Crystallization Behavior of Isotactic Propene-Octene Random Copolymers
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gahleitner, M.; Tranninger, C.; Doshev, P. Polypropylene Copolymers. In Polypropylene Handbook, Morphology, Blends and Composites; Karger-Kocsis, J., Bárány, T., Eds.; Springer Nature: Cham, Switzerland, 2019; Chapter 6; p. 295. [Google Scholar]
- Galli, P.; Haylock, J.C.; Simonazzi, T. Manufacturing and properties of polypropylene copolymers. In Polypropylene: Structure, Blends and Composites. Vol. 2 Copolymers and Blends; Karger-Kocsis, J., Ed.; Chapman & Hall: London, UK, 1995; pp. 1–24. [Google Scholar]
- Pasquini, N. (Ed.) Polypropylene Handbook; Hanser Publishers: Munich, Germany, 2005. [Google Scholar]
- De Rosa, C.; Auriemma, F.; Ruiz de Ballesteros, O.; Resconi, L.; Camurati, I. Tailoring the Physical Properties of Isotactic Polypropylene through Incorporation of Comonomers and the Precise Control of Stereo- and Regio-Regularity by Metallocene Catalysts. Chem. Mater. 2007, 19, 5122. [Google Scholar] [CrossRef]
- De Rosa, C.; Scoti, M.; Di Girolamo, R.; Ruiz de Ballesteros, O.; Auriemma, F.; Malafronte, A. Polymorphism in polymers: A tool to tailor material’s properties. Polym. Cryst. 2020, 3, e10101. [Google Scholar] [CrossRef]
- Turner-Jones, A. Development of the γ-crystal form in random copolymers of propylene and their analysis by DSC and X-ray methods. Polymer 1971, 12, 487–508. [Google Scholar] [CrossRef]
- Cimmino, S.; Martuscelli, E.; Nicolais, L.; Silvestre, C. Thermal and mechanical properties of isotactic random propylene-butene-1 copolymers. Polymer 1978, 19, 1222. [Google Scholar] [CrossRef]
- Crispino, L.; Martuscelli, E.; Pracella, M. Influence of composition on the melt crystallization of isotactic random propylene/1-butene copolymers. Makromol. Chem. 1980, 181, 1747. [Google Scholar] [CrossRef]
- Cavallo, P.; Martuscelli, E.; Pracella, M. Effect of thermal treatment on solution grown crystals of isotactic propylene/butene-1 copolymers. Polymer 1997, 18, 891–896. [Google Scholar] [CrossRef]
- Starkweather, H.W., Jr.; Van-Catledge, F.A.; MacDonald, R.N. Crystalline order in copolymers of ethylene and propylene. Macromolecules 1982, 15, 1600–1604. [Google Scholar] [CrossRef]
- Guidetti, G.P.; Busi, P.; Giulianetti, I.; Zanetti, R. Structure-properties relationships in some random copolymers of propylene. Eur. Polym. J. 1983, 19, 757–759. [Google Scholar] [CrossRef]
- Busico, V.; Corradini, P.; De Rosa, C.; Di Benedetto, E. Physico-chemical and structural characterization of ethylene-propene copolymers with low ethylene content from isotactic-specific Ziegler-Natta catalysts. Eur. Polym. J. 1985, 21, 239–244. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Della Volpe, G.; Segre, A.; Rossi, E.; Simonazzi, T. Composition-properties relationships in propene-ethene random copolymers obtained with high-yield Ziegler-Natta supported catalysts. Makromol. Chem. 1986, 187, 1927. [Google Scholar] [CrossRef]
- Marigo, A.; Marega, C.; Zanetti, R.; Paganetto, G.; Canossa, E.; Coletta, F.; Gottardi, F. Crystallization of the γ-form of isotactic poly(propylene). Makromol. Chem. 1989, 190, 2805. [Google Scholar] [CrossRef]
- Monasse, B.; Haudin, J.M. Effect of random copolymerization on growth transition and morphology change in polypropylene. Colloid Polym. Sci. 1988, 266, 679–687. [Google Scholar] [CrossRef]
- Xu, Z.K.; Feng, L.X.; Wang, D.; Yang, S.L. Copolymerization of propene with 1-alkenes using a MgCl2/TiCl4 catalyst. Makromol. Chem. 1991, 192, 1835–1840. [Google Scholar] [CrossRef]
- Yang, S.L.; Xu, Z.K.; Feng, L.X. Copolymerization of propene with high-1-olefin using a MgCl2/TiCl4 catalyst. Makromol. Chem. Macromol. Symp. 1992, 63, 233–243. [Google Scholar] [CrossRef]
- Zimmermann, H.J. Structural analysis of random propylene-ethylene copolymers. J. Macromol. Sci. Phys. 1993, 32, 141–161. [Google Scholar] [CrossRef]
- Sugano, T.; Gotoh, Y.; Fujita, T. Effect of catalyst isospecificity on the copolymerization of propene with 1-hexene. Makromol. Chem. 1992, 193, 43. [Google Scholar] [CrossRef]
- Hingmann, R.; Rieger, J.; Kersting, M. Rheological Properties of a Partially Molten Polypropylene Random Copolymer during Annealing. Macromolecules 1995, 28, 3801–3806. [Google Scholar] [CrossRef]
