Effects of Radiation on Cross-Linking Reaction, Microstructure, and Microbiological Properties of Whey Protein-Based Tissue Adhesive Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Model System Preparation
2.3. Microbial Analysis
2.4. Viscosity
2.5. Gelation during Storage
2.6. Soluble Nitrogen
2.7. Lap-Shear Bonding Strength
2.8. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.9. Scanning Electron Microscopy (SEM)
2.10. Molecular Docking of WPI and GTA
2.11. Statistical Analysis
3. Results
3.1. Effect of Radiation on Microbial Properties
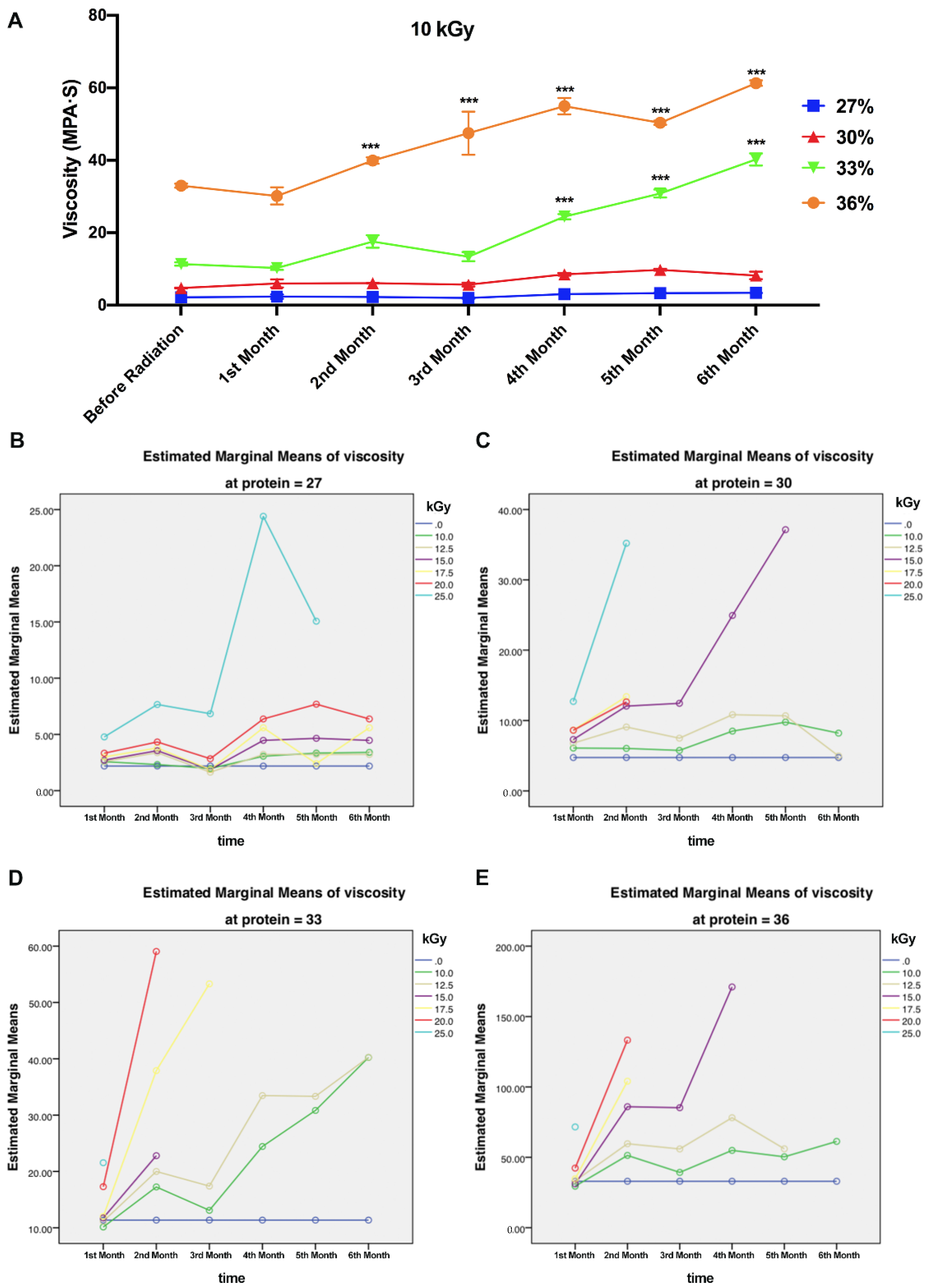
3.2. Viscosity
3.3. Gelation
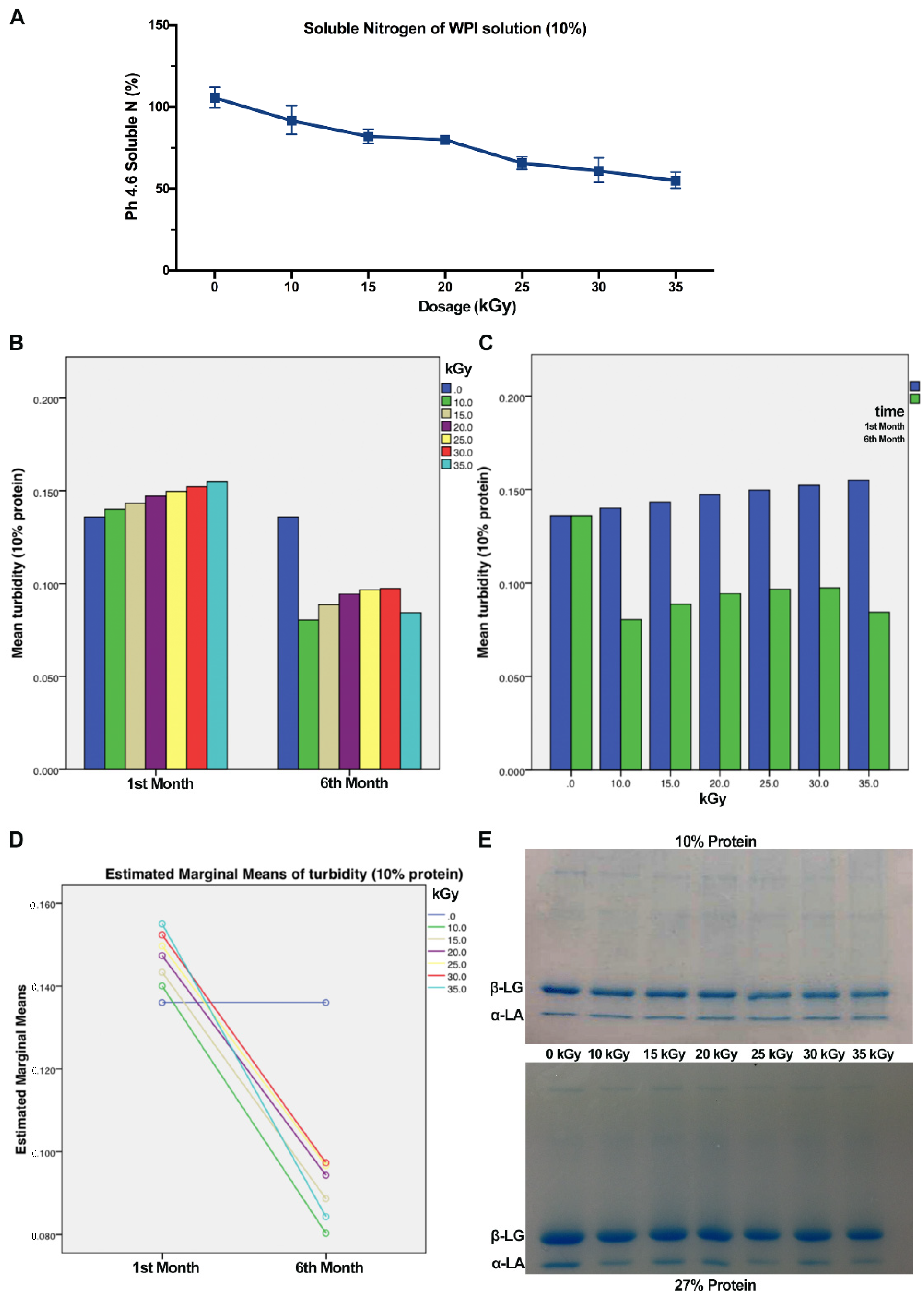
3.4. Soluble Nitrogen
3.5. Turbidity
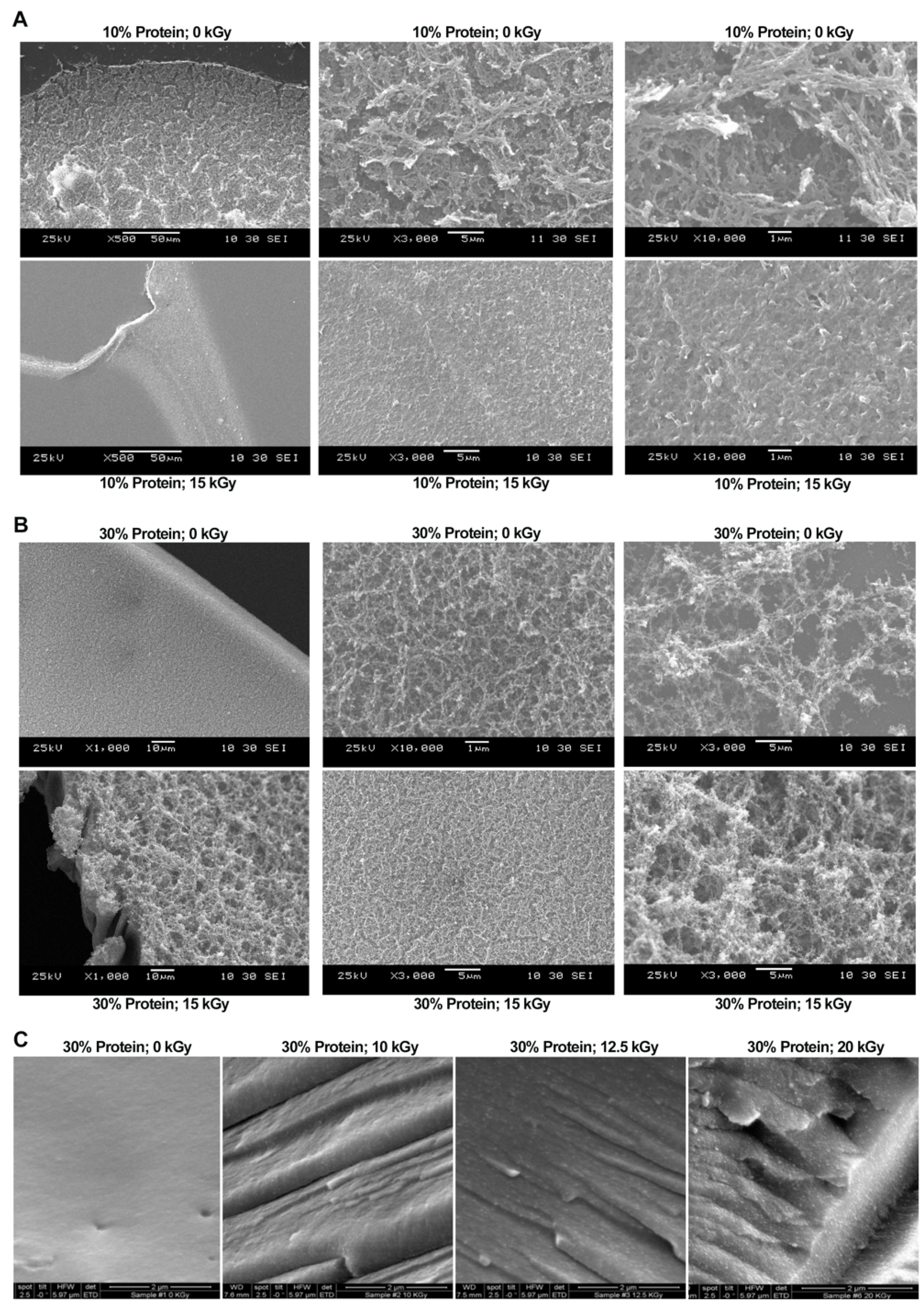
3.6. Microstructure Observation
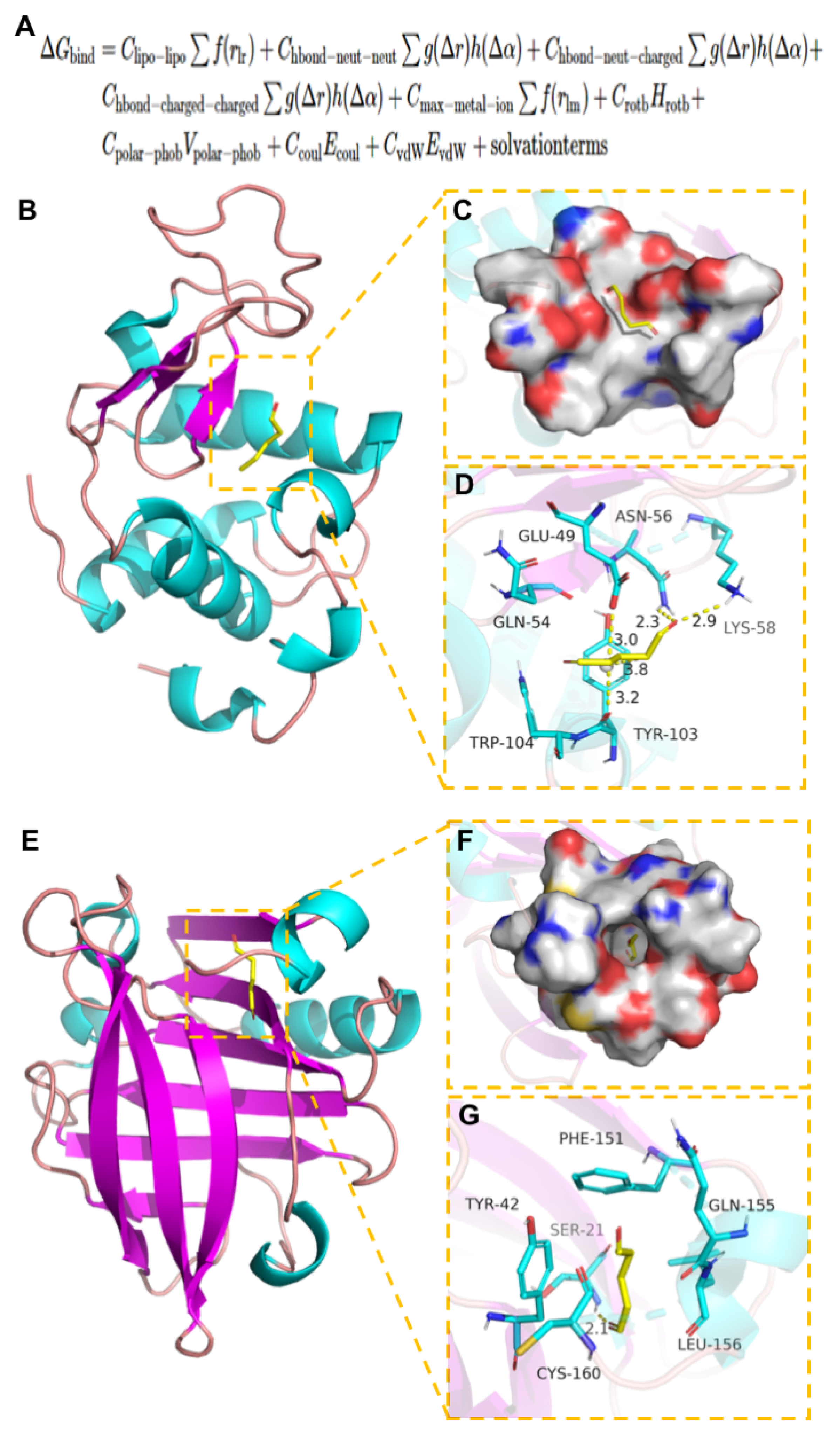
3.7. Molecular Docking of WPI and GTA
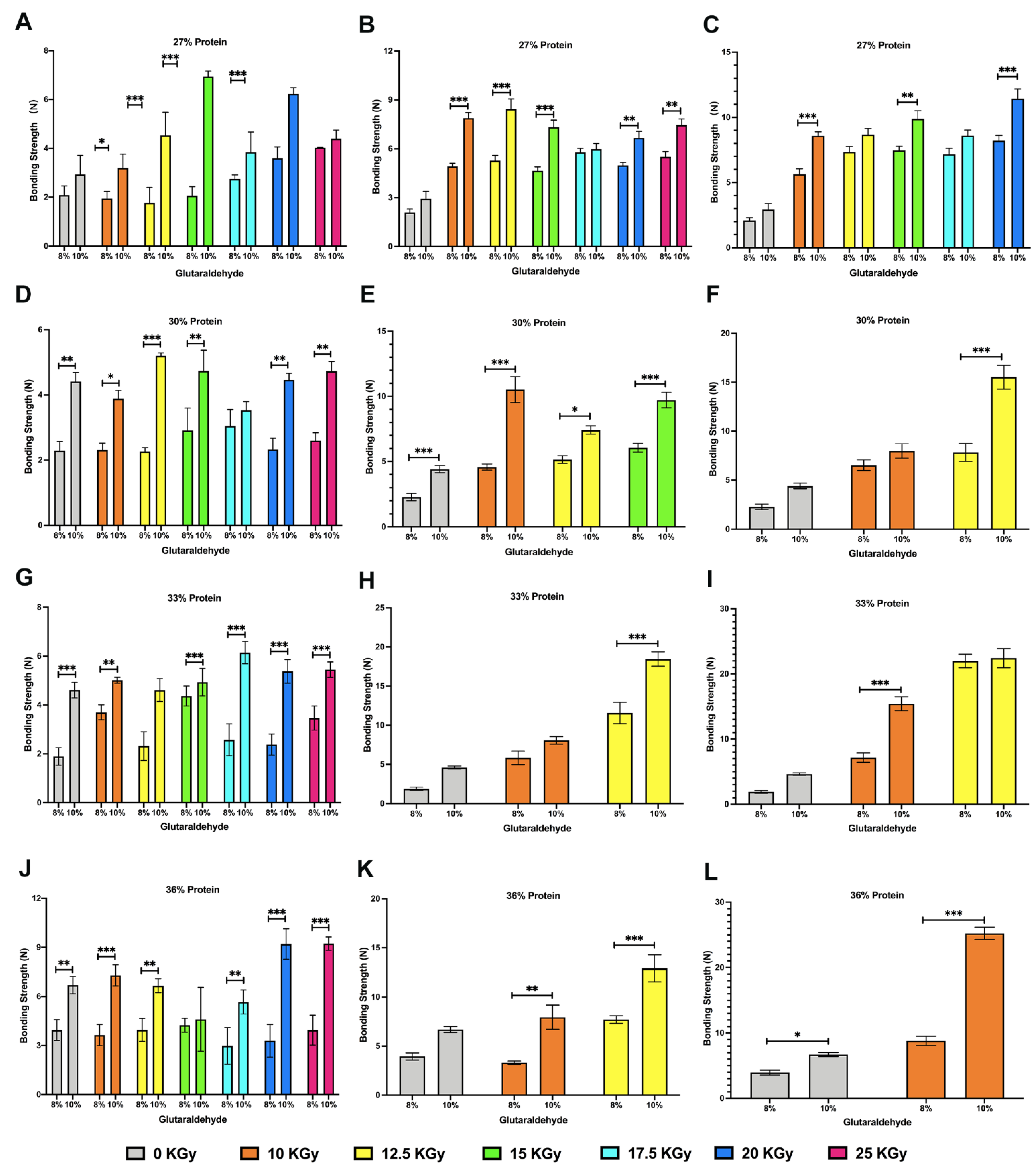
3.8. Lap-Shear Bonding Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Zhang, X.; Hong, Y.; Fu, Q.; He, Q.; Mechakra, A.; Zhu, Q.; Zhou, F.; Liang, R.; Li, C.; et al. Tissue-Adhesive Paint of Silk Microparticles for Articular Surface Cartilage Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 22467–22478. [Google Scholar] [CrossRef] [PubMed]
