Supramolecular Structure and Mechanical Performance of κ-Carrageenan–Gelatin Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions and Gels
2.3. X-ray Powder Diffraction
2.4. Small-Angle X-ray Scattering
2.5. Atomic Force Microscopy (AFM)
2.6. Rheological Measurements
3. Results
3.1. PXRD Overview of the Hydrogel Phase State
3.2. SAXS Structural Characterization of Hydrogel Sol and Gel States
3.3. AFM Study of Gelatin and κ-Carrageenan–Gelatin Gels
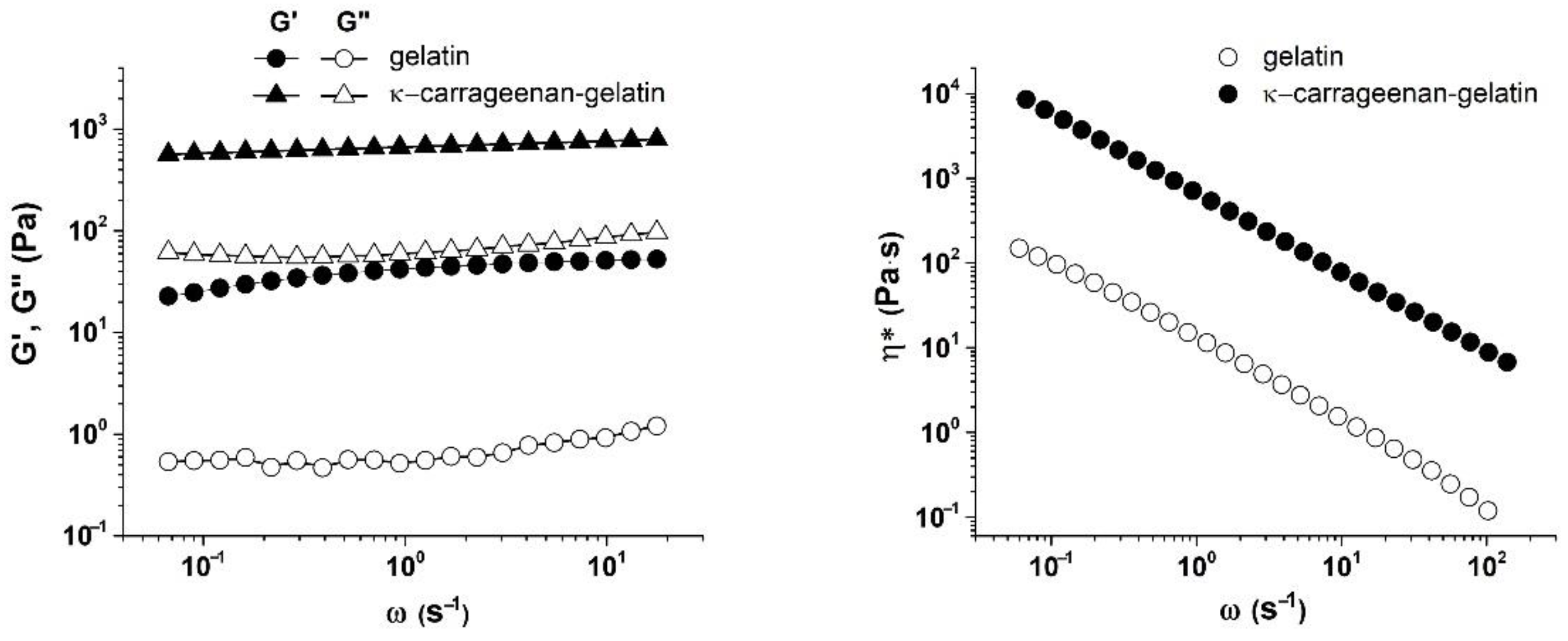
3.4. Rheological Characterization of Gelatin and κ-Carrageenan–Gelatin Gels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makshakova, O.N.; Zuev, Y.F. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels 2022, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, L.R.; Zelenikhin, P.V.; Makarova, A.O.; Zueva, O.S.; Salnikov, V.V.; Zuev, Y.F.; Ilinskaya, O.N. Alginate-Based Hydrogel as Delivery System for Therapeutic Bacterial RNase. Polymers 2022, 14, 2461. [Google Scholar] [CrossRef]
- Qi, X.; Su, T.; Zhang, M.; Tong, X.; Pan, W.; Zeng, Q.; Zhou, Z.; Shen, L.; He, X.; Shen, J. Macroporous Hydrogel Scaffolds with Tunable Physicochemical Properties for Tissue Engineering Constructed Using Renewable Polysaccharides. ACS Appl. Mater. Interfaces 2020, 12, 13256–13264. [Google Scholar] [CrossRef] [PubMed]
- Ozel, B.; Aydin, O.; Grunin, L.; Oztop, M.H. Physico-Chemical Changes of Composite Whey Protein Hydrogels in Simulated Gastric Fluid Conditions. J. Agric. Food Chem. 2018, 66, 9542–9555. [Google Scholar] [CrossRef] [PubMed]
