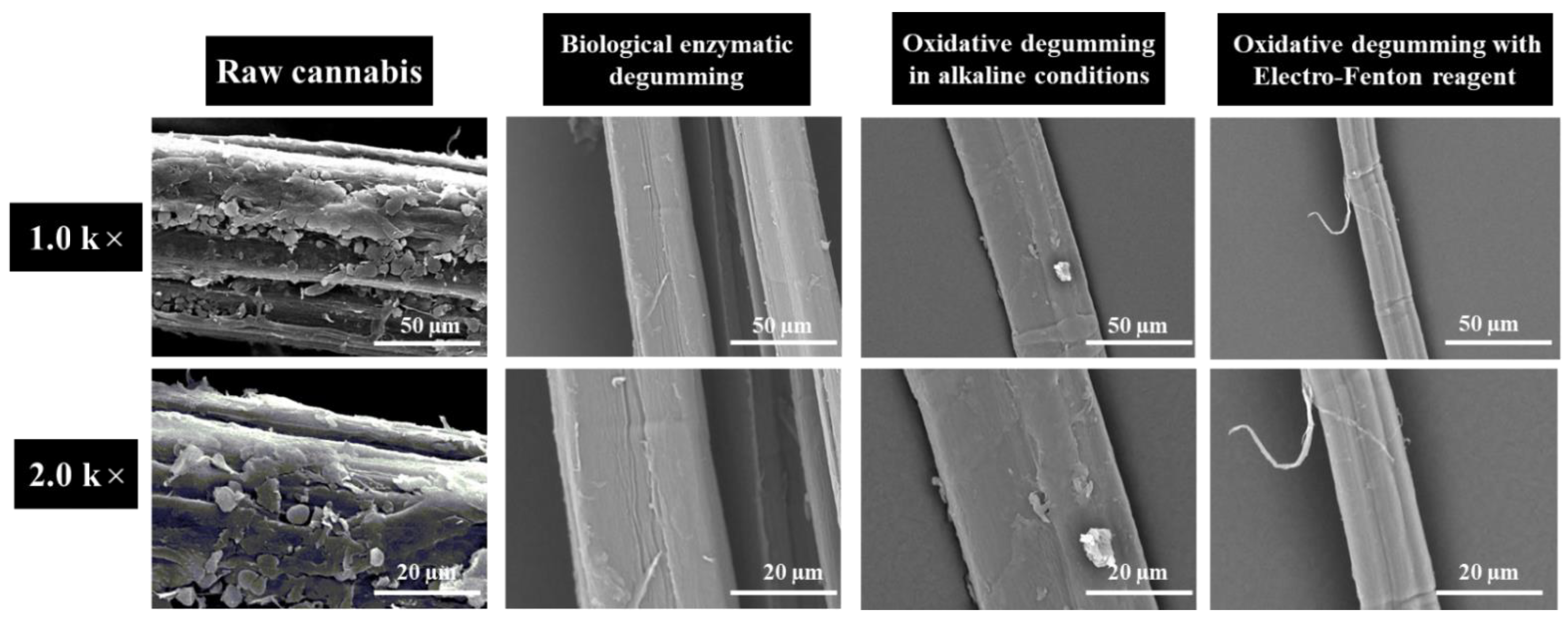
3.6. SEM Analysis
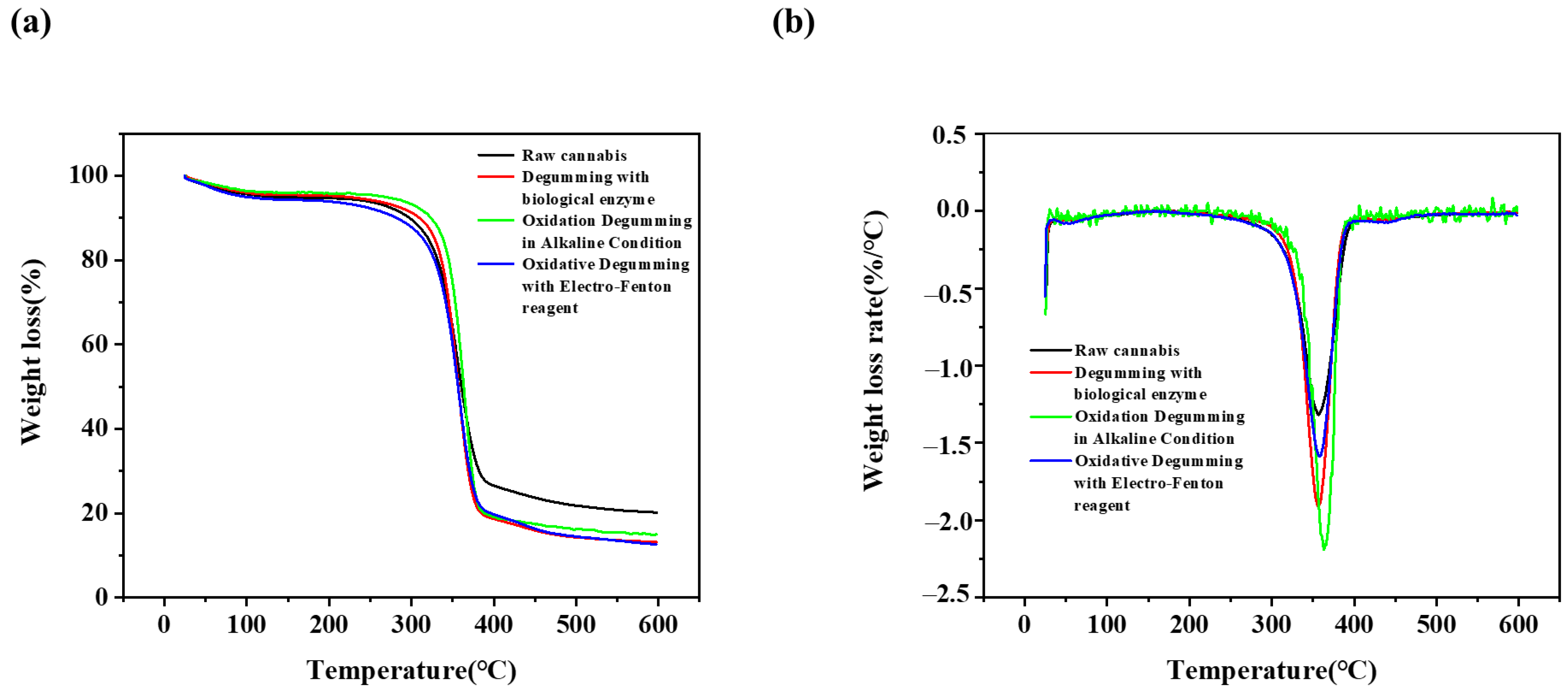
3.7. TGA Analysis
3.8. Antibacterial Test
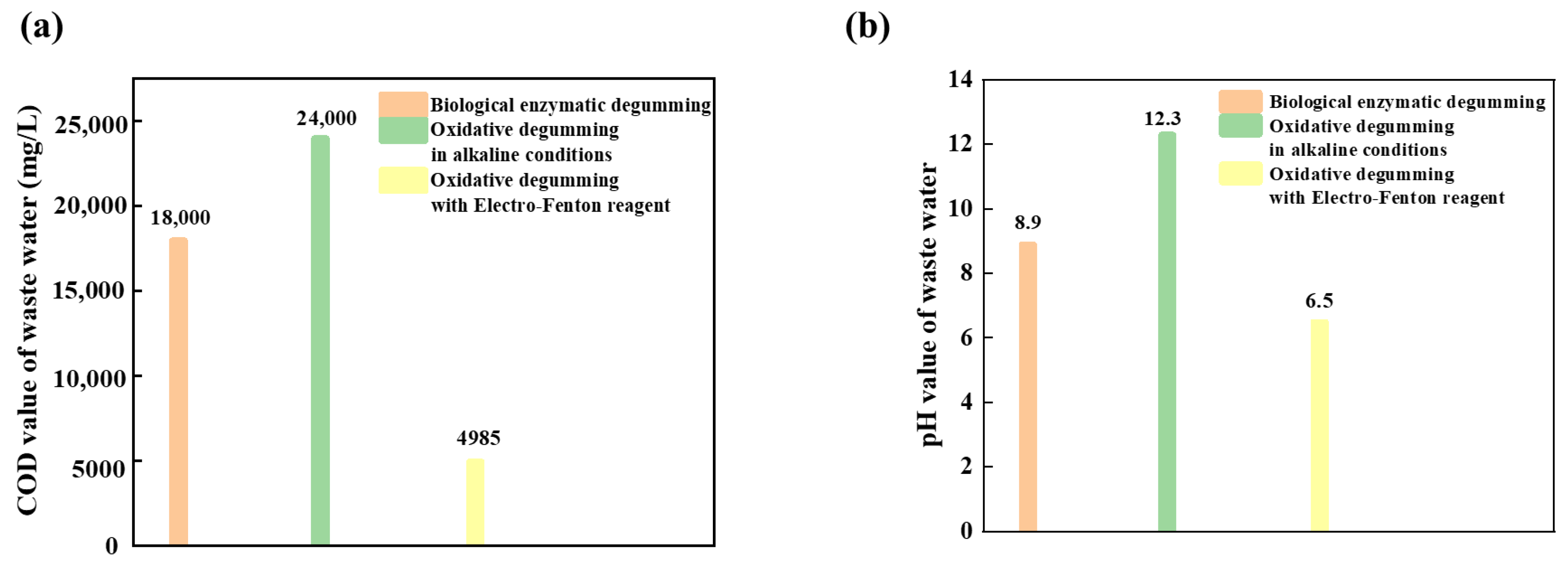
3.9. Degumming Waste Liquid Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Xu, R.; Yang, J.; Nie, S.; Liu, D.; Liu, Y.; Si, C. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgradation. Ind. Crops Prod. 2019, 130, 197. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Fernando, A.; Gao, Y.; Li, G.; Jin, L.; Zhai, H.; Yi, Y.; Xu, L.; Zheng, Y.; et al. Permeable graphited hemp fabrics-based, wearing-comfortable pressure sensors for monitoring human activities. Chem. Eng. J. 2021, 403, 126191. [Google Scholar] [CrossRef]
- Chouhan, S.; Guleria, S. Green synthesis of AgNPs using Cannabis sativa leaf extract: Characterization, antibacterial, anti-yeast and α-amylase inhibitory activity. Mater. Sci. Energy Technol. 2020, 3, 536–544. [Google Scholar] [CrossRef]
- Boccarusso, L.; Durante, M.; Iucolano, F.; Mocerino, D.; Langella, A. Production of hemp-gypsum composites with enhanced flexural and impact resistance. Constr. Build. Mater. 2020, 260, 120476. [Google Scholar] [CrossRef]
- Ingrao, C.; Giudice, A.L.; Bacenetti, J.; Tricase, C.; Dotelli, G.; Fiala, M.; Siracusa, V.; Mbohwa, C. Energy and environmental assessment of industrial hemp for building applications: A review. Renew. Sustain. Energy Rev. 2015, 51, 29–42. [Google Scholar] [CrossRef]
- Vandepitte, K.; Vasile, S.; Vermeire, S.; Vanderhoeven, M.e.; Borght, W.V.d.; Latr´e, J.; Raeve, A.D.; Troch, V. Hemp (Cannabis sativa L.) for high-value textile applications: The effective long fiber yield and quality of different hemp varieties, processed using industrial flax equipment. Ind. Crops Prod. 2020, 158, 112969. [Google Scholar] [CrossRef]