- Morini, G.; Albizzati, E.; Balbontin, G.; Mingozzi, I.; Sacchi, M.C.; Forlini, F.; Tritto, I. Microstructure Distribution of Polypropylenes Obtained in the Presence of Traditional Phthalate/Silane and Novel Diether Donors: A Tool for Understanding the Role of Electron Donors in MgCl2-Supported Ziegler−Natta Catalysts. Macromolecules 1996, 29, 5770. [Google Scholar] [CrossRef]
- Pérez, E.; Benavente, R.; Bello, A.; Pereña, J.M.; Zucchi, D.; Sacchi, M.C. Crystallization behavior of fractions of a copolymer of propene and 1-hexene. Polymer 1997, 38, 5411. [Google Scholar] [CrossRef]
- Laihonen, S.; Gedde, U.W.; Werner, P.-E.; Martinez-Salazar, J. Crystallization kinetics and morphology of poly(propylene-stat-ethylene) fractions. Polymer 1997, 38, 361–369. [Google Scholar] [CrossRef]
- Laihomen, S.; Gedde, U.W.; Werner, P.E.; Westdahl, M.; Jääskeläinen, P.; Martinez-Salazar, J. Crystal structure and morphology of melt-crystallized poly(propylene-stat-ethylene) fractions. Polymer 1997, 38, 371–377. [Google Scholar] [CrossRef]
- Abiru, T.; Mizuno, A.; Weigand, F. Microstructural characterization of propylene–butene-1 copolymer using temperature rising elution fractionation. J. Appl. Polym. Sci. 1998, 68, 1493–1501. [Google Scholar] [CrossRef]
- Feng, Y.; Jin, X.; Hay, J.N. Crystalline structure of propylene–ethylene copolymer fractions. J. Appl. Polym. Sci. 1998, 68, 381–386. [Google Scholar] [CrossRef]
- Feng, Y.; Hay, J.N. The characterization of random propylene–ethylene copolymer. Polymer 1998, 39, 6589–6596. [Google Scholar] [CrossRef]
- Xu, J.; Feng, Y. Application of temperature rising elution fractionation in polyolefins. Eur. Polym. J. 2000, 36, 867. [Google Scholar] [CrossRef]
- Zhao, Y.; Vaughan, A.S.; Sutton, S.J.; Swingler, S.G. On nucleation and the evolution of morphology in a propylene/ethylene copolymer. Polymer 2001, 42, 6599–6608. [Google Scholar] [CrossRef]
- Foresta, T.; Piccarolo, S.; Goldbeck-Wood, G. Competition between α and γ phases in isotactic polypropylene: Effects of ethylene content and nucleating agents at different cooling rates. Polymer 2001, 42, 1167–1176. [Google Scholar] [CrossRef]
- Marega, C.; Marigo, A.; Saini, R.; Ferrari, P. The influence of thermal treatment and processing on the structure and morphology of poly(propylene-ran-1-butene) copolymers. Polym. Int. 2001, 50, 442. [Google Scholar] [CrossRef]
- Marigo, A.; Causin, V.; Marega, C.; Ferrari, P. Crystallization of the γ form in random propylene-ethylene copolymers. Polym. Int. 2004, 53, 2001–2008. [Google Scholar] [CrossRef]
- Dimenska, A.; Phillips, P.J. High pressure crystallization of random propylene–ethylene copolymers: α–γ Phase diagram. Polymer 2006, 47, 5445–5456. [Google Scholar] [CrossRef]
- Arnold, M.; Henschke, O.; Knorr, J. Copolymerization of propene and higher α-olefins with the metallocene catalyst Et[Ind]2HfCl2/methylaluminoxane. Macromol. Chem. Phys. 1996, 197, 563–573. [Google Scholar] [CrossRef]
- Arnold, M.; Bornemann, S.; Köller, F.; Menke, T.J.; Kressler, J. Synthesis and characterization of branched polypropenes obtained by metallocene catalysis. Macromol. Chem. Phys. 1998, 199, 2647–2653. [Google Scholar] [CrossRef]
- Galimberti, M.; Destro, M.; Fusco, O.; Piemontesi, F.; Camurati, I. Ethene/Propene Copolymerization from Metallocene-Based Catalytic Systems: Role of the Alumoxane. Macromolecules 1999, 32, 258–263. [Google Scholar] [CrossRef]
- Busse, K.; Kressler, J.; Maier, R.D.; Scherble, J. Tailoring of the α-, β-, and γ-Modification in Isotactic Polypropene and Propene/Ethene Random Copolymers. Macromolecules 2000, 33, 8775–8780. [Google Scholar] [CrossRef]
- Forlini, F.; Fan, Z.-Q.; Tritto, I.; Locatelli, P.; Sacchi, M.C. Metallocene-catalyzed propene 1-hexene copolymerization-influence of amount and bulkiness of cocatalyst and of solvent polarity. Macromol. Chem. Phys. 1997, 198, 2397. [Google Scholar] [CrossRef]
- Tritto, I.; Donetti, R.; Sacchi, M.C.; Locatelli, P.; Zannoni, G. Evidence of Zircononium−Polymeryl Ion Pairs from 13C NMR in Situ 13C2H4 Polymerization with Cp2Zr(13CH3)2-Based Catalysts. Macromolecules 1999, 32, 264–269. [Google Scholar] [CrossRef]