- Farhoudi, N.; Leu, H.-Y.; Laurentius, L.B.; Magda, J.J.; Solzbacher, F.; Reiche, C.F. Smart Hydrogel Micromechanical Resonators with Ultrasound Readout for Biomedical Sensing. ACS Sens. 2020, 5, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Qian, C.; Higashigaki, T.; Asoh, T.-A.; Uyama, H. Anisotropic Conductive Hydrogels with High Water Content. ACS Appl. Mater. Interfaces 2020, 12, 27518–27525. [Google Scholar] [CrossRef] [PubMed]
- Yahia, L. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar] [CrossRef]
- Gubaidullin, A.T.; Makarova, A.O.; Derkach, S.R.; Voron’ko, N.G.; Kadyirov, A.I.; Ziganshina, S.A.; Salnikov, V.V.; Zueva, O.S.; Zuev, Y.F. Modulation of Molecular Structure and Mechanical Properties of κ-Carrageenan-Gelatin Hydrogel with Multi-Walled Carbon Nanotubes. Polymers 2022, 14, 2346. [Google Scholar] [CrossRef]
- Kanduč, M.; Kim, W.K.; Roa, R.; Dzubiella, J. How the Shape and Chemistry of Molecular Penetrants Control Responsive Hydrogel Permeability. ACS Nano 2021, 15, 614–624. [Google Scholar] [CrossRef]
- Singh, S.R. Advanced Hydrogels for Biomedical Applications. BJSTR 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Guvendiren, M.; Burdick, J.A. Stiffening Hydrogels to Probe Short- and Long-Term Cellular Responses to Dynamic Mechanics. Nat. Commun. 2012, 3, 792. [Google Scholar] [CrossRef] [Green Version]
- Beckett, L.E.; Lewis, J.T.; Tonge, T.K.; Korley, L.T.J. Enhancement of the Mechanical Properties of Hydrogels with Continuous Fibrous Reinforcement. ACS Biomater. Sci. Eng. 2020, 6, 5453–5473. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular Structure and Properties of κ-Carrageenan-Gelatin Gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Yermak, I.M.; Khotimchenko, Y.S. Chemical Properties, Biological Activities and Applications of Carrageenans from Red Algae. Recent Adv. Mar. Technol. 2003, 9, 207–255. [Google Scholar]
- De France, K.J.; Chan, K.J.W.; Cranston, E.D.; Hoare, T. Enhanced Mechanical Properties in Cellulose Nanocrystal–Poly(Oligoethylene Glycol Methacrylate) Injectable Nanocomposite Hydrogels through Control of Physical and Chemical Cross-Linking. Biomacromolecules 2016, 17, 649–660. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Chen, X.; Cui, C.; Zhang, J.; Zhang, Q.; Zhang, Y.; Wang, S.; et al. H-Bonding Supramolecular Hydrogels with Promising Mechanical Strength and Shape Memory Properties for Postoperative Antiadhesion Application. ACS Appl. Mater. Interfaces 2020, 12, 34161–34169. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhao, H.; Huang, C.; Du, Y. Mechanically and Electrically Enhanced CNT–Collagen Hydrogels As Potential Scaffolds for Engineered Cardiac Constructs. ACS Biomater. Sci. Eng. 2017, 3, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Huang, W.; Chen, L. Soluble Pea Protein Aggregates Form Strong Gels in the Presence of κ-Carrageenan. ACS Food Sci. Technol. 2021, 1, 1605–1614. [Google Scholar] [CrossRef]