- Bruhlmann, F.; Kh, L.M.E.; Fiechter, A. Enzymatic degumming of ramie bast fibers. J. Biotechnol. 2000, 76, 43–50. [Google Scholar] [CrossRef]
- Khai, N.M.; Son, T.V.; Thuy, P.T.; Giang, P.H.; Loan, H.B.; Huong, N.T.; Ha, N.T.; Huong, T.T.; Hao, N.H. Fenton/ozone-based oxidation and coagulation processes for removing metals (Cu, Ni)-EDTA from plating wastewater. J. Water Process Eng. 2021, 39, 101836. [Google Scholar]
- Jessieleena, A.A.; Priyanka, M.; Saravanakumar, M.P. Comparative study of Fenton, Fe2+/NaOCl and Fe2+/(NH4)2S2O8 on tannery sludge dewaterability, degradability of organics and leachability of chromium. J. Hazard. Mater. 2021, 402, 123495. [Google Scholar] [CrossRef]
- Eslami, A.; Moradi, M.; Ghanbari, F.; Mehdipour, F. Decolorization and COD removal from real textile wastewater by chemical and electrochemical Fenton processes: A comparative study. J. Environ. Health Sci. 2013, 11, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process Saf. Environ. Prot. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Deng, F.; Qiu, S.; Olvera-vargas, H.; Zhu, Y.; Gao, W.; Yang, J.; Ma, F. Electrocatalytic sulfathiazole degradation by a novel nickel-foam cathode coated with nitrogen-doped porous carbon. Electrochim. Acta 2019, 297, 21–30. [Google Scholar] [CrossRef]
- Liu, W.; Ai, Z.; Zhang, L. Design of a neutral three-dimensional electro-Fenton system with foam nickel as particle electrodes for wastewater treatment. J. Hazard. Mater. 2012, 243, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210. [Google Scholar] [CrossRef]
