Development of Double Hydrophilic Block Copolymer/Porphyrin Polyion Complex Micelles towards Photofunctional Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Block Copolymer Synthesis and Quaternization
2.3. Preparation of the Polyion Complex Micelles (PICs)
2.4. Methods
3. Results and Discussion
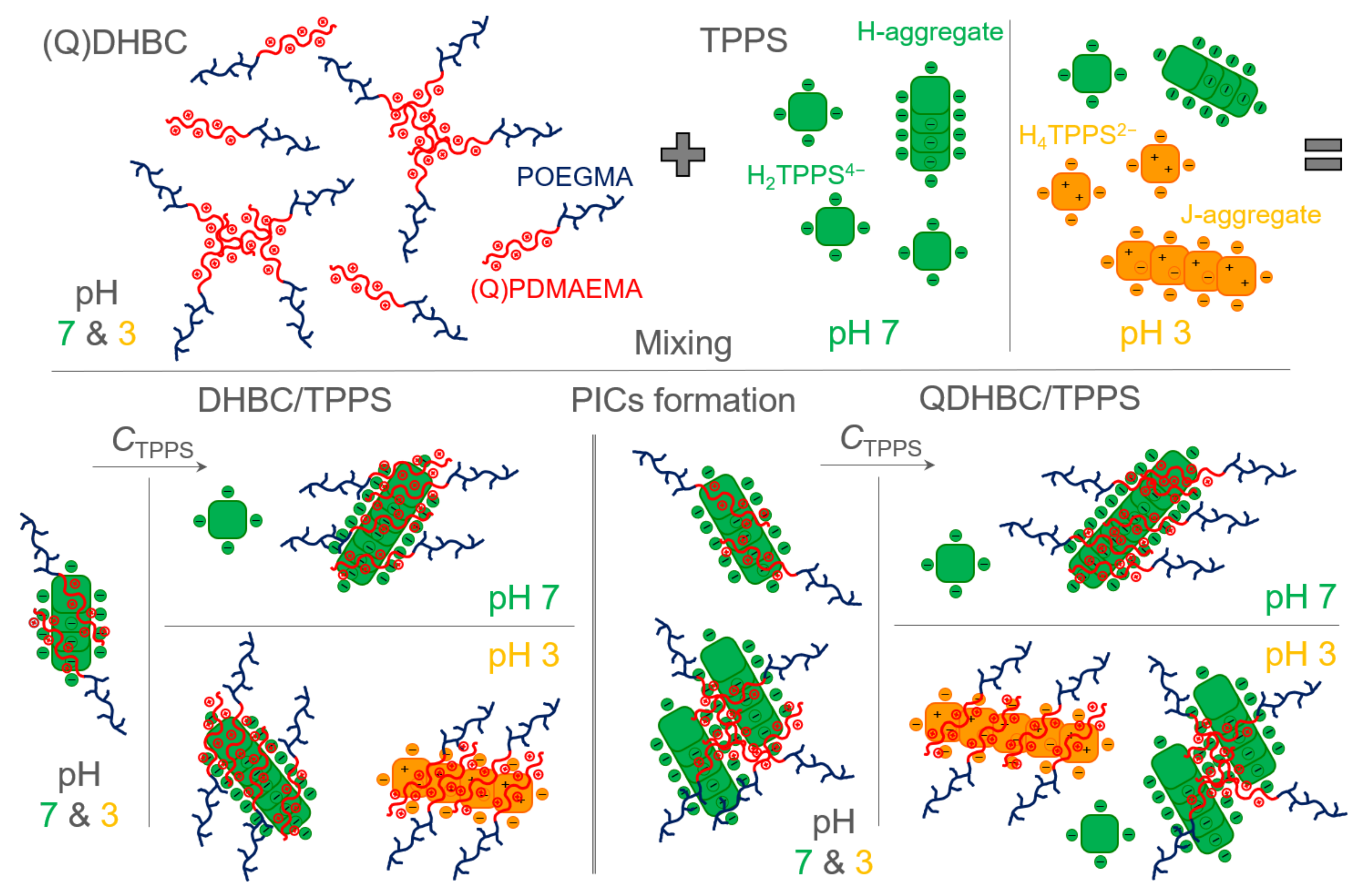
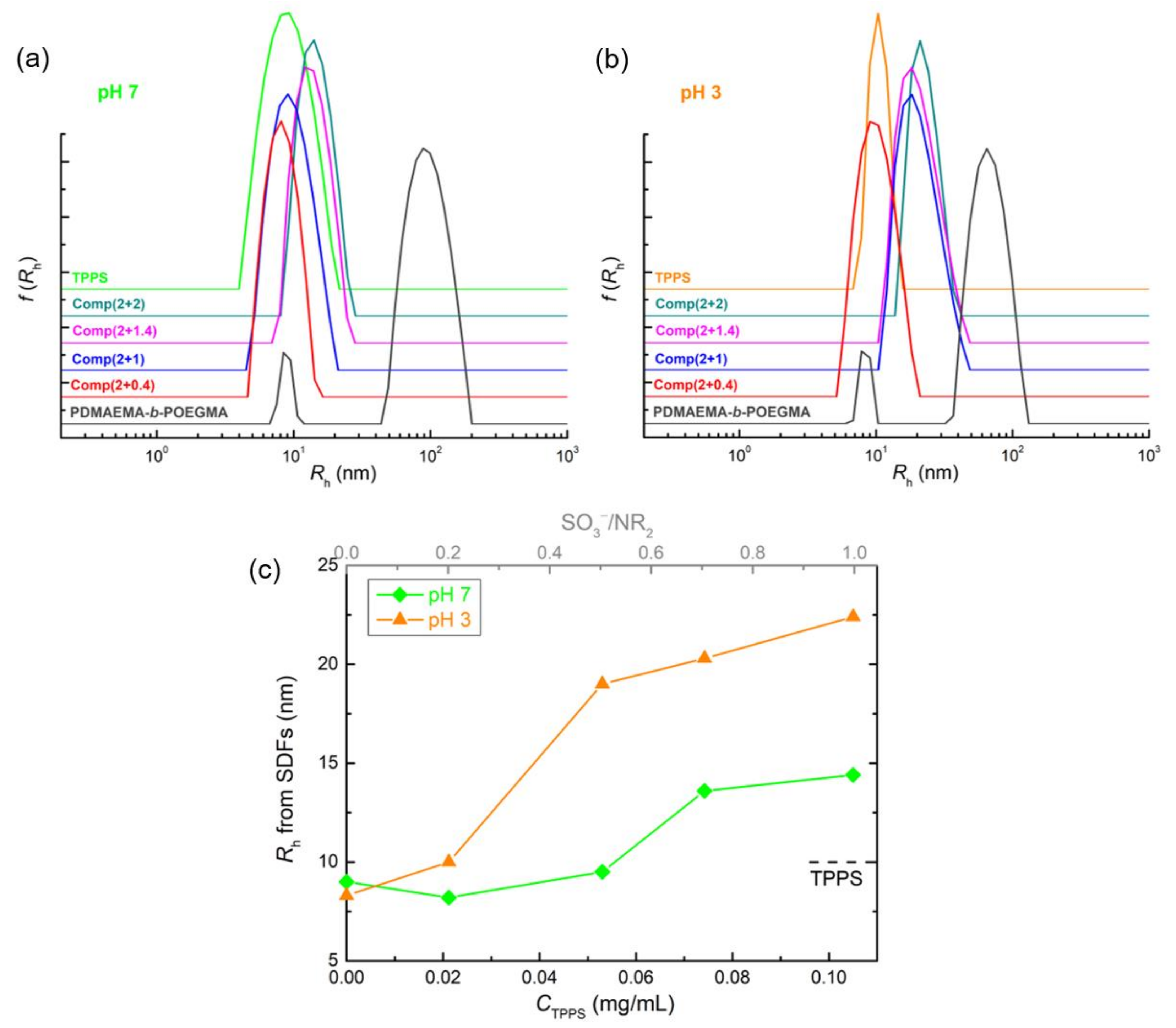
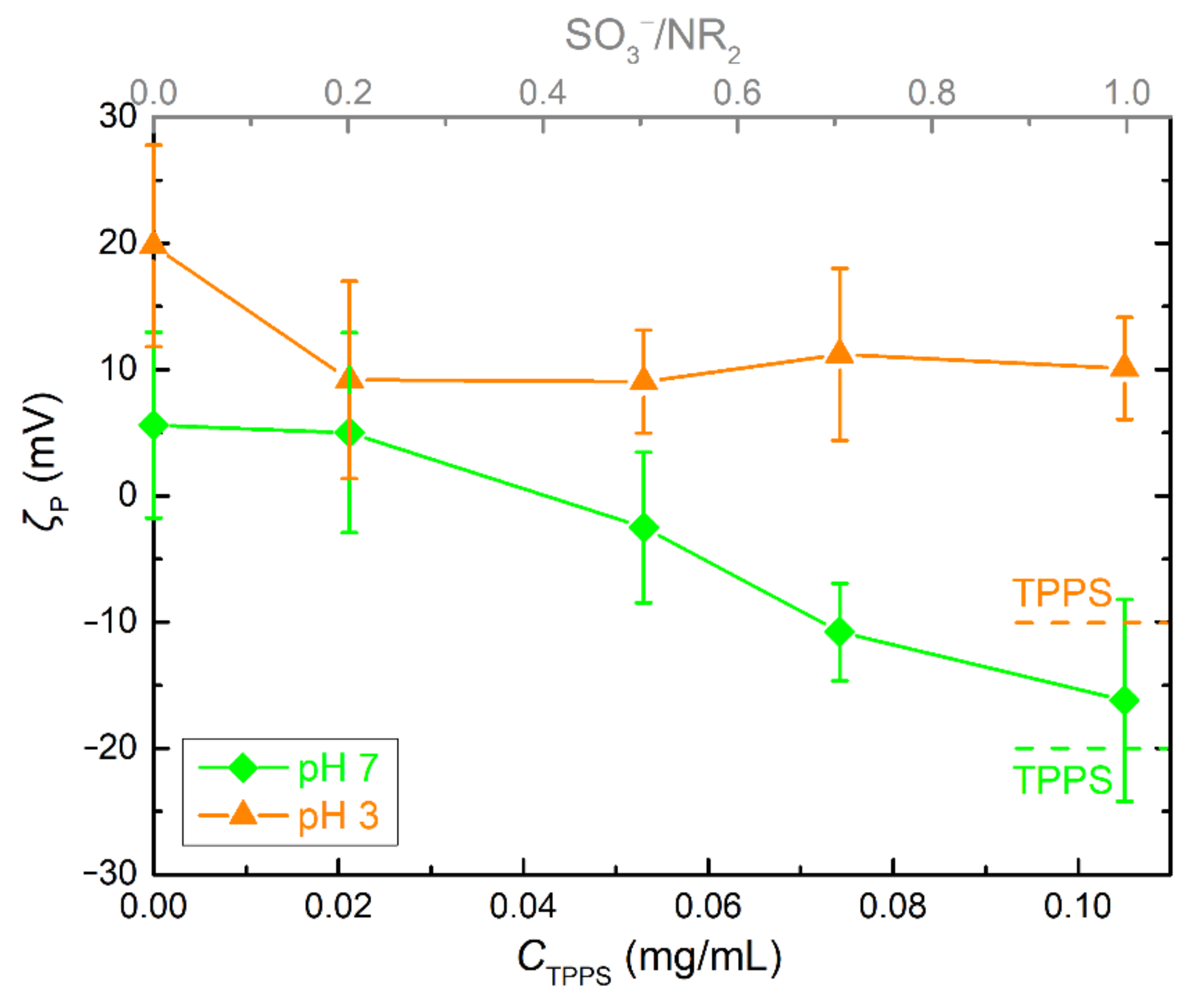
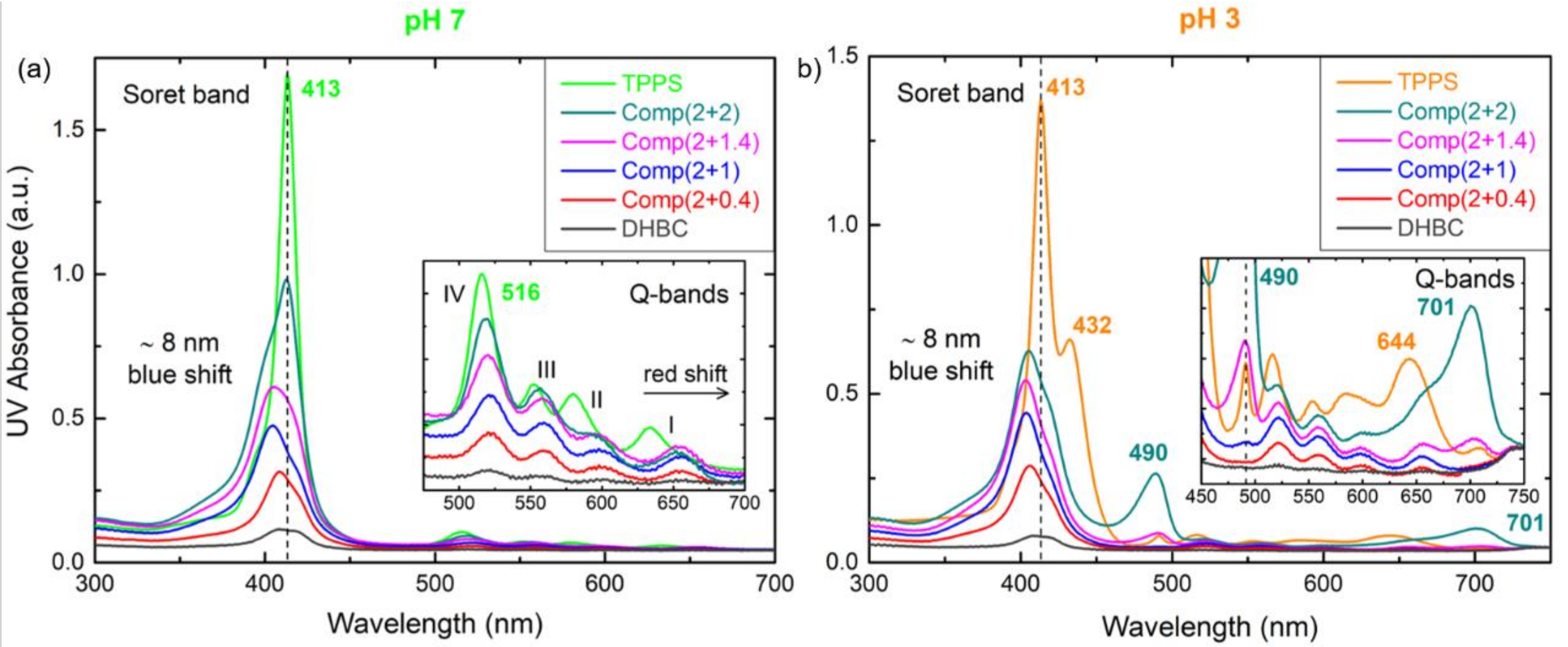
3.1. PDMAEMA-b-POEGMA and TPPS Complexation
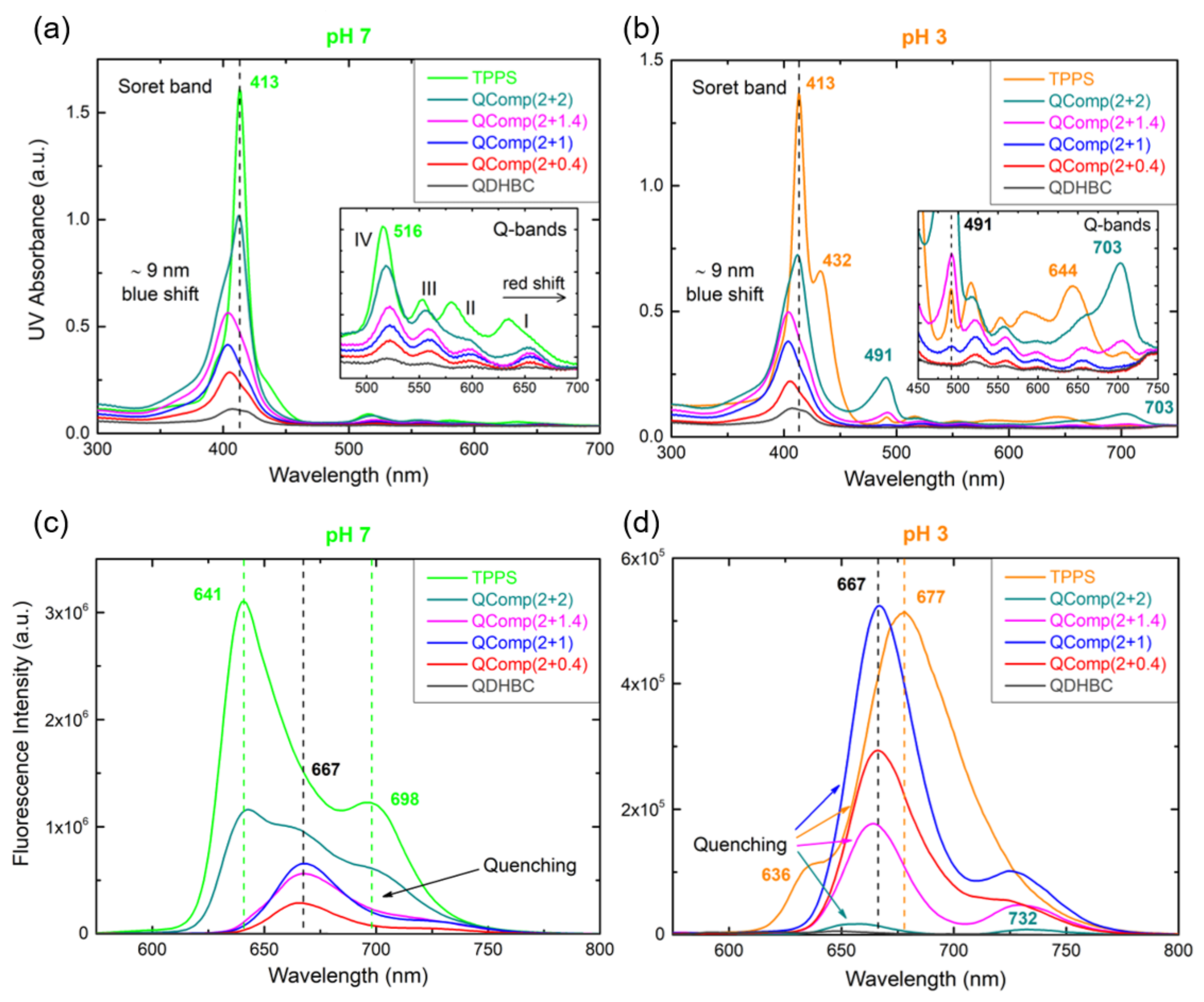
3.2. QPDMAEMA-b-POEGMA and TPPS complexation
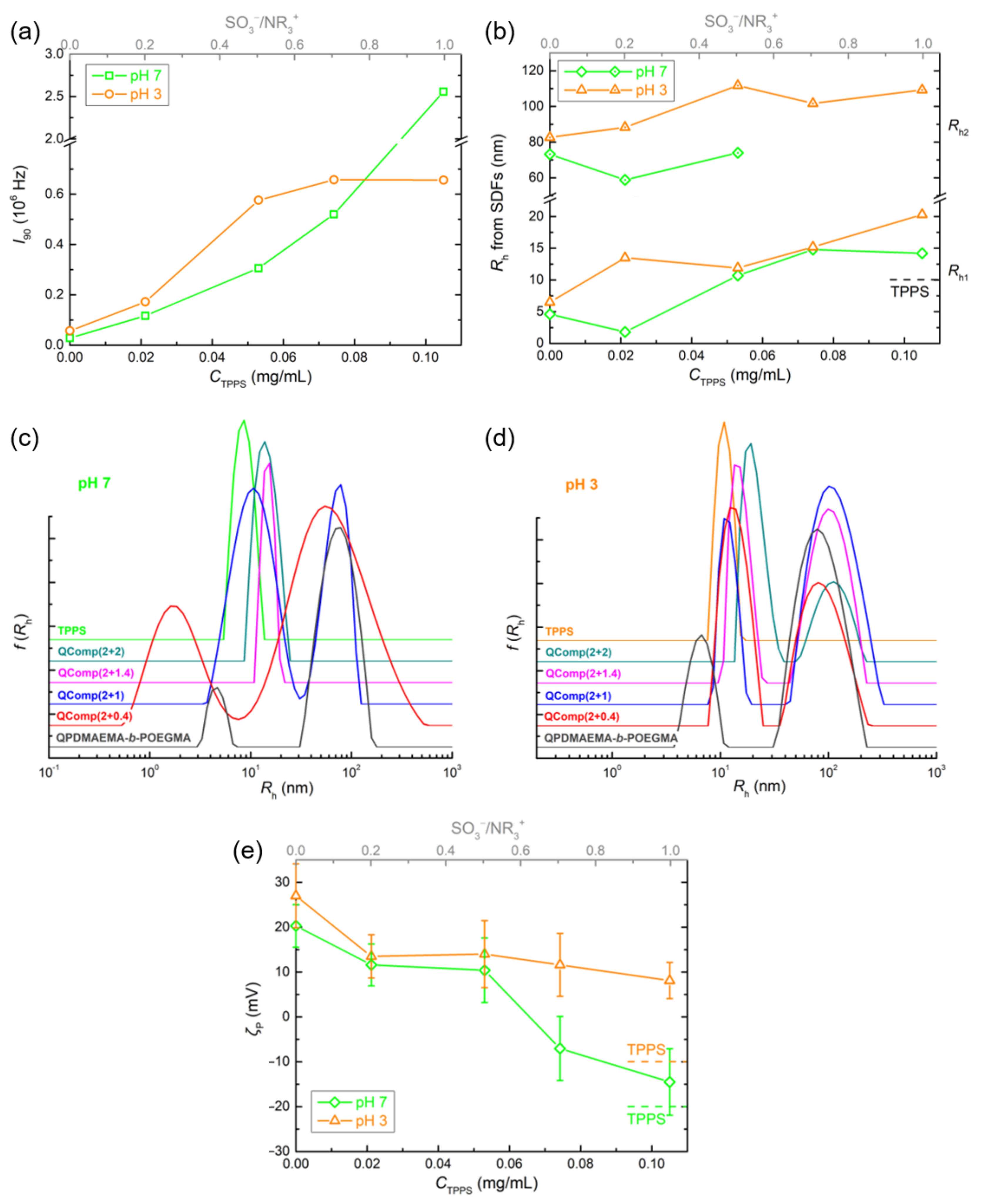
3.3. Comparison of the Two Systems in Different Conditions
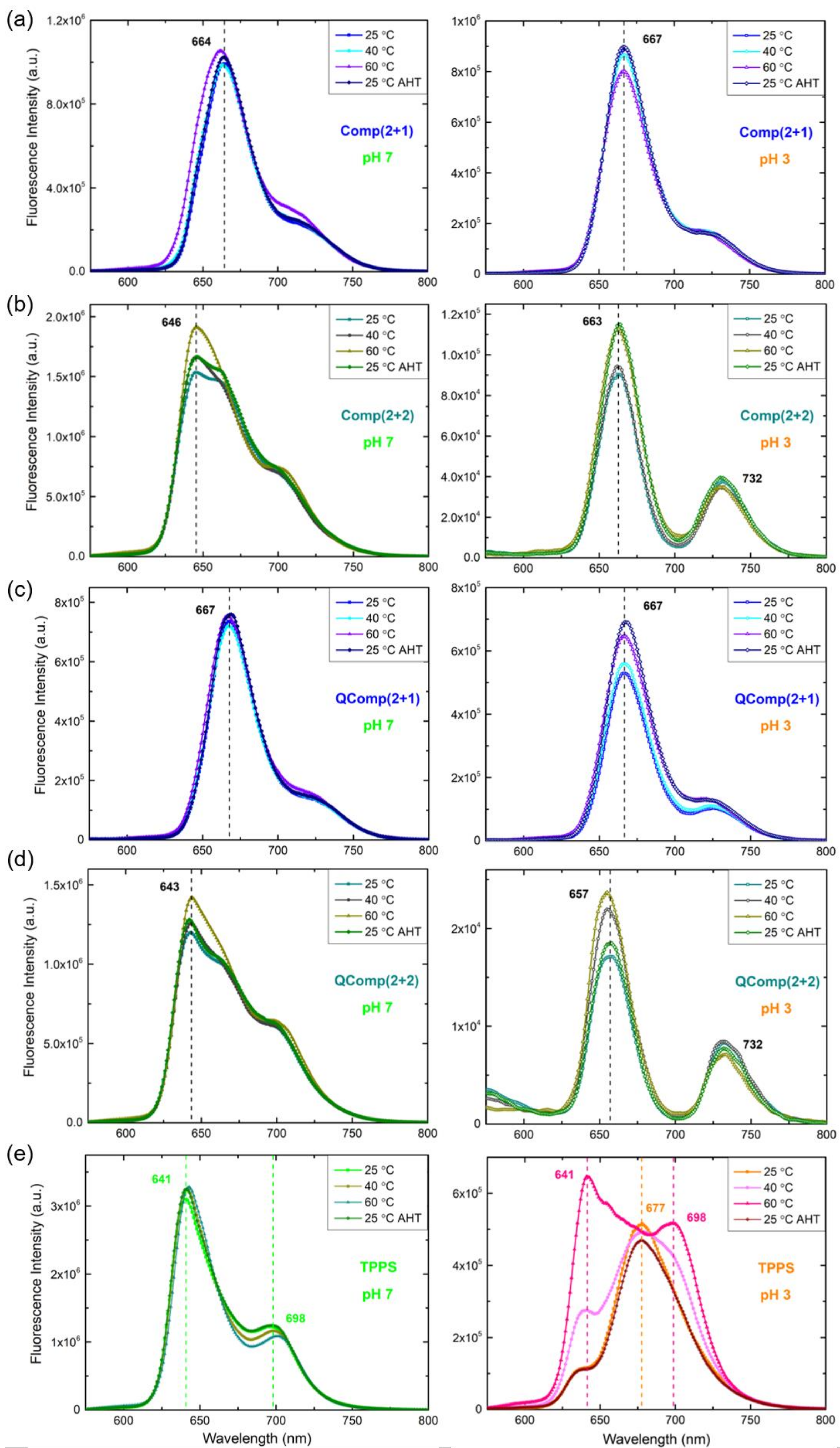
3.4. Temperature Effect on the Formed PICs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy–Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent Advances in Photodynamic Therapy for Cancer and Infectious Diseases. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2019, 11, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Recent Advances of Multi-Dimensional Porphyrin-Based Functional Materials in Photodynamic