- Tytgat, L.; Vagenende, M.; Declercq, H.; Martins, J.C.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Van Vlierberghe, S. Synergistic Effect of κ-Carrageenan and Gelatin Blends towards Adipose Tissue Engineering. Carbohydr. Polym. 2018, 189, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Lu, L.; Li, X. Enzymatic and Ionic Crosslinked Gelatin/K-Carrageenan IPN Hydrogels as Potential Biomaterials. J. Appl. Polym. Sci. 2014, 131, 21. [Google Scholar] [CrossRef]
- Padhi, J.R.; Nayak, D.; Nanda, A.; Rauta, P.R.; Ashe, S.; Nayak, B. Development of Highly Biocompatible Gelatin & I-Carrageenan Based Composite Hydrogels: In Depth Physiochemical Analysis for Biomedical Applications. Carbohydr. Polym. 2016, 153, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, L.; Zhang, K.; Fang, Y.; Nishinari, K.; Phillips, G.O. Mapping the Complex Phase Behaviors of Aqueous Mixtures of κ-Carrageenan and Type B Gelatin. J. Phys. Chem. B 2015, 119, 9982–9992. [Google Scholar] [CrossRef] [PubMed]
- Shimada, R.; Kumeno, K.; Akabane, H.; Nakahama, N. Gelation and Melting of a Mixed Carrageenan-Gelatin Gel. J. Home Econ. Jpn. 1993, 44, 999–1005. [Google Scholar] [CrossRef]
- DIFFRAC Plus Evaluation Package EVA, Version 11, User’s Manual; Bruker AXS: Karlsruhe, Germany, 2005.
- TOPAS V3: General Profile and Structure Analysis Software for Powder Diffraction Data, Technical Reference; Bruker AXS: Karlsruhe, Germany, 2005.
- Small Angle X-Ray Scattering, Version 4.0. Software Reference Manual, M86-E00005-0600; Bruker AXS Inc.: Madison, WI, USA, 2000.
- UMD, UTK, NIST, ORNL, ISIS, ESS, ILL. 2009. Available online: https://zenodo.org/record/825675#.Y0o3lUxBxPY (accessed on 30 August 2022).