- Yamazaki, I.; Piette, L.H. Stoichiometric measurements of the fenton reaction, possible non-OH oxidizing intermediate by spin-trapping. Free Radic. Biol. Med. 1990, 9, 42. [Google Scholar] [CrossRef]
- Liu, X.C.; Li, W.Q.; Wang, Y.R.; Zhou, G.N.; Wang, Y.X.; He, C.S.; Wang, G.M.; Mu, Y. Cathode-Introduced Atomic H* for Fe(II)-Complex Regeneration to Effective Electro-Fenton Process at a Natural pH. Environ. Sci. Technol. 2019, 53, 6927–6936. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Yu, C. Property of ramie fiber degummed with Fenton reagent. Fibers Polym. 2017, 18, 1891–1897. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, W.; Zhang, Y.; Ben, H.; Han, G.; Ragauskas, A.J. Isolation and characterization of cellulosic fibers from kenaf bast using steam explosion and Fenton oxidation treatment. Cellulose 2018, 25, 4979–4992. [Google Scholar] [CrossRef]
- Gu, D.Y.; Huang, T. Study on the biological enzyme degumming process of pineapple fiber. Shanghai Text. Sci. Technol. 2013, 41, 10–12. [Google Scholar]
- Meng, C.; Hu, J.; Yu, C.; Sun, F. Evaluation of the mild Mg(OH)2-AQ aided alkaline oxidation degumming process of ramie fiber at an industrial scale. Ind. Crops Prod. 2019, 137, 694–701. [Google Scholar] [CrossRef]
- Meng, C.; Yang, J.; Zhang, B.; Yu, C. Rapid and energy-saving preparation of ramie fiber in TEMPO-mediated selective oxidation system. Ind. Crops Prod. 2018, 126, 143–150. [Google Scholar] [CrossRef]