Therapy. Coord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Qindeel, M.; Sargazi, S.; Hosseinikhah, S.M.; Rahdar, A.; Barani, M.; Thakur, V.K.; Pandey, S.; Mirsafaei, R. Porphyrin-Based Nanostructures for Cancer Theranostics: Chemistry, Fundamentals and Recent Advances. ChemistrySelect 2021, 6, 14082–14099. [Google Scholar] [CrossRef]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, M.; Bae, Y. Block Copolymer Nanoassemblies for Photodynamic Therapy and Diagnosis. Ther. Deliv. 2013, 4, 1431–1441. [Google Scholar] [CrossRef]
- Insua, I.; Wilkinson, A.; Fernandez-Trillo, F. Polyion Complex (PIC) Particles: Preparation and Biomedical Applications. Eur. Polym. J. 2016, 81, 198–215. [Google Scholar] [CrossRef] [Green Version]
- Harada, A.; Kataoka, K. Polyion Complex Micelle Formation from Double-Hydrophilic Block Copolymers Composed of Charged and Non-Charged Segments in Aqueous Media. Polym. J. 2018, 50, 95–100. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [Green Version]
- Magana, J.R.; Sproncken, C.C.M.; Voets, I.K. On Complex Coacervate Core Micelles: Structure-Function Perspectives. Polymers 2020, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Stapert, H.R.; Nishiyama, N.; Jiang, D.L.; Aida, T.; Kataoka, K. Polyion Complex Micelles Encapsulating Light-Harvesting Ionic Dendrimer Zinc Porphyrins. Langmuir 2000, 16, 8182–8188. [Google Scholar] [CrossRef]