- Pérez, E.; Zucchi, D.; Sacchi, M.C.; Forlini, F.; Bello, A. Obtaining the γ phase in isotactic polypropylene: Effect of catalyst system and crystallization conditions. Polymer 1999, 40, 675–681. [Google Scholar] [CrossRef]
- Forlini, F.; Tritto, I.; Locatelli, P.; Sacchi, M.C.; Piemontesi, F. 13C NMR studies of zirconocene-catalyzed propylene/1-hexene copolymers: In-depth investigation of the effect of solvent polarity. Macromol. Chem. Phys. 2000, 201, 401–408. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Forlini, F.; Losio, S.; Tritto, I.; Wahner, U.M.; Tincul, I.; Joubert, D.J.; Sadiku, E.R. Microstructure of Metallocene-Catalyzed Propene/1-Pentene Copolymers. Macromol. Chem. Phys. 2003, 204, 1643. [Google Scholar] [CrossRef]
- Wahner, U.M.; Tincul, I.; Joubert, D.J.; Sadiku, E.R.; Forlini, F.; Losio, S.; Tritto, I.; Sacchi, M.C. 13C NMR Study of Copolymers of Propene with Higher 1-Olefins with New Microstructures by ansa-Zirconocene Catalysts. Macromol. Chem. Phys. 2003, 204, 1738. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Stagnaro, P. Unexpected Formation of Atactic Blocks in Propylene/1-Pentene Copolymers from rac-Me2Si(2-MeBenz[e]Ind)2ZrCl2. Macromol. Chem. Phys. 2004, 205, 1804. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Forlini, F.; Losio, S.; Tritto, I.; Costa, G.; Stagnaro, P.; Tincul, I.; Wahner, U.M. Microstructural characteristics and thermal properties of ansa-zirconocene catalyzed copolymers of propene with higher α-olefins. Macromol. Symp. 2004, 213, 57. [Google Scholar] [CrossRef]
- Costa, G.; Stagnaro, P.; Trefiletti, V.; Sacchi, M.C.; Forlini, F.; Alfonso, G.C.; Tincul, I.; Wahner, U.M. Thermal Behavior and Structural Features of Propene/1-Pentene Copolymers by Metallocene Catalysts. Macromol. Chem. Phys. 2004, 205, 383–389. [Google Scholar] [CrossRef]
- Stagnaro, P.; Costa, G.; Trefiletti, V.; Canetti, M.; Forlini, F.; Alfonso, G.C. Thermal Behavior, Structure and Morphology of Propene/Higher 1-Olefin Copolymers. Macromol. Chem. Phys. 2006, 207, 2128–2141. [Google Scholar] [CrossRef]
- Stagnaro, P.; Boragno, L.; Canetti, M.; Forlini, F.; Azzurri, F.; Alfonso, G.C. Crystallization and morphology of the trigonal form in random propene/1-pentene copolymers. Polymer 2009, 50, 5242–5249. [Google Scholar] [CrossRef]
- Kim, I. Copolymerization of propene and 1-hexene using metallocene amide compounds. Macromol. Rapid Commun. 1998, 19, 299. [Google Scholar] [CrossRef]
- Kim, I.; Kim, Y.J. Copolymerization of propene and 1-hexene with isospecific and syndiospecific metallocene catalysts. Polym. Bull. 1998, 40, 415–421. [Google Scholar] [CrossRef]
- Alamo, R.G.; VanderHart, D.L.; Nyden, M.R.; Mandelkern, L. Morphological Partitioning of Ethylene Defects in Random Propylene−Ethylene Copolymers. Macromolecules 2000, 33, 6094–6105. [Google Scholar] [CrossRef]
- Hosier, I.L.; Alamo, R.G.; Esteso, P.; Isasi, G.R.; Mandelkern, L. Formation of the α and γ Polymorphs in Random Metallocene-Propylene Copolymers. Effect of Concentration and Type of Comonomer. Macromolecules 2003, 36, 5623–5636. [Google Scholar] [CrossRef]
- Hosier, I.L.; Alamo, R.G.; Lin, J.S. Lamellar morphology of random metallocene propylene copolymers studied by atomic force microscopy. Polymer 2004, 45, 3441–3455. [Google Scholar] [CrossRef]
- Alamo, R.G.; Ghosal, A.; Chatterjee, J.; Thompson, K.L. Linear growth rates of random propylene ethylene copolymers. The change over from γ dominated growth to mixed (α+γ) polymorphic growth. Polymer 2005, 46, 8774–8789. [Google Scholar] [CrossRef]
- Fan, Z.; Yasin, T.; Feng, L. Copolymerization of propylene with 1-octene catalyzed by rac-Me2Si(2,4,6-Me3-Ind)2ZrCl2/methyl aluminoxane. J. Polym. Sci. Part A 2000, 38, 4299. [Google Scholar] [CrossRef]
- Brull, R.; Pasch, H.; Rauberheimer, H.G.; Sanderson, R.D.; Wahner, U.M. Polymerization of higher linear α-olefins with (CH3)2Si(2-methylbenz[e]indenyl)2ZrCl2. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2333–2339. [Google Scholar] [CrossRef]
- Van Reenen, A.J.; Brull, R.; Wahner, U.M.; Raubenheimer, H.G.; Sanderson, R.D.; Pasch, H. The copolymerization of propylene with higher, linear α-olefins. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4110–4118. [Google Scholar] [CrossRef]
