Plasma-Initiated Grafting of Bioactive Peptide onto Nano-CuO/Tencel Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
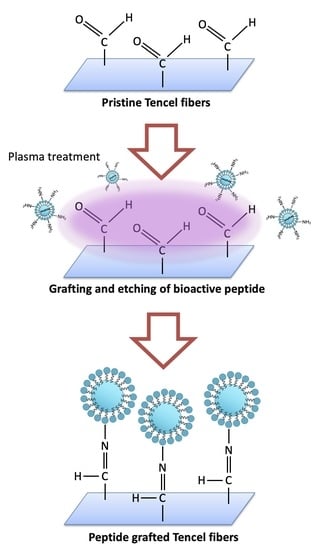
2.2. Membranes Fabrication
2.3. Scanning Electron Microscopy (SEM)
2.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.5. Contact Angle Measurements
2.6. Cytotoxicity Evaluation
2.7. Antimicrobial Assay
3. Results and Discussion
3.1. Surface Morphology of Nano-CuO/Tencel Membranes
3.2. FT-IR Analysis of Nano-CuO/Tencel Membranes
3.3. Cytotoxicity and Contact Angle Measurements of Nano-CuO/Tencel Membranes
3.4. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Gulland, A. Emergency Medicine: Lessons from the battlefield. BMJ 2008, 336, 1098–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Hohy-Eldin, M.S.; Hassan, M. Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin–streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, W.; Deng, W.; Xu, C.; Cai, Y.; Wang, X. Antibacterial and Hemostatic Thiol-Modified Chitosan-Immobilized AgNPs Composite Sponges. ACS Appl. Mater. Interfaces 2020, 12, 20307–20320. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional Tissue-Adhesive Cryogel Wound Dressing for Rapid Nonpressing Surface Hemorrhage and Wound Repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; Mertz, M. The effect of a new tissue-adhesive wound dressing on the healing of traumatic abrasions. Dermatology 2000, 201, 343–346. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Taguchi, T. Designing an anti-inflammatory and tissue-adhesive colloidal dressing for wound treatment. Colloids Surf. B Biointerfaces 2020, 188, 110737. [Google Scholar] [CrossRef]
- Kirwan, H.; Pignataro, R. The Skin and Wound Healing. In Pathology and Intervention in Musculoskeletal Rehabilitation, 2nd ed.; Elsevier: London, UK, 2016; pp. 25–62. [Google Scholar]
- Weller, C. Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Woodhead Publishing: Sawston, UK, 2009; pp. 97–113. [Google Scholar]
- Aderibigbe, B.; Byuana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Weigelt, M.A.; Sanchez, D.P.; Lev-Tov, H. Dressings and Wound Care Supplies for Hidradenitis Suppurativa. In A Comprehensive Guide to Hidradenitis Suppurativa; Elsevier: London, UK, 2022; pp. 201–207. [Google Scholar]
- Derbyshire, A. Using a silicone-based dressing as a primary wound contact layer. Br. J. Nurs. 2014, 23, S14–S20. [Google Scholar] [CrossRef]
- Morris, C.; Emsley, P.; Marland, E.; Meuleneire, F.; White, R. Use of wound dressings with soft silicone adhesive technology. Paediatr. Nurs. 2009, 21, 38–43. [Google Scholar] [PubMed] [Green Version]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M.J. Wound healing dressings and drug delivery systems: A review. Pharm. Sci. 2008, 97, 2892. [Google Scholar] [CrossRef]
- Jain, S.; Domb, A.J.; Kumar, N. Focal Controlled Drug Delivery; Domb, A.J., Khan, W., Eds.; Springer: Dordrecht, The Netherlands, 2014; p. 585. [Google Scholar]
- Kim, H. Moisture Vapor Permeability and Thermal Wear Comfort of Ecofriendly Fiber-Embedded Woven Fabrics for High-Performance Clothing. Materials 2021, 14, 6205. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.A.; El-Nahrawy, A.; Hassanin, A.; Morsy, M. Comfort and Protection Properties of Tencel/Cotton Blends. In Beltwide Cotton Conference Proceedings; National Cotton Council Omnipress: Madison, WI, USA, 2014. [Google Scholar]
- Gomez, M.; Alvarez, H.; Berdasco, A.; Andres, F. Paving the Way to Eco-Friendly IoT Antennas: Tencel-Based Ultra-Thin Compact Monopole and Its Applications to ZigBee. Sensors 2020, 20, 3658. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, J.G.; Nalakilli, G.; Shanmugasundaram, O.L.; Prakash, C. Moisture Management Properties of Bamboo Viscose/Tencel Single Jersey Knitted Fabrics. J. Nat. Fibers 2016, 14, 143–152. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, R. The Study of Moisture Absorption and Release Properties of Tencel Fiber. Adv. Mater. Res. 2012, 627, 138–142. [Google Scholar] [CrossRef]