- Jang, W.-D.; Nishiyama, N.; Zhang, G.-D.; Harada, A.; Jiang, D.-L.; Kawauchi, S.; Morimoto, Y.; Kikuchi, M.; Koyama, H.; Aida, T.; et al. Supramolecular Nanocarrier of Anionic Dendrimer Porphyrins with Cationic Block Copolymers Modified with Polyethylene Glycol to Enhance Intracellular Photodynamic Efficacy. Angew. Chemie Int. Ed. 2005, 44, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, R.; Li, J.; Li, Y.; An, Y.; Shi, L. J- and H-Aggregates of 5,10,15,20-Tetrakis-(4-Sulfonatophenyl)-Porphyrin and Interconversion in PEG- b -P4VP Micelles. Biomacromolecules 2008, 9, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiang, R.; Zhang, L.; Wu, C.; Ma, R.; An, Y.; Shi, L. Micellization of Copolymers via Noncovalent Interaction with TPPS and Aggregation of TPPS. Sci. China Chem. 2011, 54, 343–350. [Google Scholar] [CrossRef]
- Zhao, L.; Xiang, R.; Ma, R.; Wang, X.; An, Y.; Shi, L. Chiral Conversion and Memory of TPPS J-Aggregates in Complex Micelles: PEG-b-PDMAEMA/TPPS. Langmuir 2011, 27, 11554–11559. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Gao, H.; Ren, J.; An, Y.; Shi, L. MgTPPS/Block Copolymers Complexes for Enhanced Stability and Photoactivity. RSC Adv. 2013, 3, 18351–18358. [Google Scholar] [CrossRef]