- Lovisi, H.; Tavares, M.I.B.; da Silva, N.M.; de Menezes, S.M.C.; de Santa Maria, L.C.; Coutinho, F.M.B. Influence of comonomer content and short branch length on the physical properties of metallocene propylene copolymers. Polymer 2001, 42, 9791–9799. [Google Scholar] [CrossRef]
- Shin, Y.-W.; Uozumi, T.; Terano, M.; Nitta, K.-H. Synthesis and characterization of ethylene–propylene random copolymers with isotactic propylene sequence. Polymer 2001, 42, 9611–9615. [Google Scholar] [CrossRef]
- Shin, Y.-W.; Hashiguchi, H.; Terano, M.; Nitta, K. Synthesis and characterization of propylene-α-olefin random copolymers with isotactic propylene sequence. II. Propylene– hexene-1 random copolymers. J. Appl. Polym. Sci. 2004, 92, 2949. [Google Scholar] [CrossRef]
- Hosoda, S.; Hori, H.; Yada, K.; Tsuji, M.; Nakahara, S. Degree of comonomer inclusion into lamella crystal for propylene/olefin copolymers. Polymer 2002, 43, 7451–7460. [Google Scholar] [CrossRef]
- Fujiyama, M.; Inata, H. Crystallization and melting characteristics of metallocene isotactic polypropylenes. J. Appl. Polym. Sci. 2002, 85, 1851–1857. [Google Scholar] [CrossRef]
- Xu, J.-T.; Xue, L.; Fan, Z.-Q. Nonisothermal crystallization of metallocene propylene–decene- 1 copolymers. J. Appl. Polym. Sci. 2004, 93, 1724–1730. [Google Scholar] [CrossRef]
- Poon, B.; Rogunova, M.; Chum, S.P.; Hiltner, A.; Baer, E. Classification of Homogeneous Copolymers of Propylene and 1-Octene Based on Comonomer Content. J. Polym. Sci. Polym. Phys. 2004, 42, 4357–4370. [Google Scholar] [CrossRef]
- Poon, B.; Rogunova, M.; Hiltner, A.; Baer, E.; Chum, S.P.; Galeski, A.; Piorkowska, E. Structure and Properties of Homogeneous Copolymers of Propylene and 1-Hexene. Macromolecules 2005, 38, 1232–1243. [Google Scholar] [CrossRef]
- Palza, H.; López-Majada, J.M.; Quijada, R.; Benavente, R.; Pérez, E.; Cerrada, M.L. Metallocenic Copolymers of Isotactic Propylene and 1-Octadecene: Crystalline Structure and Mechanical Behavior. Macromol. Chem. Phys. 2005, 206, 1221–1230. [Google Scholar] [CrossRef]
- López-Majada, J.M.; Palza, H.; Guevara, J.L.; Quijada, R.; Martinez, M.C.; Benavente, R.; Pereña, J.M.; Pérez, E.; Cerrada, M.L. Metallocene Copolymers of Propene and 1-Hexene: The Influence of the Comonomer Content and Thermal History on the Structure and Mechanical Properties. J. Polym. Sci. Polym. Phys. Ed. 2006, 44, 1253–1267. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Corradini, P.; Tarallo, O.; Dello Iacono, S.; Ciaccia, E.; Resconi, L. Crystal Structure of the Trigonal Form of Isotactic Polypropylene as an Example of Density-Driven Polymer Structure. J. Am. Chem. Soc. 2006, 128, 80–81. [Google Scholar] [CrossRef]
- De Rosa, C.; Dello Iacono, S.; Auriemma, F.; Ciaccia, E.; Resconi, L. Crystal Structure of Isotactic Propylene-Hexene Copolymers: The Trigonal Form of Isotactic Polypropylene. Macromolecules 2006, 39, 6098–6109. [Google Scholar] [CrossRef]
- Lotz, B.; Ruan, J.; Thierry, A.; Alfonso, G.C.; Hiltner, A.; Baer, E.; Piorkowska, E.; Galeski, A. A Structure of Copolymers of Propene and Hexene Isomorphous to Isotactic Poly(1-butene) Form I. Macromolecules 2006, 39, 5777–5781. [Google Scholar] [CrossRef]
- Toki, S.; Sics, I.; Burger, C.; Fang, D.; Liu, L.; Hsiao, B.S.; Datta, S.; Tsou, A.H. Structure Evolution during Cyclic Deformation of an Elastic Propylene-Based Ethylene-Propylene Copolymer. Macromolecules 2006, 39, 3588–3597. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Ruiz de Ballesteros, O.; Resconi, L.; Camurati, I. Crystallization Behavior of Isotactic Propylene-Ethylene and Propylene-Butene Copolymers: Effect of Comonomers versus Stereodefects on Crystallization Properties of Isotactic Polypropylene. Macromolecules 2007, 40, 6600–6616. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Talarico, G.; Ruiz de Ballesteros, O. Structure of Isotactic Propylene-Pentene Copolymers. Macromolecules 2007, 40, 8531–8532. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Ruiz de Ballesteros, O.; De Luca, D.; Resconi, L. The double role of comonomers on the crystallization behavior of isotactic polypropylene: Propylene-hexene Copolymers. Macromolecules 2008, 41, 2172–2177. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Ruiz de Ballesteros, O.; Dello Iacono, S.; De Luca, D.; Resconi, L. Stress-induced polymorphic transformations and mechanical properties of isotactic propylene-hexene copolymers. Cryst. Grow Des. 2009, 9, 165–176. [Google Scholar] [CrossRef]