- Konarev, P.V.; Volkov, V.V.; Sokolova, A.V.; Koch, M.H.J.; Svergun, D.I. PRIMUS: A Windows PC-Based System for Small-Angle Scattering Data Analysis. J. Appl. Crystallogr. 2003, 36, 1277–1282. [Google Scholar] [CrossRef]
- Evmenenko, G.; Theunissen, E.; Mortensen, K.; Reynaers, H. SANS Study of Surfactant Ordering in κ-Carrageenan/Cetylpyridinium Chloride Complexes. Polymer 2001, 42, 2907–2913. [Google Scholar] [CrossRef]
- Shibayama, M.; Tanaka, T.; Han, C.C. Small Angle Neutron Scattering Study on Poly(N-isopropyl Acrylamide) Gels near Their Volume-phase Transition Temperature. J. Chem. Phys. 1992, 97, 6829–6841. [Google Scholar] [CrossRef]
- Yeh, F.; Sokolov, E.L.; Walter, T.; Chu, B. Structure Studies of Poly(Diallyldimethylammonium Chloride- Co -Acrylamide) Gels/Sodium Dodecyl Sulfate Complex. Langmuir 1998, 14, 4350–4358. [Google Scholar] [CrossRef]
- Zhuang, C.; Tao, F.; Cui, Y. Anti-Degradation Gelatin Films Crosslinked by Active Ester Based on Cellulose. RSC Adv. 2015, 5, 52183–52193. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; He, Y.-G.; Ren, F.-X.; Wang, G. Synthesis, Characterization, and Applied Properties of Carboxymethyl Cellulose and Polyacrylamide Graft Copolymer. Carbohydr. Polym. 2009, 78, 95–99. [Google Scholar] [CrossRef]
- Guinier, A.; Fournet, G. Small-Angle Scattering of X-Rays; Wiley: New York, NY, USA, 1955. [Google Scholar]
- Wei, Y.; Hore, M.J.A. Characterizing Polymer Structure with Small-Angle Neutron Scattering: A Tutorial. J. Appl. Phys. 2021, 129, 171101. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y. A Review of the Application of Atomic Force Microscopy (AFM) in Food Science and Technology. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 62, pp. 201–240. ISBN 978-0-12-385989-1. [Google Scholar]