- Zhao, L.; Li, A.; Xiang, R.; Shen, L.; Shi, L. Interaction of FeIII-Tetra-(4-Sulfonatophenyl)-Porphyrin with Copolymers and Aggregation in Complex Micelles. Langmuir 2013, 29, 8936–8943. [Google Scholar] [CrossRef]
- Chai, Z.; Jing, C.; Liu, Y.; An, Y.; Shi, L. Spectroscopic Studies on the Photostability and Photoactivity of Metallo-Tetraphenylporphyrin in Micelles. Colloid Polym. Sci. 2014, 292, 1329–1337. [Google Scholar] [CrossRef]
- Bo, Q.; Zhao, Y. Double-Hydrophilic Block Copolymer for Encapsulation and Two-Way pH Change-Induced Release of Metalloporphyrins. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1734–1744. [Google Scholar] [CrossRef]
- Sorrells, J.L.; Shrestha, R.; Neumann, W.L.; Wooley, K.L. Porphyrin-Crosslinked Block Copolymer Assemblies as Photophysically-Active Nanoscopic Devices. J. Mater. Chem. 2011, 21, 8983–8986. [Google Scholar] [CrossRef]
- Liu, S.; Hu, C.; Wei, Y.; Duan, M.; Chen, X.; Hu, Y. Transformation of H-Aggregates and J-Dimers of Water-Soluble Tetrakis (4-Carboxyphenyl) Porphyrin in Polyion Complex Micelles. Polymers 2018, 10, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhao, P.; Jiang, D.; Yang, G.; Xue, Y.; Tang, Z.; Zhang, M.; Wang, H.; Jiang, X.; Wu, Y.; et al. In Situ Catalytic Reaction for Solving the Aggregation of Hydrophobic Photosensitizers in Tumor. ACS Appl. Mater. Interfaces 2020, 12, 5624–5632. [Google Scholar] [CrossRef] [PubMed]