- Palza, H.; López-Majada, J.M.; Quijada, R.; Pereña, J.M.; Benavente, R.; Pérez, E.; Cerrada, M.L. Comonomer Length Influence on the Structure and Mechanical Response of Metallocenic Polypropylenic Materials. Macromol. Chem. Phys. 2008, 209, 2259–2267. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Polo-Corpa, M.J.; Benavente, R.; Pérez, E.; Velilla, T.; Quijada, R. Formation of the new trigonal polymorph in iPP-1-hexene copolymers. Competition with the mesomorphic phase. Macromolecules 2009, 42, 702–708. [Google Scholar] [CrossRef]
- Polo-Corpa, M.J.; Benavente, R.; Velilla, T.; Quijada, R.; Pérez, E.; Cerrada, M.L. Development of the mesomorphic phase in isotactic propene/higher α-olefin copolymers at intermediate comonomer content and its effect on properties. Eur. Polym. J. 2010, 46, 1345–1354. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Di Girolamo, R.; Romano, L.; De Luca, R.M. A New Mesophase of Isotactic Polypropylene in Copolymers of Propylene with Long Branched Comonomers. Macromolecules 2010, 43, 8559–8569. [Google Scholar] [CrossRef]
- Pérez, E.; Cerrada, M.L.; Benavente, R.; Gómez-Elvira, J.M. Enhancing the formation of the new trigonal polymorph in isotactic propene-1-pentene copolymers: Determination of the X-ray crystallinity. Macromol. Res. 2011, 19, 1179–1185. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Vollaro, P.; Resconi, L.; Guidotti, S.; Camurati, I. Crystallization Behavior of Propylene-Butene Copolymers: The Trigonal Form of Isotactic Polypropylene and Form I of Isotactic Poly(1-butene). Macromolecules 2011, 44, 540–549. [Google Scholar] [CrossRef]
- De Rosa, C.; Ruiz de Ballesteros, O.; Auriemma, F.; Di Caprio, M.R. Crystal Structure of the Trigonal Form of Isotactic Propylene−Pentene Copolymers: An Example of the Principle of Entropy−Density Driven Phase Formation in Polymers. Macromolecules 2012, 45, 2749–2763. [Google Scholar] [CrossRef]
- Pérez, E.; Gómez-Elvira, J.M.; Benavente, R.; Cerrada, M.L. Tailoring the formation rate of the mesophase in random propylene-co-1-pentene copolymers. Macromolecules 2012, 45, 6481–6490. [Google Scholar] [CrossRef]
- Boragno, L.; Stagnaro, P.; Forlini, F.; Azzurri, F.; Alfonso, G.C. The trigonal form of i-PP in random C3/C5/C6 terpolymers. Polymer 2013, 54, 1656–1662. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Elvira, J.M.; Pérez, E.; Cerrada, M.L. Isotactic poly(propylene-co-1-pentene-co-1-hexene) terpolymers: Synthesis, molecular characterization, and evidence of the trigonal polymorph. J. Polym. Sci. A Polym. Chem. 2013, 51, 3251–3259. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Elvira, J.M.; Barranco-García, R.; Pérez, E.; Cerrada, M.L. Trigonal δ form as a tool for tuning mechanical behavior in poly(propylene-co-1-pentene-co-1-heptene) terpolymers. Polymer 2016, 99, 112–121. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Elvira, J.M.; Lorenzo, V.; Pérez, E.; Cerrada, M.L. Unprecedented dependence of stiffness parameters and crystallinity on comonomer content in rapidly cooled propylene-co-1-pentene copolymers. Polymer 2017, 130, 17–25. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Di Girolamo, R.; Malafronte, A.; Scoti, M.; Cipullo, R. Yield behavior of random copolymers of isotactic polypropylene. Polymer 2017, 129, 235–246. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Di Girolamo, R.; Malafronte, A.; Scoti, M.; Cioce, C. A molecular view of properties of random copolymers of isotactic polypropene. Adv. Polym. Sci. 2017, 276, 45–92. [Google Scholar]
- De Rosa, C.; Scoti, M.; Auriemma, F.; Ruiz de Ballesteros, O.; Talarico, G.; Malafronte, A.; Di Girolamo, R. Mechanical Properties and Morphology of Propene−Pentene Isotactic Copolymers. Macromolecules 2018, 51, 3030–3040. [Google Scholar] [CrossRef]
- De Rosa, C.; Scoti, M.; Auriemma, F.; Ruiz de Ballesteros, O.; Talarico, G.; Di Girolamo, R.; Cipullo, R. Relationships among lamellar morphology parameters, structure and thermal behavior of isotactic propene-pentene copolymers: The role of incorporation of comonomeric units in the crystals. Eur. Polym. J. 2018, 103, 251–259. [Google Scholar] [CrossRef]