- Gillman, N.; Lloyd, D.; Bindra, R.; Ruan, R.; Zheng, M. Surgical applications of intracorporal tissue adhesive agents: Current evidence and future development. Expert Rev. Med. Devices 2020, 17, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, N.; Guo, M. Use of Whey Protein as a Natural Polymer for Tissue Adhesive: Preliminary Formulation and Evaluation In Vitro. Polymers 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Gao, M.; Sun, Y.; Nian, R.; Xian, M. The recent progress of tissue adhesives in design strategies, adhesive mechanism and applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110796. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Taguchi, T. Designing an anti-inflammatory and tissue-adhesive colloidal dressing for wound treatment. Colloids Surf. B Biointerfaces 2020, 188, 110737. [Google Scholar] [CrossRef] [PubMed]
- Fiore, V.F.; Lofton, M.C.; Roser-Page, S.; Yang, S.C.; Roman, J.; Murthy, N.; Barker, T.H. Polyketal microparticles for therapeutic delivery to the lung. Biomaterials 2010, 31, 810–817. [Google Scholar] [CrossRef]
- Sharifirad, A.; Mohammadian, S.; Yakhchali, B.; Mehrpooyan, S.; Fatemi, S.S. Characterization of a farnesyl diphosphate synthase gene from Penicillium brevicompactum MUCL 19011. Biotechnol. Lett. 2016, 38, 71–79. [Google Scholar] [CrossRef]
- Cornacchia, L.; Roos, Y.H. Stability of beta-carotene in protein-stabilized oil-in-water delivery systems. J. Agric. Food Chem. 2011, 59, 7013–7020. [Google Scholar] [CrossRef]