- Yang, Z.; Yang, H.; Yang, H. Effects of Sucrose Addition on the Rheology and Microstructure of κ-Carrageenan Gel. Food Hydrocoll. 2018, 75, 164–173. [Google Scholar] [CrossRef]
- Ferry, J.D.; Rice, S.A. Viscoelastic Properties of Polymers. Phys. Today 1962, 15, 76–78. [Google Scholar] [CrossRef] [Green Version]
- Rial, R.; Soltero, J.F.A.; Verdes, P.V.; Liu, Z.; Ruso, J.M. Mechanical Properties of Composite Hydrogels for Tissue Engineering. CTMC 2018, 18, 1214–1223. [Google Scholar] [CrossRef]
- Zhu, W.; Chu, C.; Kuddannaya, S.; Yuan, Y.; Walczak, P.; Singh, A.; Song, X.; Bulte, J.W.M. In Vivo Imaging of Composite Hydrogel Scaffold Degradation Using CEST MRI and Two-Color NIR Imaging. Adv. Funct. Mater. 2019, 29, 1903753. [Google Scholar] [CrossRef]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The Rheology of Gelatin Hydrogels Modified by κ-Carrageenan. LWT Food Sci. Technol. 2015, 63, 612–619. [Google Scholar] [CrossRef]
- He, Q.; Yu, W.; Wu, Y.; Zhou, C. Shear Induced Phase Inversion of Dilute Smectic Liquid Crystal/Polymer Blends. Soft Matter 2012, 8, 2992. [Google Scholar] [CrossRef]
- Pan, A.; Roy, S.G.; Haldar, U.; Mahapatra, R.D.; Harper, G.R.; Low, W.L.; De, P.; Hardy, J.G. Uptake and Release of Species from Carbohydrate Containing Organogels and Hydrogels. Gels 2019, 5, 43. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Z.; Wang, J.; Yu, W. Stress Bifurcation in Large Amplitude Oscillatory Shear of Yield Stress Fluids. J. Rheol. 2018, 62, 89–106. [Google Scholar] [CrossRef]