- De Rosa, C.; Scoti, M.; Ruiz de Ballesteros, O.; Di Girolamo, R.; Auriemma, F.; Malafronte, A. Propylene-Butene Copolymers: Tailoring Mechanical Properties from Isotactic Polypropylene to Polybutene. Macromolecules 2020, 53, 4407–4421. [Google Scholar] [CrossRef]
- Scoti, M.; De Stefano, F.; Di Girolamo, R.; Talarico, G.; Malafronte, A.; De Rosa, C. Crystallization of Propene-Pentene Isotactic Copolymers as an Indicator of the General View of the Crystallization Behavior of Isotactic Polypropylene. Macromolecules 2022, 55, 241–251. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Bárány, T. Polypropylene Handbook, Morphology, Blends and Composites; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Lotz, B.; Graff, S.; Wittmann, J.C. Crystal morphology of the γ (triclinic) phase of isotactic polypropylene and its relation to the α phase. J. Polym. Sci. Polym. Phys. Ed. 1986, 24, 2017. [Google Scholar] [CrossRef]
- Kardos, J.L.; Christiansen, A.W.; Baer, E. Structure of pressure crystallized polypropylene. J. Polym. Sci. Part B Polym. Phys. 1966, 4, 777–788. [Google Scholar] [CrossRef]
- Pal, K.D.; Morrow, D.R.; Sauer, J.A. Interior morphology of bulk polypropylene. Nature 1966, 211, 514–515. [Google Scholar]
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene: III. The equilibrium melting point and the phase diagram. Polymer 1998, 39, 3735. [Google Scholar] [CrossRef]
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene. II. The morphology of the γ-form crystallized at 200 MPa. Polymer 1997, 38, 5725. [Google Scholar] [CrossRef]
- Brückner, S.; Phillips, P.J.; Mezghani, K.; Meille, S.V. On the crystallization of γ-isotactic polypropylene. A high pressure study. Macromol. Rapid Commun. 1997, 18, 1–7. [Google Scholar] [CrossRef]
- Ewen, J.A. Mechanisms of stereochemical control in propylene polymerizations with soluble Group 4B metallocene/methylalumoxane catalysts. J. Am. Chem. Soc. 1984, 106, 6355. [Google Scholar] [CrossRef]
- Kaminsky, W.; Kulper, K.; Brintzinger, H.H.; Wild, F.R.W. Polymerization of propene and butene with a chiral zirconocene and methylaluminoxane as cocatalyst. Angew. Chem. 1985, 97, 507–508. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D.; Mulhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Fischer, D.; Mülhaupt, R. The influence of regio-and stereoirregularities on the crystallization behavior of isotactic poly(propylene)s prepared with homogeneous group IVa metallocene/methylaluminoxane Ziegler-Natta catalysts. Macromol. Chem. Phys. 1994, 195, 1433. [Google Scholar] [CrossRef]
- Thomann, R.; Wang, C.; Kressler, J.; Mülhaupt, R. On the γ-Phase of Isotactic Poypropylene. Macromolecules 1996, 29, 8425–8434. [Google Scholar] [CrossRef]
- Thomann, R.; Semke, H.; Maier, R.D.; Thomann, Y.; Scherble, J.; Mülhaupt, R.; Kressler, J. Influence of stereoirregularities on the formation of the γ-phase in isotactic polypropylene. Polymer 2001, 42, 4597–4603. [Google Scholar] [CrossRef]
- Alamo, R.G.; Kim, M.H.; Galante, M.J.; Isasi, J.R.; Mandelkern, L. Structural and Kinetic Factors Governing the Formation of the γ Polymorph of Isotactic Polypropylene. Macromolecules 1999, 32, 4050–4064. [Google Scholar] [CrossRef]
- VanderHart, D.L.; Alamo, R.G.; Nyden, M.R.; Kim, M.H.; Mandelkern, L. Observation of Resonances Associated with Stereo and Regio Defects in the Crystalline Regions of Isotactic Polypropylene: Toward a Determination of Morphological Partioning. Macromolecules 2000, 33, 6078–6093. [Google Scholar] [CrossRef]