- Platania, V.; Douglas, T.E.L.; Zubko, M.K.; Ward, D.; Pietryga, K.; Chatzinikolaidou, M. Phloroglucinol-enhanced whey protein isolate hydrogels with antimicrobial activity for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112412. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Y.; Li, Z.; Li, T.; Shi, Y.; Xie, H.; Li, Y.; Su, H.; Li, Z. Application of whey protein isolate fibrils in encapsulation and protection of beta-carotene. Food Chem. 2021, 346, 128963. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.; Brennan, C.; Zheng, H. Influence of whey protein isolate on pasting, thermal, and structural characteristics of oat starch. J. Dairy Sci. 2022, 105, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yuan, F.; Gao, Y.; McClements, D.J.; Decker, E.A. Influence of pH, metal chelator, free radical scavenger and interfacial characteristics on the oxidative stability of beta-carotene in conjugated whey protein-pectin stabilised emulsion. Food Chem. 2013, 139, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, W.; Guo, M.; Li, Y.; Meng, F.; Liu, D. Whey protein isolate-gum Acacia Maillard conjugates as emulsifiers for nutraceutical emulsions: Impact of glycation methods on physicochemical stability and in vitro bioaccessibility of beta-carotene emulsions. Food Chem. 2022, 375, 131706. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, C.; Guo, M. Effects of high intensity ultrasound on acid-induced gelation properties of whey protein gel. Ultrason Sonochem. 