- Firgo, H.; Schuster, K.C.; Suchomel, F.; Männer, J.; Burrow, T.; Abu-Rous, M. The functional properties of Tencel—A current update. Lenzing. Ber. 2006, 85, 22–30. [Google Scholar]
- Lou, C.W.; Lin, J.H.; Lai, M.F.; Huang, C.H.; Shiu, B.C. Lay-Up Compound Matrices for Application of Medical Protective Clothing: Manufacturing Techniques and Property Evaluations. Polymers 2022, 14, 1179. [Google Scholar] [CrossRef]
- Li, T.; Lou, C.; Chen, A.; Lee, M.; Ho, T.; Chen, Y.; Lin, J. Highly Absorbent Antibacterial Hemostatic Dressing for Healing Severe Hemorrhagic Wounds. Materials 2016, 9, 793. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Williams, G.R.; Wu, J.; Lv, Y.; Sun, X.; Wu, H.; Zhu, L.M. Thermosensitive nanofibers loaded with ciprofloxacin as antibacterial wound dressing materials. Int. J. Pharm. 2017, 517, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test 2020, 82, 106298. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Yang, S.; Liu, P.; Zhang, B. Electrospun PCL/mupirocin and chitosan/lidocaine hydrochloride multifunctional double layer nanofibrous scaffolds for wound dressing applications. Int. J. Nanomed. 2018, 13, 5287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasubbu, D.A.; Smith, V.; Hayden, F.; Cronin, P. Systemic antibiotics for treating malignant wounds. Cochrane Database Syst. Rev. 2017, 8, CD011609. [Google Scholar] [CrossRef]
- Marchant, J. When antibiotics turn toxic. Nature 2018, 555, 431–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Jung, H.C.; Baek, Y.J.; Kim, B.Y.; Lee, M.W.; Kim, H.D.; Kim, S.W. Antibacterial Activity of Green-Synthesized Silver Nanoparticles Using Areca catechu Extract against Antibiotic-Resistant Bacteria. Nanomaterials 2021, 11, 205. [Google Scholar] [CrossRef]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef]
- Tormena, R.P.; Rosa, E.V.; Mota, B.; Chaker, J.; Fagg, C.W.; Freire, D.O.; Martins, P.M.; Rodrigues da Silva, I.C.; Sousa, M.H. Evaluation of the antimicrobial activity of silver nanoparticles obtained by microwave-assisted green synthesis using Handroanthus impetiginosus (Mart. ex DC.) Mattos underbark extract. RSC Adv. 2020, 10, 20676–20681. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Sathiyaraj, S.; Suriyakala, G.; Gandhi, A.D.; Babujanarthanam, R.; Almaary, K.S.; Chen, T.W.; Kaviyarasu, K. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J. Infect. Public Health 2021, 14, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, K.S.; Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- de Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance; InTechOpen: London, UK, 2020. [Google Scholar]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-J.; Seo, Y.-B.; Seo, J.-Y.; Ryu, J.-H.; Ahn, H.-J.; Kim, K.-M.; Kwon, J.-S.; Choi, S.-H. Development of a Bioactive Flowable Resin Composite Containing a Zinc-Doped Phosphate-Based Glass. Nanomaterials 2020, 10, 2311. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Liang, J.; Cui, L.; Li, J.; Guan, S.; Zhang, K.; Li, J. Aloe vera: A Medicinal Plant Used in Skin Wound Healing. Tissue Eng. Part B Rev. 2021, 27, 455–474. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835. [Google Scholar] [CrossRef] [Green Version]
- Gniadecki, R. Regulation of keratinocyte proliferation. Gen. Pharmacol. 1998, 30, 619. [Google Scholar] [CrossRef]