- Alkbir, M.F.M.; Sapuan, S.M.; Nuraini, A.A.; Ishak, M.R. Fiber properties and crashworthiness parameters of natural fiber-reinforced composite structure: A literature review. Compos. Struct. 2016, 148, 59–73. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A. Some of Physical and Mechanical Properties of Particleboard Panels bonded with Phenol- Lignin- Glyoxal Resin. J. Adhes 2019, 96, 1–11. [Google Scholar] [CrossRef]
- Sara, R.; Hallanib, G.E.; Bakkardoucha, F.; Nouric, A.; Laallama, L.; Libab, A.; Barrouga, A.; Jouaitia, A. Influence of cellulose on the thermal conductivity of cellulose based composite thin films. Therm. Sci. Eng. Prog. 2021, 21, 100790. [Google Scholar]
- Banerjee, P.; Ray, D.P.; Satya, P.; Debnath, S.; Mondal, D.; Saha, S.C.; Biswas, P.K. Evaluation of Ramie Fibre Quality: A Review. Int. J. Bioresour. Sci. 2015, 2, 25–69. [Google Scholar]
- Lai, C.Y.; Sapuan, S.M.; Ahmad, M.; Yahya, N.; Dahlan, K.Z.H.M. Mechanical and Electrical Properties of Coconut Coir Fiber-Reinforced Polypropylene Composites. J. Macromol. Sci. D Rev. Polym. Process 2005, 44, 619–632. [Google Scholar] [CrossRef]
- Mahjoub, R.; Yatim, J.M.; Sam, A.R.M.; Hashemi, S.H. Tensile properties of kenaf fiber due to various conditions of chemical fiber surface modifications. Constr. Build. Mater. 2014, 55, 103–113. [Google Scholar] [CrossRef]
- Soghomonyan, D.; Akopyan, K.; Trchounian, A. pH and Oxidation-Reduction Potential Change of Environment during Growth of Lactic Acid Bacteria: Effects of Oxidizers and Reducers. Appl. Biochem. Microbiol. 2011, 47, 27–31. [Google Scholar] [CrossRef]