- Kubát, P.; Henke, P.; Raya, R.K.; Štěpánek, M.; Mosinger, J. Polystyrene and Poly(Ethylene Glycol)-b-Poly(ε-Caprolactone) Nanoparticles with Porphyrins: Structure, Size, and Photooxidation Properties. Langmuir 2020, 36, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Y.; Yang, G.; Xia, L.; Yu, F.; Chen, C.; Zhang, L.; Cao, H. A pH/H2O2 Dual Triggered Nanoplatform for Enhanced Photodynamic Antibacterial Efficiency. J. Mater. Chem. B 2021, 9, 5076–5082. [Google Scholar] [CrossRef] [PubMed]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-Aggregates of Porphyrin−Surfactant Complexes: Time-Resolved Fluorescence and Other Spectroscopic Studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, H.; Zhu, J.; Yang, Y.; Liu, X.; Wang, D.; Zhang, X.; Zhuo, L. Self-Assembly into Temperature Dependent Micro-/Nano-Aggregates of 5,10,15,20-Tetrakis(4-Carboxyl Phenyl)-Porphyrin. Mater. Sci. Eng. C 2013, 33, 4944–4951. [Google Scholar] [CrossRef]
- Toncelli, C.; Pino-Pinto, J.P.; Sano, N.; Picchioni, F.; Broekhuis, A.A.; Nishide, H.; Moreno-Villoslada, I. Controlling the Aggregation of 5,10,15,20-Tetrakis-(4-Sulfonatophenyl)-Porphyrin by the Use of Polycations Derived from Polyketones Bearing Charged Aromatic Groups. Dye. Pigment. 2013, 98, 51–63. [Google Scholar] [CrossRef]
- Steinbeck, C.A.; Hedin, N.; Chmelka, B.F. Interactions of Charged Porphyrins with Nonionic Triblock Copolymer Hosts in Aqueous Solutions. Langmuir 2004, 20, 10399–10412. [Google Scholar] [CrossRef]
- Wang, M.; Yan, F.; Zhao, L.; Zhang, Y.; Sorci, M. Preparation and Characterization of a pH-Responsive Membrane Carrier for Meso-Tetraphenylsulfonato Porphyrin. RSC Adv. 2017, 7, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Haladjova, E.; Chrysostomou, V.; Petrova, M.; Ugrinova, I.; Pispas, S.; Rangelov, S. Physicochemical Properties and Biological Performance of Polymethacrylate Based Gene Delivery Vector Systems: Influence of Amino Functionalities. Macromol. Biosci. 2021, 21, 2000352. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic Light Scattering; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Chu, B. Laser Light Scattering; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Karayianni, M.; Radeva, R.; Koseva, N.; Pispas, S. Electrostatic Complexation of a Double Hydrophilic Block Polyelectrolyte and Proteins of Different Molecular Shape. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1515–1529. [Google Scholar] [CrossRef]