- Gupta, A.; Keddie, D.J.; Kannappan, V.; Gibson, H.; Khalil, I.R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Mutlu, G.; Calamak, S.; Ulubayram, K.; Guven, E. Curcumin-loaded electrospun PHBV nanofibers as potential wound-dressing material. J. Drug Deliv. Sci. Technol. 2018, 43, 185–193. [Google Scholar] [CrossRef]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol. 2019, 122, 452–460. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, Y.; Chen, W.; Wei, Q. Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mater. Sci. Eng. C 2020, 109, 110536. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S. Electrospun Nanofibrous Membranes with Essential Oils for Wound Dressing Applications. Fibers Polym. 2020, 21, 999–1012. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly Porous and Superabsorbent Biomaterial Made of Marine-Derived Polysaccharides and Ascorbic Acid as an Optimal Dressing for Exuding Wound Management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef]
- Farzanfar, S.; Kouzekonan, G.S.; Mirjani, R.; Shekarchi, B. Vitamin B12-loaded polycaprolacton/gelatin nanofibrous scaffold as potential wound care material. Biomed. Eng. Lett. 2020, 10, 547–554. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Sahrapeyma, H.; Ghorbani, S. A promising wound dressing based on alginate hydrogels containing vitamin D3 cross-linked by calcium carbonate/d-glucono-δ-lactone. Biomed. Eng. Lett. 2020, 10, 309–319. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Williams, G.R.; Wu, J.; Sun, X.; Lv, Y.; Zhu, L.-M. Electrospun gelatin nanofibers loaded with vitamins A and E as antibacterial wound dressing materials. RSC Adv. 2016, 6, 50267–50277. [Google Scholar] [CrossRef]
- Eulálio, H.Y.C.; Vieira, M.; Fideles, T.B.; Tomás, H.; Silva, S.M.L.; Peniche, C.A.; Fook, M.V.L. Physicochemical Properties and Cell Viability of Shrimp Chitosan Films as Affected by Film Casting Solvents. I-Potential Use as Wound Dressing. Materials 2020, 13, 5005. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, S.; Kny, E. Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chem. Eng. J. 2019, 358, 1262–1278. [Google Scholar] [CrossRef]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.C.; Wu, S.-L.; Tsai, C.-S. Green and Rapid Fabrication of Copper Oxide in Enhanced Electrode Liquid Phase Plasma System. Plasma Process. Polym. 2022, e2200078. [Google Scholar] [CrossRef]
- Lou, C.W.; Lu, C.T.; Chen, Y.S.; Lin, M.C.; Li, T.T.; Lin, J.H. The Primary Study on PLA/Tencel Nonwoven Fabric. Appl. Mech. Mater. 2012, 184–185, 1333–1336. [Google Scholar] [CrossRef]
- Gomathi, N.; Chanda, A.K.; Neogi, S. Atmospheric Plasma Treatment of Polymers for Biomedical Applications. In Atmospheric Pressure Plasma Treatment of Polymers: Relevance to Adhesion; Scrivener Publishing: Salem, MA, USA, 2013. [Google Scholar]
- Švorčík, V.; Hnatowicz, V. Properties of polymers modified by plasma discharge and ion beam. In Polymer Degradation and Stability; Nova Science Publishers: New York, NY, USA, 2007. [Google Scholar]
- Marletta, G.; Iacona, F. Chemical and Physical Property Modifications Induced by Ion Irradiation in Polymers. In Materials and Processes for Surface and Interface Engineering; Springer: Dordrecht, The Netherlands, 1995; pp. 597–640. [Google Scholar]
- Lewis, K.M.; Spazierer, D.; Urban, M.D.; Lin, L.; Redl, H.; Goppelt, A. Comparison of regenerated and non-regenerated oxidized cellulose hemostatic agents. Eur. Sur. 2013, 45, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Mengal, N.; Sahito, I.A.; Arbab, A.A.; Sun, K.C.; Qadir, M.B.; Memon, A.A.; Jeong, S.H. Fabrication of a flexible and conductive lyocell fabric decorated with graphene nanosheets as a stable electrode material. Carbohydr. Polym. 2016, 152, 19–25. [Google Scholar] [CrossRef]
- Bodade, A.; Taiwade, M.; Chaudhari, G. Bioelectrode based chitosan-nano copper oxide for application to lipase biosensor. J. Appl. Pharm. Res. 2017, 5, 30–39. [Google Scholar]
- Vidal, B.; Mello, M. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Martinez, G.; Millhauser, G. FTIR spectroscopy of alanine-based peptides: Assignment of the amide I′ modes for random coil and helix. J. Struct. Biol. 1995, 114, 23–27. [Google Scholar] [CrossRef]