- Liu, G.L.; Li, Z.F.; Ding, R.Y.; Zhong, X.M.; Yu, C.W. Urea Peroxide:New Degumming Agent Impact on the Effect of Oxidation Degumming of Ramie. Appl. Mech. Mater. 2012, 121–126, 3039–3043. [Google Scholar] [CrossRef]
- Mcdonagh, B.; Carrasco, G. Characterization of Porous Structures of Cellulose Nanofibrils Loaded with Salicylic Acid. Polymers 2020, 12, 2538. [Google Scholar] [CrossRef]
- Cui, G.; Dong, Y.; Li, B.; Li, Y.; Wang, P. A novel heterogeneous Fenton photocatalyst prepared using waste wool fiber combined with Fe3+ ions for dye degradation. Fibers Polym. 2017, 18, 713–719. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, X. Study on H2O2/TAED and H2O2/TBCC bleaching mechanism related to hydroxyl radical with a fluorescent probe. Carbohydr. Polym. 2014, 103, 581–586. [Google Scholar]
- Choi, H.Y.; Lee, J.S. Effects of surface treatment of ramie fibers in a ramie/poly(lactic acid) composite. Fibers Polym. 2012, 13, 217–223. [Google Scholar] [CrossRef]
- Deng, F.; Olvera-Vargas, H.; Garcia-Rodriguez, O.; Qiu, S.; Lefebvre, O. Unconventional electro-Fenton process operating at a wide pH range with Ni foam cathode and tripolyphosphate electrolyte. J. Hazard. Mater. 2020, 396, 122641. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Sarkar, B.K.; Basak, R.K.; Rana, A.K. Study of the thermal behavior of alkali-treated jute fibers. J. Appl. Polym. Sci. 2010, 85, 2594–2599. [Google Scholar] [CrossRef]
- Rodríguez, E.S.; Wladyka-Przybylak, M.; Vázquez, A. Thermal degradation and fire resistance of unsaturated polyester, modified acrylic resins and their composites with natural fibres. Polym. Degrad. Stab. 2006, 91, 255–261. [Google Scholar]