- Nyden, M.R.; Vanderhart, D.L.; Alamo, R.G. The conformational structures of defect-containing chains in the crystalline regions of isotactic polypropylene. Comput. Theor. Comput. Sci. 2001, 11, 175–189. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Di Capua, A.; Resconi, L.; Guidotti, S.; Camurati, I.; Nifant’ev, I.E.; Laishevtsev, I.P. Structure-property correlations in polypropylene from metallocene catalysts: Stereodefective, regioregular isotactic polypropylene. J. Am. Chem. Soc. 2004, 126, 17040–17049. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, C.; Auriemma, F.; Circelli, T.; Waymouth, R.M. Crystallization of the α and γ Forms of Isotactic Polypropylene as a Tool to Test the Degree of Segregation of Defects in the Polymer Chains. Macromolecules 2002, 35, 3622–3629. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C. Crystallization of Metallocene-Made Isotactic Polypropylene: Disordered Modifications Intermediate between the α and γ Forms. Macromolecules 2002, 35, 9057–9068. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Spera, C.; Talarico, G.; Tarallo, O. Comparison between Polymorphic Behaviors of Ziegler-Natta and Metallocene-Made Isotactic Polypropylene: The Role of the Distribution of Defects in the Polymer Chains. Macromolecules 2004, 37, 1441–1454. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Spera, C.; Talarico, G.; Gahleitner, M. Crystallization Properties of Elastomeric Polypropylene from Alumina-Supported Tetraalkyl Zirconium Catalysts. Polymer 2004, 45, 5875–5888. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Paolillo, M.; Resconi, L.; Camurati, I. Crystallization Behavior and Mechanical Properties of Regiodefective, Highly Stereoregular Isotactic Polypropylene: Effect of Regiodefects versus Stereodefects and Influence of the Molecular Mass. Macromolecules 2005, 38, 9143–9144. [Google Scholar] [CrossRef]
- Gahleitner, M.; Jääskeläinen, P.; Ratajski, E.; Paulik, C.; Reussner, J.; Wolfschwenger, J.; Neißl, W. Propylene-ethylene random copolymers: Comonomer effects on crystallinity and application properties. J. Appl. Polym. Sci. 2005, 95, 1073–1081. [Google Scholar] [CrossRef]
- Caveda, S.; Pérez, E.; Blázquez-Blázquez, E.; Peña, B.; van Grieken, R.; Suárez, I.; Benavente, R. Influence of structure on the properties of polypropylene copolymers and terpolymers. Polym.Test. 2017, 62, 23–32. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Di Girolamo, R.; Malafronte, A.; Scoti, M.; Mitchell, G.R.; Esposito, S. Relationship between molecular configuration and stress induced phase transitions. In Controlling the Morphology of Polymers-Multiple Scales of Structure and Processing; Mitchell, G.R., Tojeira, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; p. 287. [Google Scholar]
- Auriemma, F.; De Rosa, C.; Di Girolamo, R.; Malafronte, A.; Scoti, M.; Mitchell, G.R.; Esposito, S. Deformation of Stereoirregular Isotactic Polypropylene across Length Scales. Influence of Temperature. Macromolecules 2017, 50, 2856–2870. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Di Girolamo, R.; Malafronte, A.; Scoti, M.; Mitchell, G.R.; Esposito, S. Time-resolving study of stress-induced transformations of isotactic polypropylene through wide angle X-ray scattering measurements. Polymers 2018, 10, 162. [Google Scholar] [CrossRef]
- Alamo, R.G.; Blanco, J.A.; Agarwal, P.K.; Randall, J.C. Crystallization Rates of Matched Fractions of MgCl2-Supported Ziegler Natta and Metallocene Isotactic Poly(Propylene)s. 1. The Role of Chain Microstructure. Macromolecules 2003, 36, 1559–1571. [Google Scholar] [CrossRef]
- Randall, J.C.; Alamo, R.G.; Agarwal, P.K.; Ruff, C.J. Crystallization Rates of Matched Fractions of MgCl2-Supported Ziegler-Natta and Metallocene Isotactic Poly(propylene)s. 2. Chain Microstructures from a Supercritical Fluid Fractionation of a MgCl2-Supported Ziegler-Natta Isotactic Poly(propylene). Macromolecules 2003, 36, 1572–1584. [Google Scholar] [CrossRef]
- De Rosa, C.; Ruiz de Ballesteros, O.; Auriemma, F.; Talarico, G.; Scoti, M.; Di Girolamo, R.; Malafronte, A.; Piemontesi, F.; Liguori, D.; Camurati, I.; et al. Crystallization Behavior of Copolymers of Isotactic Poly(1-butene) with Ethylene from Ziegler-Natta Catalyst: Evidence of the Blocky Molecular Structure. Macromolecules 2019, 52, 9114–9127. [Google Scholar] [CrossRef]
- De Rosa, C.; Ruiz de Ballesteros, O.; Di Girolamo, R.; Malafronte, A.; Auriemma, F.; Talarico, G.; Scoti, M. The blocky structure of Ziegler-Natta “random” copolymers: Myths and experimental evidence. Polym. Chem. 2020, 11, 34–38. [Google Scholar] [CrossRef]
- Di Girolamo, R.; Santillo, C.; Malafronte, A.; Scoti, M.; De Stefano, F.; Talarico, G.; Coates, G.W.; De Rosa, C. Structure and morphology of isotactic polypropylene-polyethylene block copolymers prepared with living and stereoselective catalyst. Polym. Chem. 2022, 13, 2950–2963. [Google Scholar] [CrossRef]