- Giaouzi, D.; Pispas, S. Synthesis and Self-assembly of Thermoresponsive Poly(N-isopropylacrylamide)-b-poly(Oligo Ethylene Glycol Methyl Ether Acrylate) Double Hydrophilic Block Copolymers. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1467–1477. [Google Scholar] [CrossRef]
- Hollingsworth, J.V.; Richard, A.J.; Vicente, M.G.H.; Russo, P.S. Characterization of the Self-Assembly of Meso-Tetra(4-Sulfonatophenyl)Porphyrin (H2TPPS4−) in Aqueous Solutions. Biomacromolecules 2012, 13, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Bolzonello, L.; Albertini, M.; Collini, E.; Di Valentin, M. Delocalized Triplet State in Porphyrin J-Aggregates Revealed by EPR Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 27173–27177. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Tian, J.; Jiang, D.; Gao, Y.; Zhang, W. NIR-Activated “OFF/ON” Photodynamic Therapy by a Hybrid Nanoplatform with Upper Critical Solution Temperature Block Copolymers and Gold Nanorods. Biomacromolecules 2019, 20, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
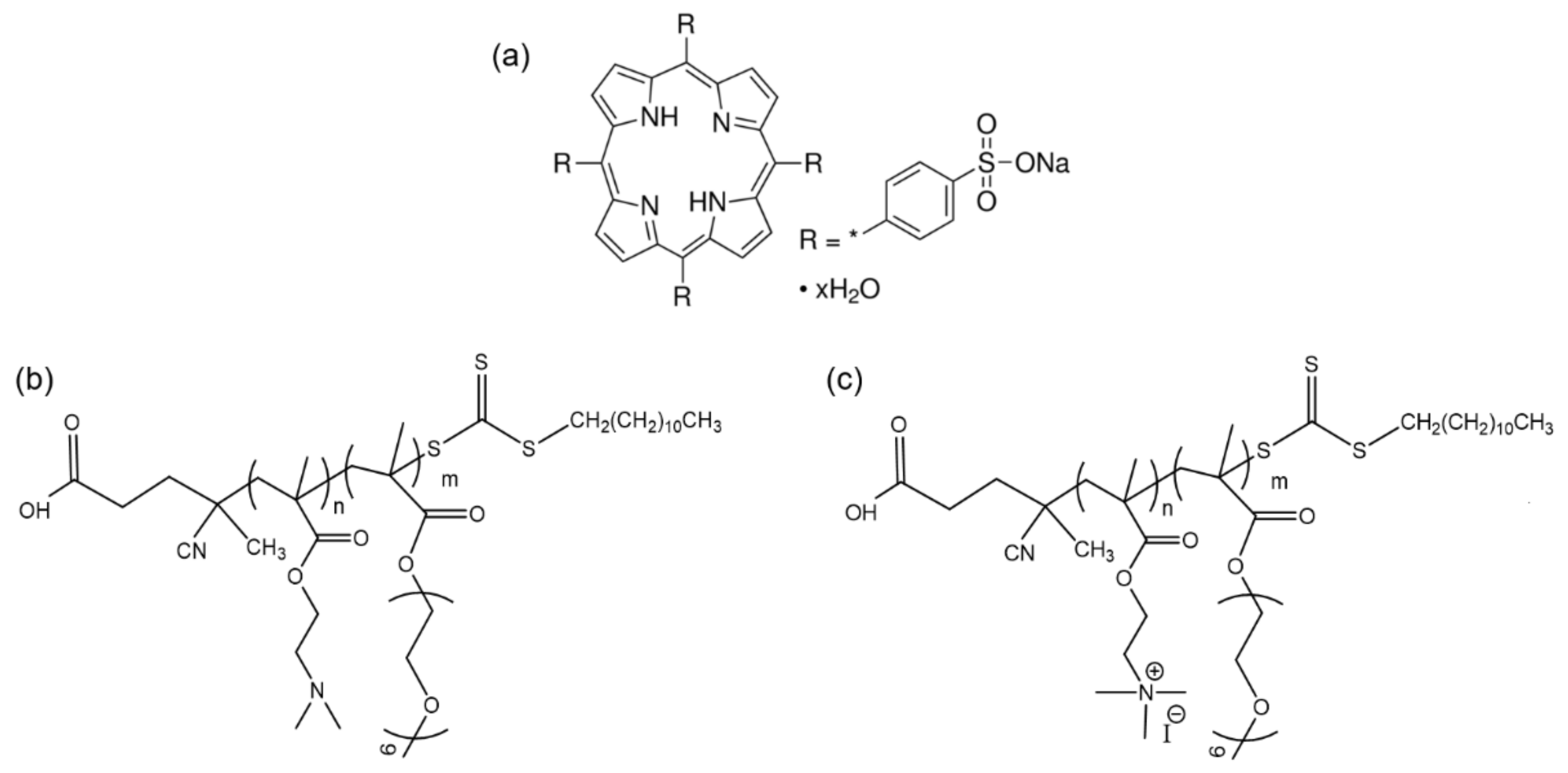