- Shebani, A.N.; Reenen, A.J.v.; Meincken, M. The effect of wood extractives on the thermal stability of different wood species-ScienceDirect. Thermochim. Acta 2008, 471, 43–50. [Google Scholar] [CrossRef]
- Nguyen, T.; Zavarin, E.; Barrall, E. Thermal Analysis of Lignocellulosic Materials. Part II. Modified Materials. J. Macromol. Sci.—Rev. Macromol. Chem. 1981, 21, 1–60. [Google Scholar] [CrossRef]
- Amalraja, S.; Krupaa, J.; Sriramavaratharajanb, V.; Mariyammala, V.; Muruganc, R.; Ayyanara, M. Chemical characterization, antioxidant, antibacterial and enzyme inhibitory properties of Canthium coromandelicum, a valuable source for bioactive compounds. J. Pharm. Biomed. Anal. 2020, 192, 113620. [Google Scholar] [CrossRef]
- Tong, M.; Yuan, S.; Ma, S.; Jin, M.; Liu, D.; Cheng, D.; Liu, X.; Gan, Y.; Wang, Y. Production of Abundant Hydroxyl Radicals from Oxygenation of Subsurface Sediments. Environ. Sci. Technol. 2015, 50, 4890–4891. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.D.; Misra, R.; Williams, L.B. Unearthing the Antibacterial Mechanism of Medicinal Clay: A Geochemical Approach to Combating Antibiotic Resistance. Sci. Rep. 2016, 6, 19043. [Google Scholar] [CrossRef] [PubMed]


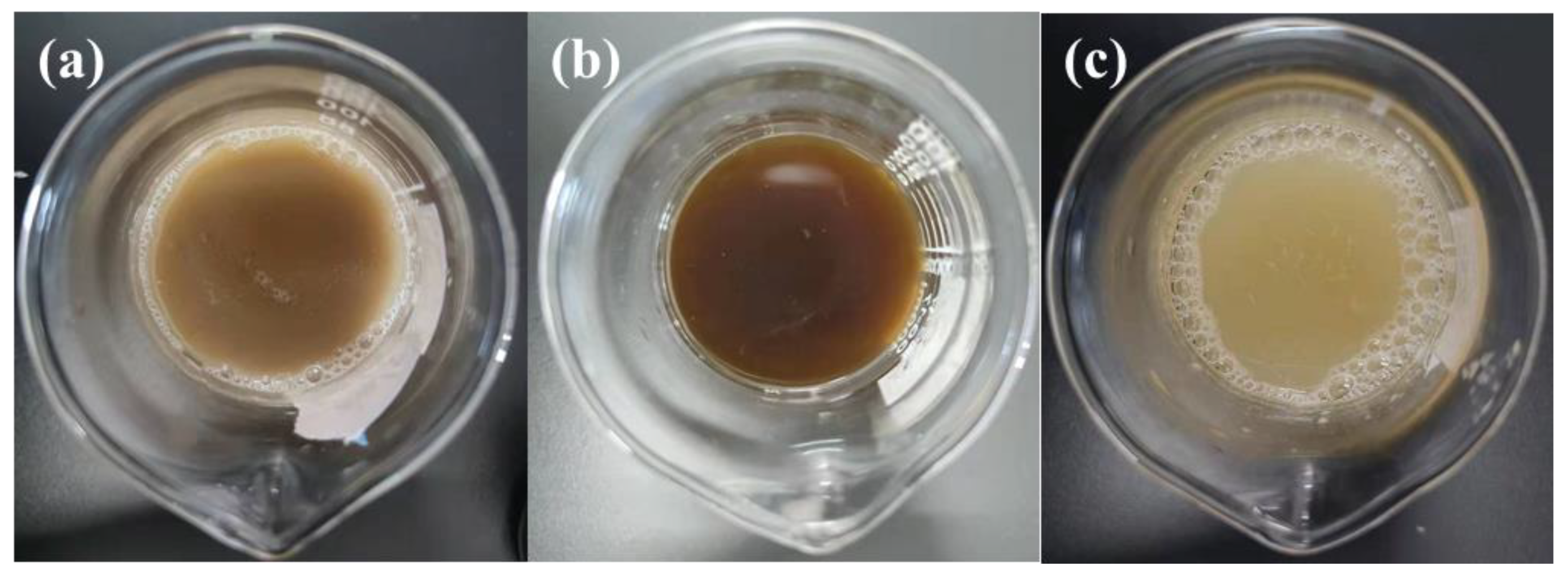
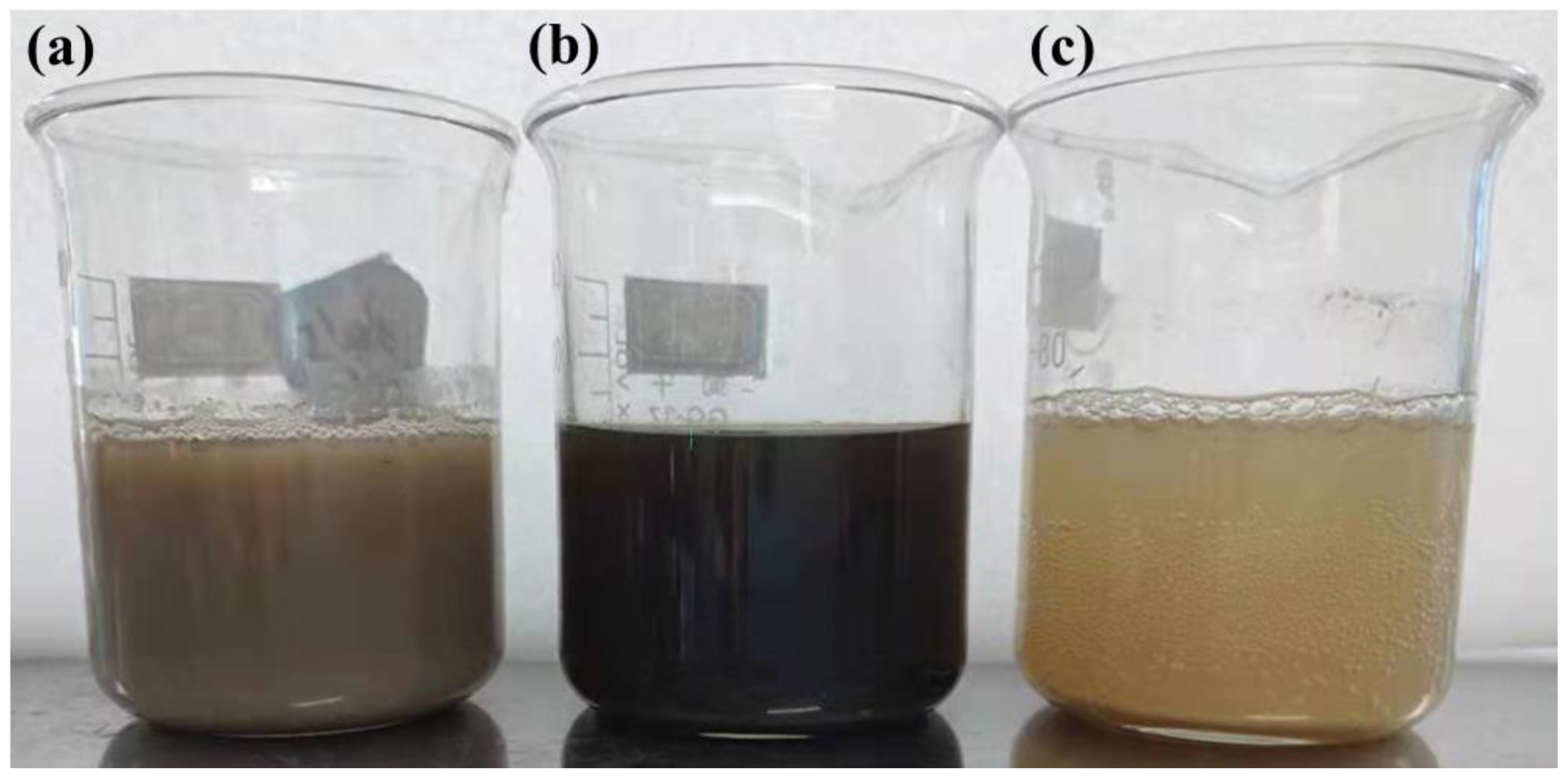
| Ingredient | Cellulose | Hemicellulose | Lignin | Pectin | Wax | Ash | Water Solubles |
|---|---|---|---|---|---|---|---|
| Content (%) | 49.63 | 19.32 | 18.98 | 6.79 | 1.32 | 1.63 | 2.33 |
| Immersing in Acid Solution | Biological Enzyme Treatment | Oxidation Degumming in Alkaline Condition | |
|---|---|---|---|
| Chemical dosage (%) | H2SO4:1 | Laccase:Xylanase:Hemicellulase = 29.0:16.6:54.4 | NaOH:5 H2O2:10 Sulfite:1 |