2017, 39, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Wang, C.N.; Zhang, Y.C.; Liu, T.T.; Lv, J.P.; Shen, X.; Guo, M.R. Effects of gamma radiation on microbial, physicochemical, and structural properties of whey protein model system. J. Dairy Sci. 2018, 101, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, K.; Salmieri, S.; Lacroix, M. Gamma-irradiation influence on the structure and properties of calcium caseinate-whey protein isolate based films. Part 1. Radiation effect on the structure of proteins gels and films. J. Agric. Food Chem. 2006, 54, 6374–6384. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Guner, A.R.; Caporaso, F.; Foley, D.M. Effect of low-dose gamma irradiation on the shelf life and quality characteristics of cut romaine lettuce packaged under modified atmosphere. J. Food Sci. 2010, 65, 549–553. [Google Scholar] [CrossRef]
- Oliveira, C.L.; de la Hoz, L.; Silva, J.C.; Torriani, I.L.; Netto, F.M. Effects of gamma radiation on beta-lactoglobulin: Oligomerization and aggregation. Biopolymers 2007, 85, 284–294. [Google Scholar] [CrossRef]
- Ouattara, B.; Canh, L.T.; Vachon, C.; Mateescu, M.A.; Lacroix, M. Use of γ-radiation cross-linking to improve the water vapor permeability and the chemical stability of milk protein films. Radiat. Phys. Chem. 2002, 63, 821–825. [Google Scholar] [CrossRef]
- Atlay, R.D.; Alfred, C. Effect of combined treatments on viscosity of whey dispersions. Radiat. Phys. Chem. 2004, 71, 105–108. [Google Scholar]