| Copolymer | Mw (104 g/mol) a | Mw/Mn a | (Q)PDMAEMA Content (wt%) | POEGMA Content (wt%) | (Q)PDMAEMA Monomeric Units | POEGMA Monomeric Units |
|---|---|---|---|---|---|---|
| PDMAEMA-b-POEGMA | 2.29 | 1.34 | 33 b | 67 b | 48 c | 32 c |
| QPDMAEMA-b-POEGMA | 2.97 | 48 d | 52 d |
| Sample Name | C(Q)DHBC (mg/mL) | CTPPS (mg/mL) | SO3−/NR2 or NR3+ a |
|---|---|---|---|
| (Q)Comp(2 + 0.4) | 0.2 | 0.021 | 20% |
| (Q)Comp(2 + 1) | 0.053 | 50% | |
| (Q)Comp(2 + 1.4) | 0.074 | 70% | |
| (Q)Comp(2 + 2) | 0.105 | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karayianni, M.; Koufi, D.; Pispas, S. Development of Double Hydrophilic Block Copolymer/Porphyrin Polyion Complex Micelles towards Photofunctional Nanoparticles. Polymers 2022, 14, 5186. https://doi.org/10.3390/polym14235186
Karayianni M, Koufi D, Pispas S. Development of Double Hydrophilic Block Copolymer/Porphyrin Polyion Complex Micelles towards Photofunctional Nanoparticles. Polymers. 2022; 14(23):5186. https://doi.org/10.3390/polym14235186
Chicago/Turabian StyleKarayianni, Maria, Dimitra Koufi, and Stergios Pispas. 2022. "Development of Double Hydrophilic Block Copolymer/Porphyrin Polyion Complex Micelles towards Photofunctional Nanoparticles" Polymers 14, no. 23: 5186. https://doi.org/10.3390/polym14235186