| Bath ratio | 1:20 | 1:20 | 1:20 |
| pH | 5.0 | ||
| Temperature (°C) | Room temperature | 50 | 80 |
| Treatment time (min) | 1440 | 50 | 60 |
| First Oxidation | Second Oxidation | Oxidation Degumming in Alkaline Condition | |
|---|---|---|---|
| Chemical dosage (%) | H2O2:1 NaOH:2 | H2O2:1 NaOH:1 | NaOH:1 |
| Bath ratio | 1:30 | 1:30 | 1:30 |
| Temperature (°C) | 80 | 90 | 50 |
| Treatment time (min) | 10 | 25 | 40 |
| Immersing in Acid Solution | Oxidation | Oxidation Degumming in Alkaline Condition | |
|---|---|---|---|
| Chemical dosage (%) | NaOH:1 | FeSO4∙7H2O:5 H2O2:5 Tripolyphosphate (3-PP):1 | NaOH:5 H2O2:10 Sulfite:1 |
| Bath ratio | 1:10 | 1:10 | 1:10 |
| pH | 6.0 | ||
| Temperature (°C) | 60 | 80 | 80 |
| Treatment time (min) | 10 | 60 | 40 |
| Voltage(V) | 15 | ||
| Cathode electrode | Ni-F |
| Cellulose (%) | Lignin (%) | Hemicellulose (%) | Pectin (%) | Wax (%) | Water Solubles (%) | Residual Glue Rate (%) | |
|---|---|---|---|---|---|---|---|
| Raw cannabis | 49.63 | 18.98 | 19.32 | 6.79 | 1.21 | 4.07 | — |
| Biological enzyme degumming | 77.98 | 9.43 | 6.84 | 2.99 | 0.75 | 2.01 | 9.43 |
| Oxidation degumming in alkaline condition | 75.5 | 10.44 | 11.25 | 1.62 | 0.22 | 0.97 | 8.81 |
| Oxidative degumming with electro-Fenton reagent | 84.57 | 6.54 | 4.96 | 1.43 | 0.58 | 1.92 | 4.77 |
| Tenacity (cN) | Elongation (%) | Diameter (µm) | Whiteness (%) | |
|---|---|---|---|---|
| Raw cannabis | 100.03 ± 2.2 | 0.77 ± 0.5 | 120.457 ± 2.6 | 17.32 ± 0.6 |
| Biological enzyme degumming | 26.58 ± 0.3 | 5.03 ± 0.7 | 64.459 ± 1.7 | 33.50 ± 0.4 |