- Meutter, J.; Goormaghtigh, E. Amino acid side chain contribution to protein FTIR spectra: Impact on secondary structure evaluation. Eur. Biophys. J. 2021, 50, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Vaideki, K.; Jayakumar, S.; Rajendran, R.; Thilagavathi, G. Investigation on the effect of RF air plasma and neem leaf extract treatment on the surface modification and antimicrobial activity of cotton fabric. Appl. Surf. Sci. 2008, 254, 2472–2478. [Google Scholar] [CrossRef]
- Clougherty, L.; Sousa, J.; Wyman, G. Notes-C=N Stretching Frequency in Infrared Spectra of Aromatic Azomethines. J. Org. Chem. 1957, 22, 462. [Google Scholar] [CrossRef]
- Tanvir, N.B.; Yurchenko, O.; Wilbertz, C.; Urban, G. Investigation of CO2 reaction with copper oxide nanoparticles for room temperature gas sensing. J. Mater. Chem. A 2016, 4, 5294–5302. [Google Scholar] [CrossRef] [Green Version]
- Nyquist, R.A.; Kagel, R.O. Infrared Spectra of Inorganic Compounds; Academic Press Inc.: New York, NY, USA; London, UK, 1971; p. 220. [Google Scholar]
- Huang, C.; Chen, Y.; Liu, H. Characterization of Composite Nano-Bioscaffolds Based on Collagen and Supercritical Fluids-Assisted Decellularized Fibrous Extracellular Matrix. Polymers 2021, 13, 4326. [Google Scholar] [CrossRef]
- Veleirinho, B.; Berti, F.V.; Dias, P.F.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Lopes-da-Silva, J.A. Manipulation of chemical composition and architecture of non-biodegradable poly(ethylene terephthalate)/chitosan fibrous scaffolds and their effects on L929 cell behavior. Mater. Sci. Eng. C 2013, 33, 37–46. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nair, S.V.; Furuike, T.; Tamura, H. Perspectives of Chitin and Chitosan Nanofibrous Scaffolds in Tissue Engineering. In Tissue Engineering; InTechOpen: London, UK, 2010. [Google Scholar]
- Rezaei, A.; Khanamani Falahati-Pour, S.; Mohammadizadeh, F.; Hajizadeh, M.R.; Mirzaei, M.R.; Khoshdel, A.; Fahmidehkar, M.A.; Mahmoodi, M. Effect of a Copper (II) Complex on The Induction of Apoptosis in Human Hepatocellular Carcinoma Cells. Asian Pac. J. Cancer Prev. 2018, 19, 2877–2884. [Google Scholar]
- Bhavana, S.; Kusuma, C.G.; Gubbiveeranna, V.; Sumachirayu, C.K.; Ravikumar, H.; Nagaraju, S. Green route synthesis of copper oxide nanoparticles using Vitex altissima [L] leaves extract and their potential anticancer activity against A549 cell lines and its apoptosis induction. Inorg. Nano-Met. Chem. 2022, 1, 2470. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Ameer, F.S.; Raval, Y.S.; Huang, G.; Tzeng, T.J.; Anker, J.N.; Brumaghim, J.L. Polyphenol effects on CuO-nanoparticle-mediated DNA damage, reactive oxygen species generation, and fibroblast cell death. Toxicol. Vitr. 2022, 78, 105252. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazal, A.; Ara, S.; Ishaq, M.T.; Sughra, K. Green Fabrication of Copper Oxide Nanoparticles: A Comparative Antibacterial Study against Gram-Positive and Gram-Negative Bacteria. Arab. J. Sci. Eng. 2022, 47, 523–533. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.C.; Teii, K.; Chang, C.C.; Matsumoto, S.; Rafailovich, M. Biocompatible Cubic Boron Nitride: A Noncytotoxic Ultrahard Material. Adv. Funct. Mater. 2020, 31, 2005066. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.-L.; Chen, G.-Y.; Shi, S.-C.; Yang, J.H.C. Plasma-Initiated Grafting of Bioactive Peptide onto Nano-CuO/Tencel Membrane. Polymers 2022, 14, 4497. https://doi.org/10.3390/polym14214497
Hu T-L, Chen G-Y, Shi S-C, Yang JHC. Plasma-Initiated Grafting of Bioactive Peptide onto Nano-CuO/Tencel Membrane. Polymers. 2022; 14(21):4497. https://doi.org/10.3390/polym14214497
Chicago/Turabian StyleHu, Tzer-Liang, Guan-Yu Chen, Shih-Chen Shi, and Jason Hsiao Chun Yang. 2022. "Plasma-Initiated Grafting of Bioactive Peptide onto Nano-CuO/Tencel Membrane" Polymers 14, no. 21: 4497. https://doi.org/10.3390/polym14214497