- Abu, J.O.; Muller, K.; Duodu, K.G.; Minnaar, A. Gamma irradiation of cowpea (Vigna unguiculata L. Walp) flours and pastes: Effects on functional, thermal and molecular properties of isolated proteins. Food Chem. 2006, 95, 138–147. [Google Scholar] [CrossRef]
- Demicheli, M.C.; Reis, B.S.; Goes, A.M.; de Andrade, A.S. Paracoccidioides brasiliensis: Attenuation of yeast cells by gamma irradiation. Mycoses 2006, 49, 184–189. [Google Scholar] [CrossRef]
- Davies, K.J.; Delsignore, M.E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J. Biol. Chem. 1987, 262, 9908–9913. [Google Scholar] [CrossRef]
- Liu, X.D.; Han, R.X.; Yun, H.; Jung, K.C.; Jin, D.I.; Lee, B.D.; Min, T.S.; Jo, C. Effect of irradiation on foaming properties of egg white proteins. Poult. Sci. 2009, 88, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; McCarthy, J.; Wang, G.; Liu, Y.; Guo, M. Physiochemical properties, microstructure, and probiotic survivability of nonfat goats’ milk yogurt using heat-treated whey protein concentrate as fat replacer. J. Food Sci. 2015, 80, M788–M794. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, J.; Lee, J.N.; Lee, H.; Park, W.H. Mussel-inspired poly(gamma-gl utamic acid)/nanosilicate composite hydrogels with enhanced mechanical properties, tissue adhesive properties, and skin tissue regeneration. Acta Biomater. 2021, 123, 254–262. [Google Scholar] [CrossRef]