| Oxidation degumming in alkaline condition | 18.98 ± 0.2 | 1.08 ± 0.6 | 13.329 ± 1.5 | 53.85 ± 0.7 |
| Degumming with electro-Fenton reagent | 41.79 ± 0.3 | 2.32 ± 0.5 | 15.432 ± 1.3 | 25.70 ± 0.3 |
| C1s | O1s | N1s | Fe2p | |
|---|---|---|---|---|
| Raw cannabis | 73.35 | 24.16 | 0.61 | 1.89 |
| Biological enzyme degumming | 68.59 | 31.41 | — | — |
| Oxidation degumming in alkaline condition | 80.93 | 18.29 | 0.78 | — |
| Degumming with electro-Fenton reagent | 81.08 | 16.12 | 2.8 | — |
| ZOI (Diameter, mm) | |||
|---|---|---|---|
| 24 h | 36 h | 72 h | |
| Raw cannabis | 9.4 ± 0.3 | 8.6 ± 0.4 | 7.4 ± 0.3 |
| Biological enzyme degumming | 10.2 ± 0.8 | 10.1 ± 0.7 | 9.3 ± 0.7 |
| Oxidative degumming under alkaline conditions | 9.2 ± 0.6 | 8.7 ± 0.5 | 8.4 ± 0.5 |
| Oxidative degumming of electro-Fenton reagent | 10.4 ± 0.5 | 9.8 ± 0.6 | 9.5 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, D.; Yu, Y.; Chen, J.; Fan, W. Separation and Characterization of Cellulose Fibers from Cannabis Bast Using Foamed Nickel by Cathodic Electro-Fenton Oxidation Strategy. Polymers 2022, 14, 380. https://doi.org/10.3390/polym14030380
Sun Y, Li D, Yu Y, Chen J, Fan W. Separation and Characterization of Cellulose Fibers from Cannabis Bast Using Foamed Nickel by Cathodic Electro-Fenton Oxidation Strategy. Polymers. 2022; 14(3):380. https://doi.org/10.3390/polym14030380
Chicago/Turabian StyleSun, Ying, Duanxin Li, Yang Yu, Jialin Chen, and Wanyue Fan. 2022. "Separation and Characterization of Cellulose Fibers from Cannabis Bast Using Foamed Nickel by Cathodic Electro-Fenton Oxidation Strategy" Polymers 14, no. 3: 380. https://doi.org/10.3390/polym14030380