- Malkin, A.; Kulichikhin, V.; Ilyin, S. A Modern Look on Yield Stress Fluids. Rheol. Acta 2017, 56, 177–188. [Google Scholar] [CrossRef]
- Chae, B.S.; Lee, Y.S.; Jhon, M.S. The Scaling Behavior of a Highly Aggregated Colloidal Suspension Microstructure and Its Change in Shear Flow. Colloid Polym. Sci. 2004, 282, 236–242. [Google Scholar] [CrossRef]
- Nishinari, K. Rheological and DSC Study of Sol-Gel Transition in Aqueous Dispersions of Industrially Important Polymers and Colloids. Colloid. Polym. Sci. 1997, 275, 1093–1107. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S.; Voron’ko, N.G. Polyelectrolyte Polysaccharide–Gelatin Complexes: Rheology and Structure. Polymers 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Goudoulas, T.B.; Germann, N. Phase Transition Kinetics and Rheology of Gelatin-Alginate Mixtures. Food Hydrocoll. 2017, 66, 49–60. [Google Scholar] [CrossRef]
- Picard, J.; Giraudier, S.; Larreta-Garde, V. Controlled Remodeling of a Protein-Polysaccharide Mixed Gel: Examples of Gelatin-Hyaluronic Acid Mixtures. Soft Matter 2009, 5, 4198. [Google Scholar] [CrossRef]
- Dobrynin, A.V. Solutions of Charged Polymers. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 81–132. ISBN 978-0-08-087862-1. [Google Scholar]
- Ali, I.; Shah, L.A. Rheological Investigation of the Viscoelastic Thixotropic Behavior of Synthesized Polyethylene Glycol-Modified Polyacrylamide Hydrogels Using Different Accelerators. Polym. Bull. 2021, 78, 1275–1291. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Faizullin, D.A.; Zuev, Y.F. Interplay between Secondary Structure and Ion Binding upon Thermoreversible Gelation of κ-Carrageenan. Carbohydr. Polym. 2020, 227, 115342. [Google Scholar] [CrossRef]