- Song, H.P.; Kim, D.H.; Jo, C.; Lee, C.H.; Kim, K.S.; Byun, M.W. Effect of gamma irradiation on the microbiological quality and antioxidant activity of fresh vegetable juice. Food Microbiol. 2006, 23, 372–378. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Song, K.B. Effect of gamma-irradiation on the physicochemical properties of porcine and bovine blood plasma proteins. Food Chem. 2003, 82, 521–526. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.H.; Yook, H.S.; Kang, K.O.; Lee, S.Y.; Hwang, H.J.; Byun, M.W. Effects of gamma radiation on the allergenic and antigenic properties of milk proteins. J. Food Prot. 2001, 64, 272–276. [Google Scholar] [CrossRef]
- Rajeswari, M.; Santhi, N.; Bhuvaneswari, V. Pharmacophore and Virtual Screening of JAK3 inhibitors. Bioinformation 2014, 10, 157–163. [Google Scholar] [CrossRef]
- Fazi, R.; Tintori, C.; Brai, A.; Botta, L.; Selvaraj, M.; Garbelli, A.; Maga, G.; Botta, M. Homology Model-Based Virtual Screening for the Identification of Human Helicase DDX3 Inhibitors. J. Chem. Inf. Model. 2015, 55, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.T. Beyond the ivory tower. The Victorian revolution in surgery. Science 2004, 304, 54–55. [Google Scholar] [CrossRef]
- Casarin, J.; Multinu, F.; Ubl, D.S.; Dowdy, S.C.; Cliby, W.A.; Glaser, G.E.; Butler, K.A.; Ghezzi, F.; Habermann, E.B.; Mariani, A. Adoption of Minimally Invasive Surgery and Decrease in Surgical Morbidity for Endometrial Cancer Treatment in the United States. Obstet. Gynecol. 