- De Rosa, C.; Di Girolamo, R.; Auriemma, F.; Talarico, G.; Scarica, C.; Malafronte, A.; Scoti, M. Controlling Size and Orientation of Lamellar Microdomains in Crystalline Block Copolymers. ACS Appl. Mater. Interfaces 2017, 9, 31252–31259. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, C.; Di Girolamo, R.; Malafronte, A.; Scoti, M.; Talarico, G.; Auriemma, F.; Ruiz de Ballesteros, O. Polyolefins based crystalline block copolymers: Ordered nanostructures from control of crystallization. Polymer 2020, 196, 122423. [Google Scholar] [CrossRef]
- De Rosa, C.; Malafronte, A.; Di Girolamo, R.; Auriemma, F.; Scoti, M.; Ruiz de Ballesteros, O.; Coates, G.W. Morphology of Isotactic Polypropylene-Polyethylene Block Copolymers Driven by Controlled Crystallization. Macromolecules 2020, 53, 10234–10244. [Google Scholar] [CrossRef]
- De Rosa, C.; Di Girolamo, R.; Cicolella, A.; Talarico, G.; Scoti, M. Double Crystallization and Phase Separation in Polyethylene-Syndiotactic Polypropylene Di-Block Copolymers. Polymers 2021, 13, 2589. [Google Scholar] [CrossRef]
- Di Girolamo, R.; Cicolella, A.; Talarico, G.; Scoti, M.; De Stefano, F.; Giordano, A.; Malafronte, A.; De Rosa, C. Structure and Morphology of Crystalline Syndiotactic Polypropylene-Polyethylene Block Copolymers. Polymers 2022, 14, 1534. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Scoti, M.; Di Girolamo, R.; Malafronte, A.; Talarico, G.; Carnahan, E. Unveiling the Molecular Structure of Ethylene/1-Octene Multi-block Copolymers from Chain Shuttling Technology. Polymer 2018, 154, 298–304. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Scoti, M.; Di Girolamo, R.; Malafronte, A.; Galotto Galotto, N. Structural Investigation at Nanometric Length Scale of Ethylene/1-Octene Multi-block Copolymers from Chain Shuttling Technology. Macromolecules 2018, 51, 9613. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Scoti, M.; Di Girolamo, R.; Malafronte, A.; D’Alterio, M.C.; Boggioni, L.; Losio, S.; Boccia, A.C.; Tritto, I. Structure and Mechanical Properties of Ethylene/1-Octene Multi-block Copolymers from Chain Shuttling Technology. Macromolecules 2019, 52, 2669. [Google Scholar] [CrossRef]
- Spaleck, W.; Kuber, F.; Winter, A.; Rohrmann, J.; Bachmann, B.; Antberg, M.; Dolle, V.; Paulus, E. The influence of aromatic substituents on the polymerization behavior of bridged zirconocene catalysts. Organometallics 1994, 13, 954–963. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Brandolini, A.J. 13C NMR spectra of propylene/1-hexene copolymers. Macromolecules 1991, 24, 2632–2633. [Google Scholar] [CrossRef]
- Carman, C.J.; Harrington, R.A.; Wilkes, C.E. Monomer Sequence Distribution in Ethylene-Propylene Rubber Measured by 13C NMR. 3. Use of Reaction Probability Model. Macromolecules 1977, 10, 536–544. [Google Scholar] [CrossRef]
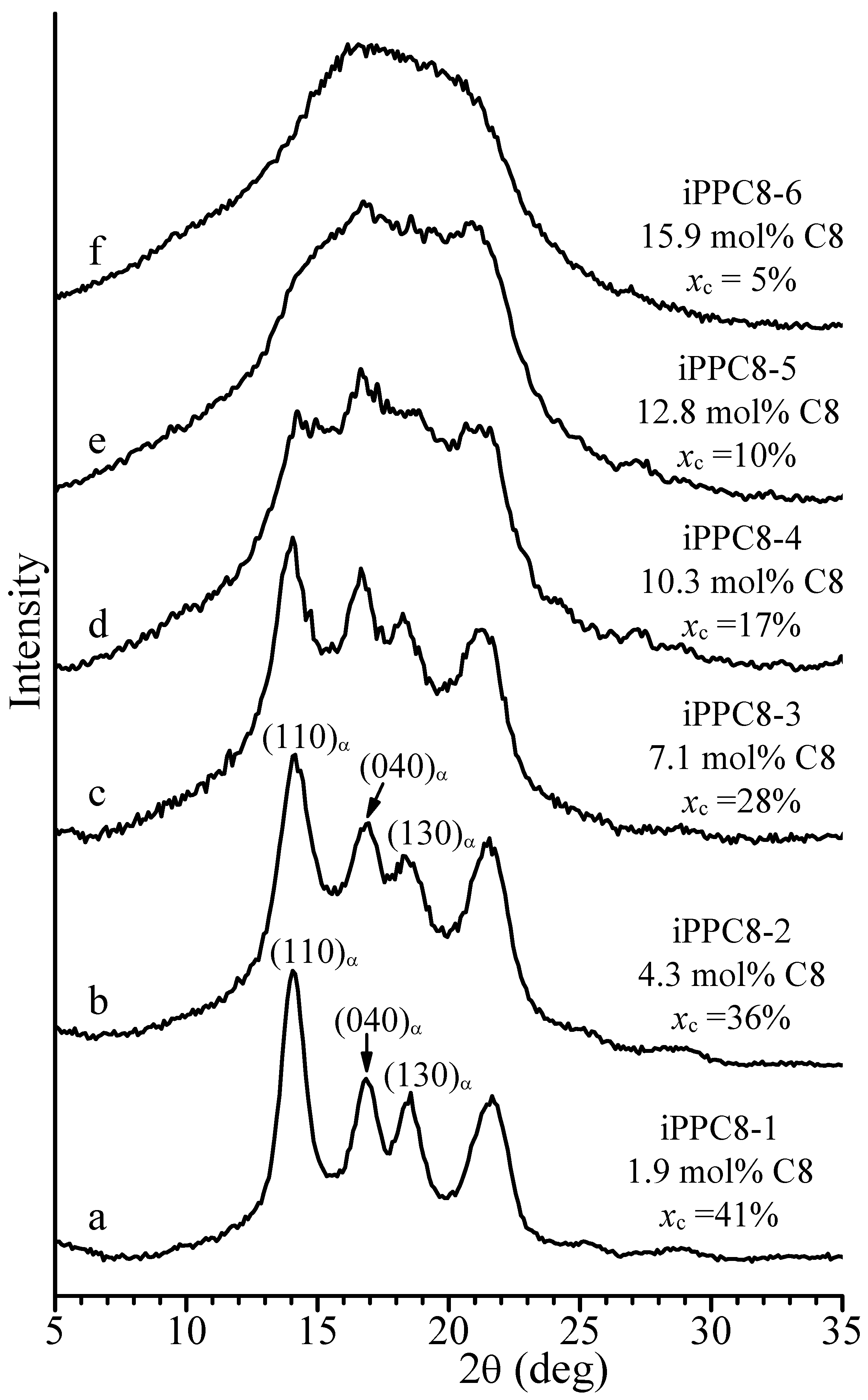
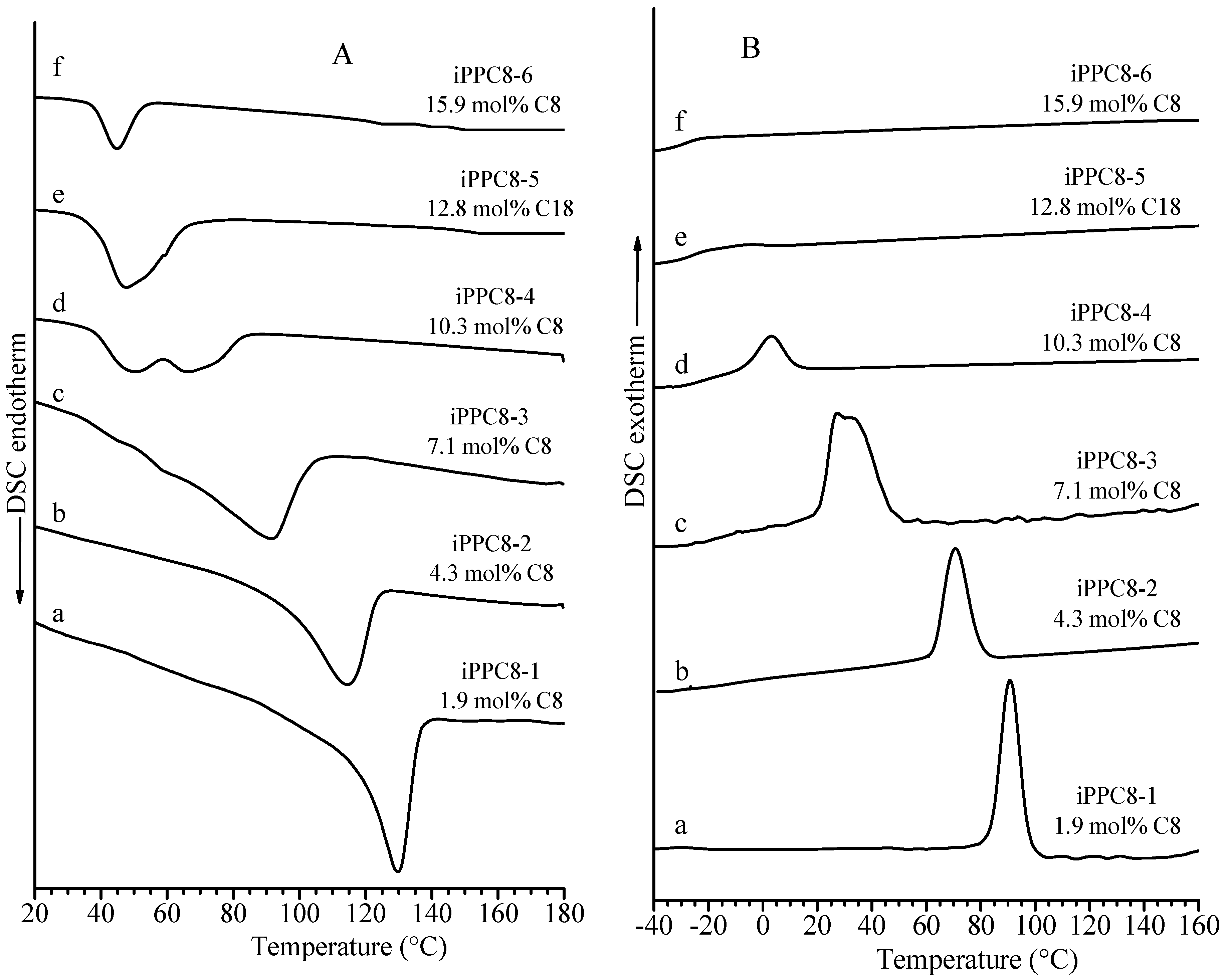
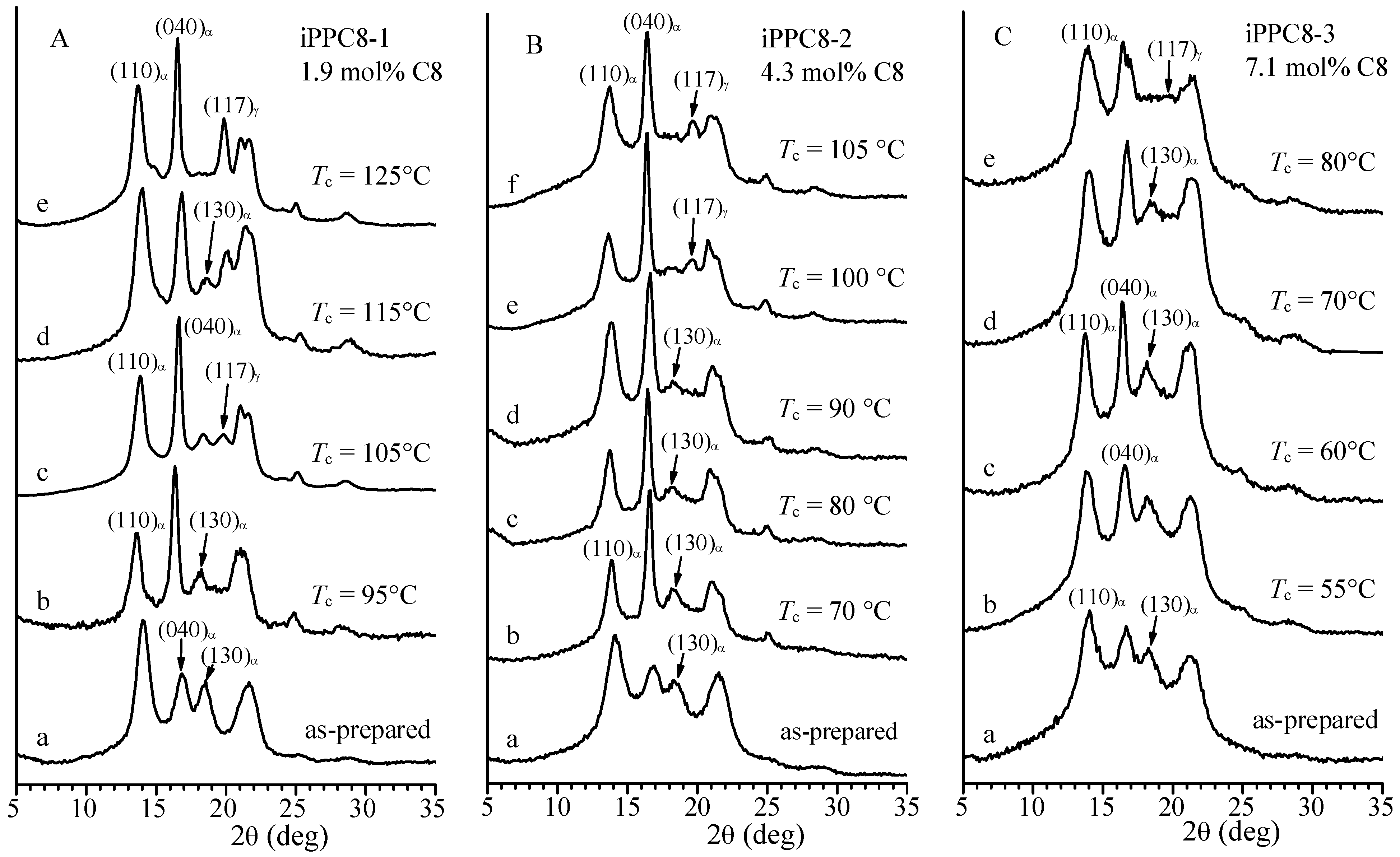

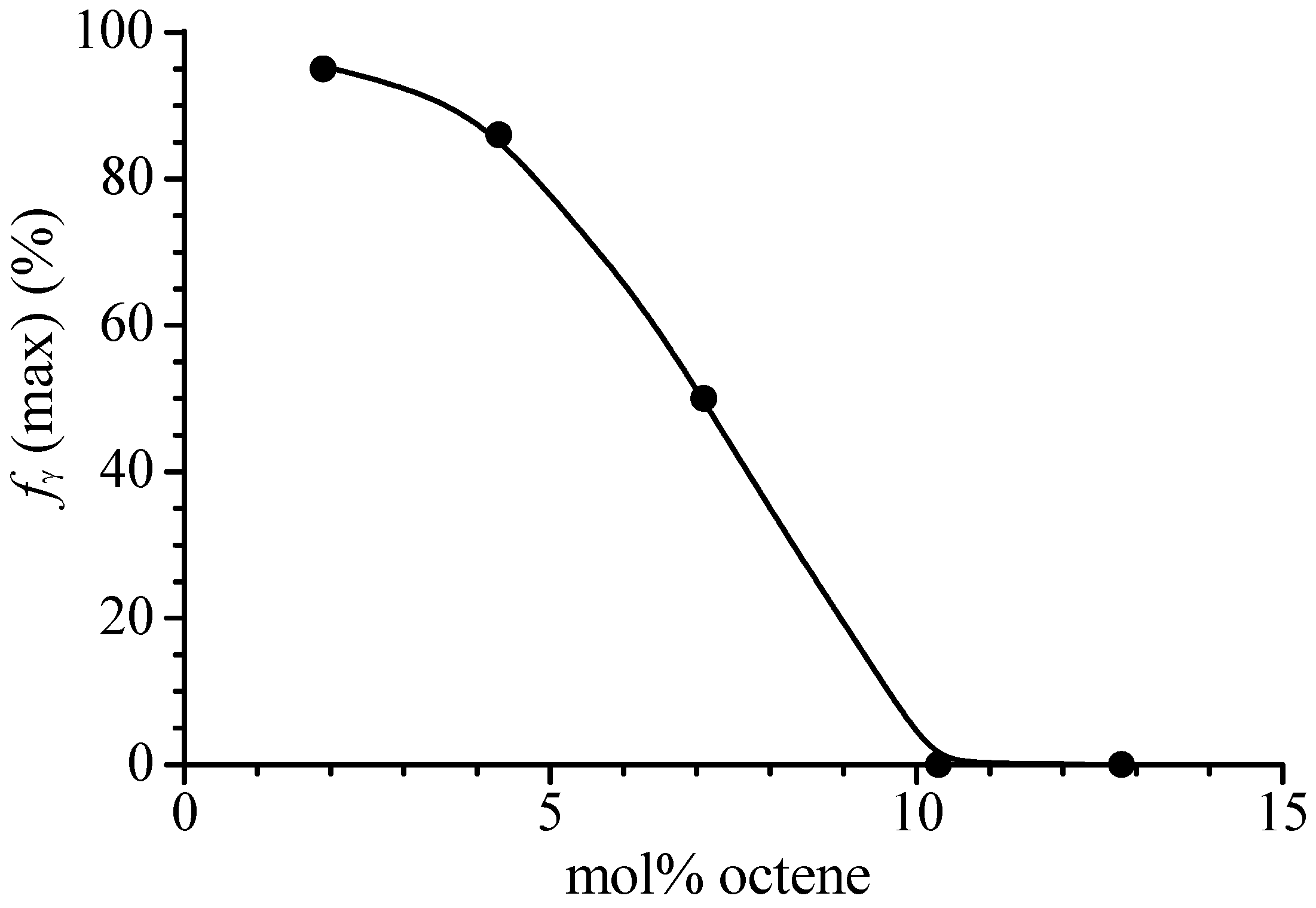

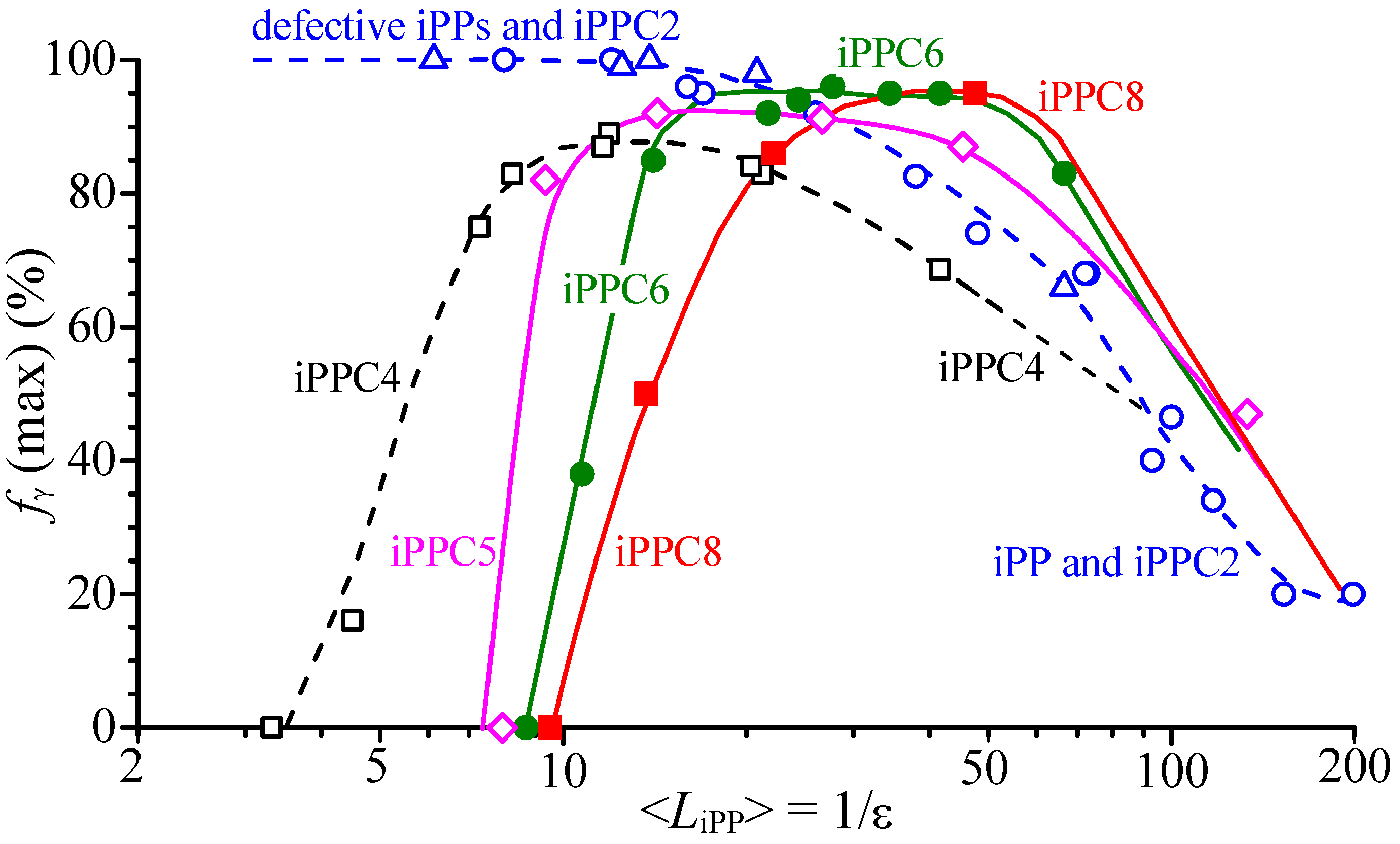
| Sample | Feed (mL Octene) | Composition (mol% Octene) | Tm (°C) a | Mwb | Mw/Mnb |
|---|---|---|---|---|---|
| iPPC8-1 | 1 | 1.9 | 132.5 | 398,576 | 2.1 |
| iPPC8-2 | 4 | 4.3 | 114.4 | 257,274 | 2.0 |
| iPPC8-3 | 6 | 7.1 | 91.8 | 302,205 | 2.1 |
| iPPC8-4 | 7 | 10.3 | 72.6/53.2 | 252,380 | 2.1 |
| iPPC8-5 | 8 | 12.8 | 47.3 | 230,985 | 2.0 |
| iPPC8-6 | 10 | 15.9 | 44.9 | 225,750 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scoti, M.; De Stefano, F.; Giordano, A.; Talarico, G.; De Rosa, C. Crystallization Behavior of Isotactic Propene-Octene Random Copolymers. Polymers 2022, 14, 4032. https://doi.org/10.3390/polym14194032
Scoti M, De Stefano F, Giordano A, Talarico G, De Rosa C. Crystallization Behavior of Isotactic Propene-Octene Random Copolymers. Polymers. 2022; 14(19):4032. https://doi.org/10.3390/polym14194032
Chicago/Turabian StyleScoti, Miriam, Fabio De Stefano, Angelo Giordano, Giovanni Talarico, and Claudio De Rosa. 2022. "Crystallization Behavior of Isotactic Propene-Octene Random Copolymers" Polymers 14, no. 19: 4032. https://doi.org/10.3390/polym14194032