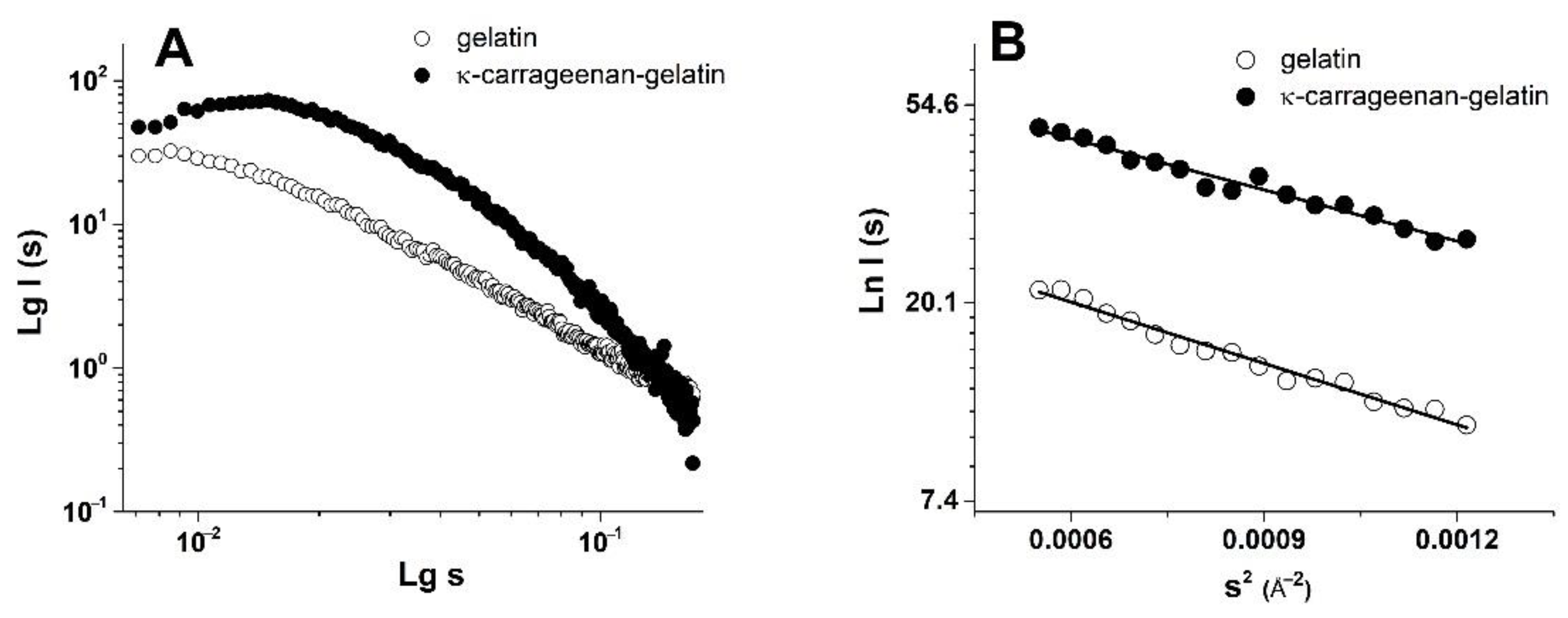
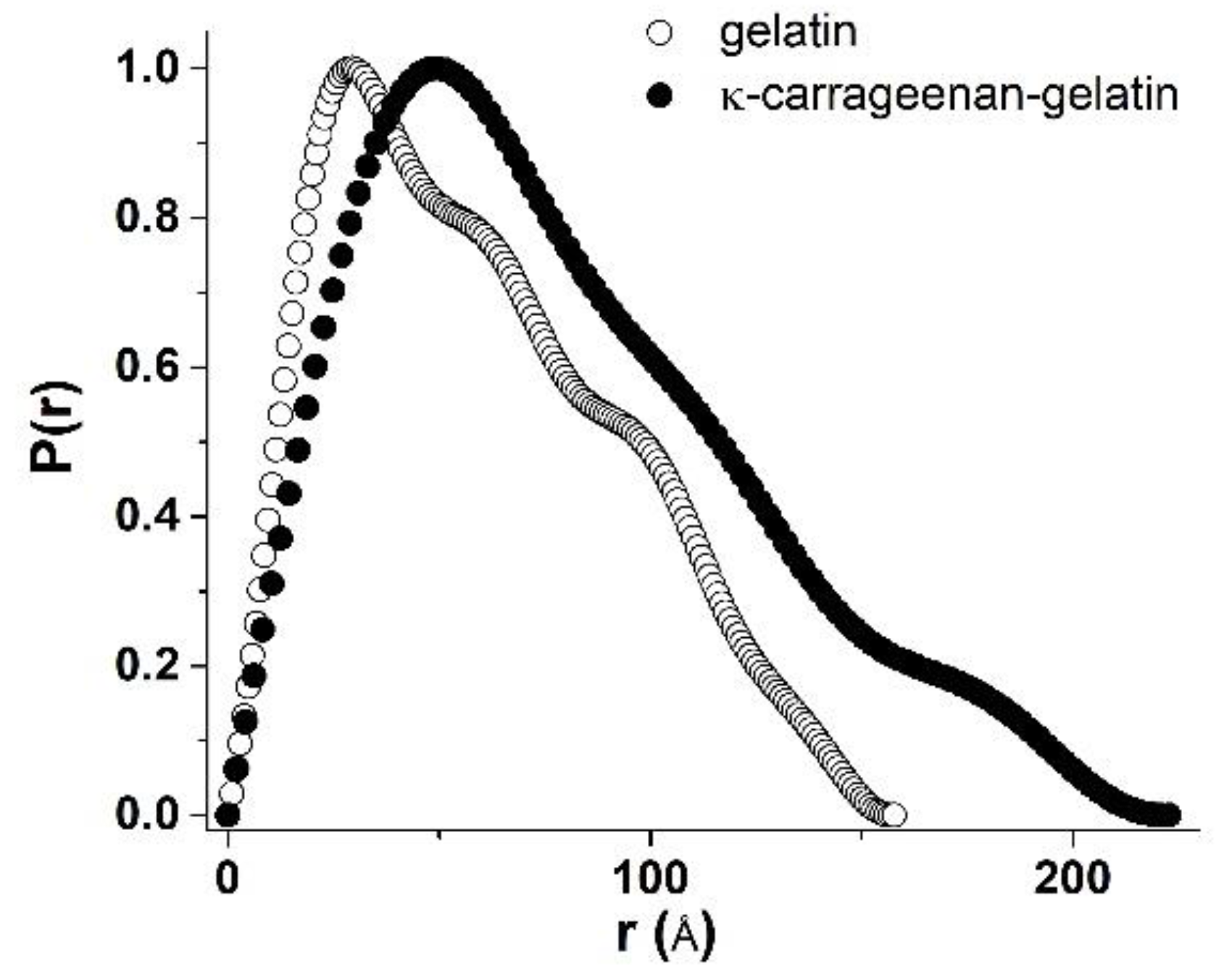
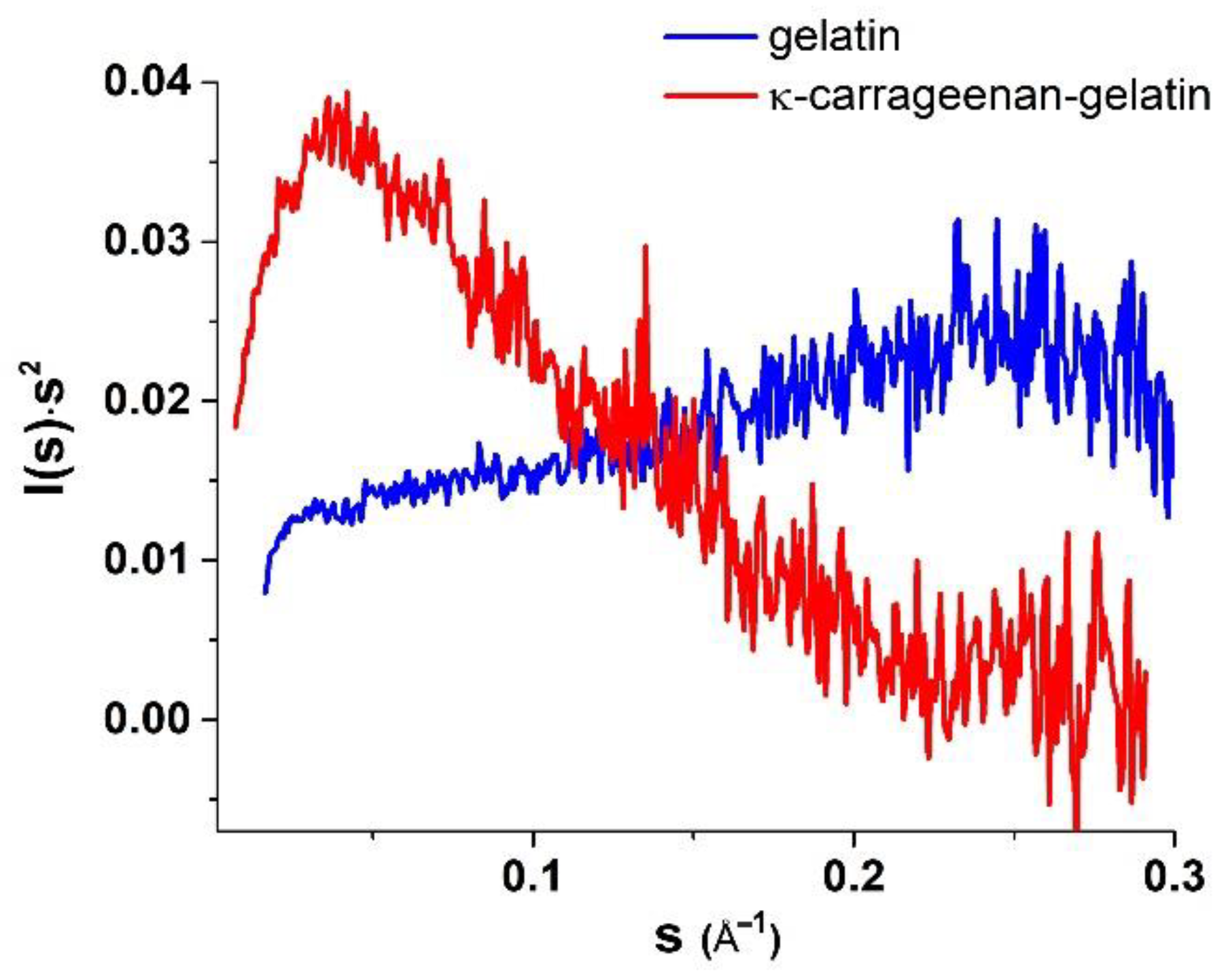
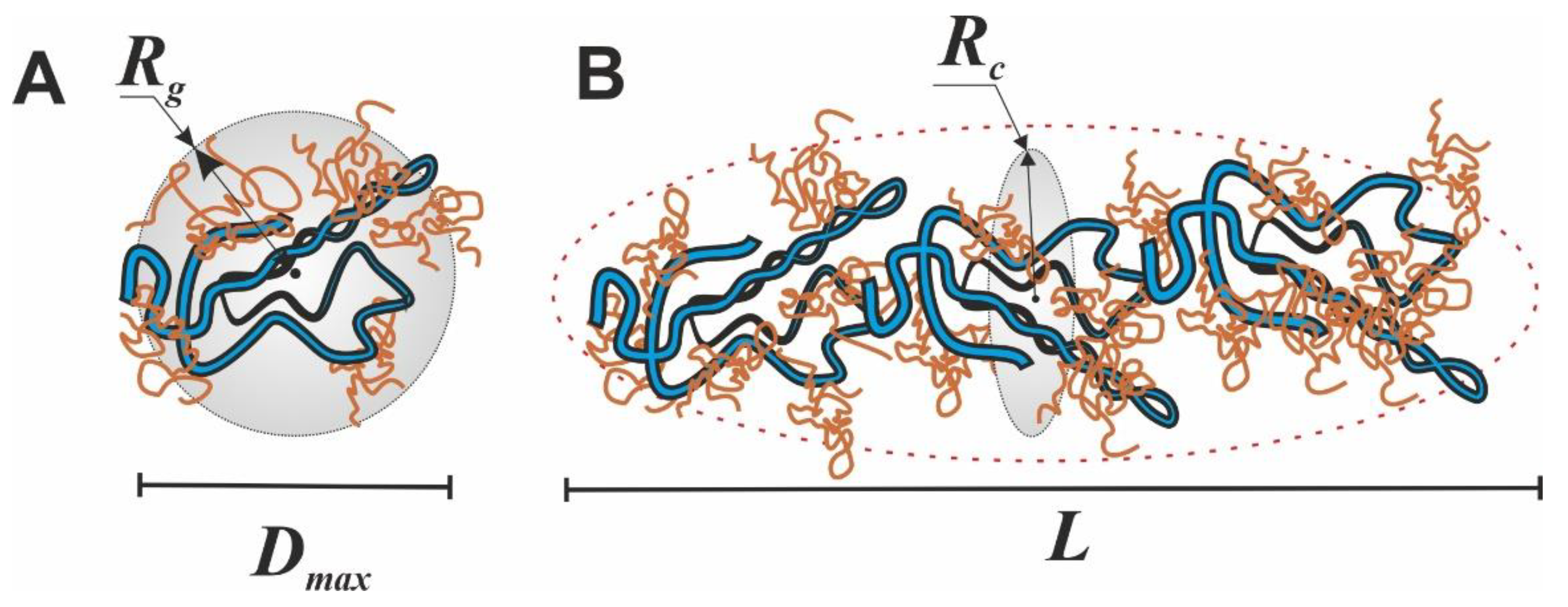




| Sample | Guinier Analysis | P(r) Analysis | |||||
|---|---|---|---|---|---|---|---|
| Rg, Å | Rsph, Å | Rc, Å | rc, Å | L, Å | Rg, Å | Dmax, Å | |
| gelatin | 47.5 | 61.3 | – | – | – | 42.5 | 149 |
| κ-carrageenan-gelatin | 56 | 72.3 | 21.2 | 27.4 | 179.5 | 63.4 | 222.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarova, A.O.; Derkach, S.R.; Kadyirov, A.I.; Ziganshina, S.A.; Kazantseva, M.A.; Zueva, O.S.; Gubaidullin, A.T.; Zuev, Y.F. Supramolecular Structure and Mechanical Performance of κ-Carrageenan–Gelatin Gel. Polymers 2022, 14, 4347. https://doi.org/10.3390/polym14204347
Makarova AO, Derkach SR, Kadyirov AI, Ziganshina SA, Kazantseva MA, Zueva OS, Gubaidullin AT, Zuev YF. Supramolecular Structure and Mechanical Performance of κ-Carrageenan–Gelatin Gel. Polymers. 2022; 14(20):4347. https://doi.org/10.3390/polym14204347
Chicago/Turabian StyleMakarova, Anastasiya O., Svetlana R. Derkach, Aidar I. Kadyirov, Sufia A. Ziganshina, Mariia A. Kazantseva, Olga S. Zueva, Aidar T. Gubaidullin, and Yuriy F. Zuev. 2022. "Supramolecular Structure and Mechanical Performance of κ-Carrageenan–Gelatin Gel" Polymers 14, no. 20: 4347. https://doi.org/10.3390/polym14204347