2018, 131, 304–311. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Vandrovcova, M.; Krocilova, N.; Keppler, J.K.; Zarubova, J.; Skirtach, A.G.; Bacakova, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef]
- Dziadek, M.; Kudlackova, R.; Zima, A.; Slosarczyk, A.; Ziabka, M.; Jelen, P.; Shkarina, S.; Cecilia, A.; Zuber, M.; Baumbach, T.; et al. Novel multicomponent organic-inorganic WPI/gelatin/CaP hydrogel composites for bone tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.; Yu, H.L.; Yefsah, R.; Alain, R.; St-Gelais, D.; Lacroix, M. Mechanical and structural properties of milk protein edible films cross-linked by heating and gamma-irradiation. J. Agric. Food Chem. 2000, 48, 3202–3209. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulanski, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Gomes, A.D.; de Oliveira, A.A.R.; Houmard, M.; Nunes, E.H.M. Gamma sterilization of collagen/hydroxyapatite composites: Validation and radiation effects. Appl. Radiat. Isot. 2021, 174, 109758. [Google Scholar] [CrossRef]
- Monboisse, J.C.; Gardès-Albert, M.; Randoux, A.; Borel, J.P.; Ferradini, C. Collagen degradation by superoxide anion in pulse and gamma radiolysis. Biochim. Biophys. Acta 1988, 965, 29–35. [Google Scholar] [CrossRef]
- Ouattara, B.; Sabato, S.F.; Lacroix, M. Combined effect of antimicrobial coating and gamma irradiation on shelf life extension of pre-cooked shrimp (Penaeus spp.). Int. J. Food Microbiol. 2001, 68, 1–9. [Google Scholar] [CrossRef]

| 10 kGy | 12.5 kGy | 15 kGy | 17.5 kGy | 20 kGy | 25 kGy | |
|---|---|---|---|---|---|---|
| 1st Month | N | N | N | N | N | N |
| 2nd Month | N | N | N | N | N | N |
| 3rd Month | N | N | N | N | N | N |
| 4th Month | N | N | N | N | N | N |
| 5th Month | N | N | N | N | N | N |
| 6th Month | N | N | N | N | N | N |
| 10 kGy | 12.5 kGy | 15 kGy | 17.5 kGy | 20 kGy | 25 kGy | |
|---|---|---|---|---|---|---|
| 27% protein | N | N | N | N | N | Y |
| 30% protein | N | N | Y | Y | Y | Y |
| 33% protein | N | N | Y | Y | Y | Y |
| 36% protein | N | Y | Y | Y | Y | Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Wang, G.; Guo, M. Effects of Radiation on Cross-Linking Reaction, Microstructure, and Microbiological Properties of Whey Protein-Based Tissue Adhesive Development. Polymers 2022, 14, 3805. https://doi.org/10.3390/polym14183805
Liu N, Wang G, Guo M. Effects of Radiation on Cross-Linking Reaction, Microstructure, and Microbiological Properties of Whey Protein-Based Tissue Adhesive Development. Polymers. 2022; 14(18):3805. https://doi.org/10.3390/polym14183805
Chicago/Turabian StyleLiu, Ning, Guorong Wang, and Mingruo Guo. 2022. "Effects of Radiation on Cross-Linking Reaction, Microstructure, and Microbiological Properties of Whey Protein-Based Tissue Adhesive Development" Polymers 14, no. 18: 3805. https://doi.org/10.3390/polym14183805





