1. Introduction
Bone injuries and unsuccessful fracture healing represent one of the most critical clinical burdens nowadays, often caused by pathological conditions, such as osteoporosis (OP), where the dynamic balance between the activity of bone-forming cells, osteoblasts (Ob), and resorptive ones, osteoclasts (Oc), is severely compromised [
1,
2]. Due to the increase in the ageing population, and the limitations associated with the currently available pharmacological treatments, the total number of people affected by impaired bone healing is predicted to sensibly grow in the coming future [
3]. This unmet clinical need results in the urgency of ad hoc medical devices and personalized treatments, not only capable of stimulating bone tissue regeneration in elderly people, but also specifically adapted for the fracture type, the anatomical location, and the specific clinical requirements [
4]. To face this challenge, in the field of bone tissue engineering (BTE), the combination of smart biomaterials, biofabrication technologies, and specific biological cues is under investigation to develop multifunctional 3D scaffolds capable of supporting cell proliferation, cell guidance, and to promote the restoration of the tissue’s innate natural balance [
5]. In this frame, the processing of biopolymers able to induce osteogenesis, such as silk fibroin [
6] and collagen [
7], is widely reported for manufacturing fibrous scaffolds mimicking both the composition and the architecture of bone extracellular matrix (ECM). To this purpose, electrospinning (ESP) technologies represent one of the most exploited tools to produce biomimetic nanofibrous constructs, with fibre diameters ranging from a few microns to less than 100 nm. Moreover, the resulting electrospun matrices are normally characterized by high flexibility and large exposed surface area which makes them particularly suitable for functionalization with bioactive molecules [
8].
Since collagen type I is the main component of bone ECM, its use alone, or in combination with other natural or synthetic polymers, has been widely reported for ESP of scaffolds able to promote cell adhesion and proliferation [
6,
9,
10,
11]. Despite the excellent biocompatibility and bioactivity of collagen, its processing with electrospinning technologies has frequently been associated with partial denaturation of the protein, mainly caused by the solvents and process parameters used. In addition, the poor stability in aqueous media and the fast degradation kinetics of collagen require an adequate chemical crosslinking of the scaffolds often compromises the original nanofibrous structure obtained by electrospinning, as well as the final biocompatibility [
12,
13,
14].
Although the inclusion of osteoconductive inorganic phases, such as nanohydroxyapatite [
15], or mesoporous bioactive glasses (MBGs) [
16] has often been chosen as an effective strategy to improve both stability and multifunctionality of collagen-based constructs, an alternative, or complementary method, is represented by the functionalization of the fibrous meshes with specific biological cues. This can be achieved through the encapsulation or the direct covalent binding of bioactive molecules to obtain specific cell stimulation [
17]. Thanks to their large exposed and accessible surface area the electrospun matrices, made of either natural or synthetic polymers, have been functionalized with growth factors and stimulatory chemicals (bone morphogenic proteins [
18] and osteogenic factors [
19]), to induce cell differentiation and stimulate regeneration [
20], when the physiological bone remodelling is compromised or delayed (i.e., osteoporosis/osteopenia, autoimmune diseases, and bone tumours).
With this perspective, the recombinant biomolecule ICOS-Fc, patented by the authors (WO/2016/189428), successfully proved to be active on bone resorption by reversibly inhibit osteoclast activity and has consequently emerged as a powerful therapeutic approach to treat osteolytic diseases. Indeed, this recombinant biomolecule is able to bind the surface receptor (ICOSL) expressed by several cell types, including osteoclasts, and, consequently, to substantially affect their activity [
17,
21,
22]. Accordingly, in vitro and in vivo findings have demonstrated that ICOS-Fc prevents differentiation and bone erosive activity of osteoclasts and the development of osteoporosis (OP) in mice [
21].
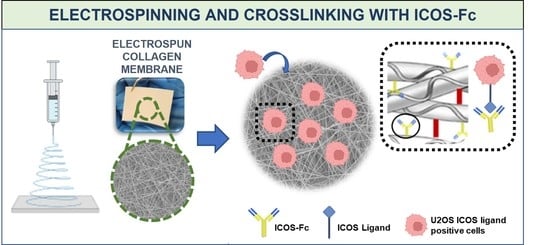
In this work, inspired by the breakthrough of these results, the authors aimed at the design of a bioactive collagen-based device biofunctionalized with ICOS-Fc molecule, potentially intended for the stimulation of physiological bone regeneration in compromised clinical situations. In addition, the peculiar flexibility provided by the electrospun membrane makes the designed device particularly suitable for the treatment of injuries not surgically mendable due to their anatomical location (e.g., pelvic fractures) and the common frailty context of the patient.
In that light, in this study the authors proved the successful development of a one-step strategy to simultaneously crosslink and functionalize the electrospun collagen membrane, with the preservation of the biomimetic nanostructure imparted by the electrospinning process and ICOS-Fc biological functionality.
At first, the retention of the collagen structural integrity upon ESP process was assessed, since the protein degradation is a commonly reported issue when organic solvents are involved [
13]. Secondly, an EDC/NHS crosslinking strategy has been optimized to promote the simultaneous crosslinking of collagen molecules and the covalent attachment of ICOS-Fc (through the carboxylic groups exposed on Fc residue) to free amine moieties exposed by the protein chains. The following scheme (
Figure 1) outlines the multistep procedure applied to obtain crosslinked biofunctionalized collagen membranes.
Finally, in vitro biological assays aimed at proving the effective binding of ICOS-Fc onto the electrospun membrane and the overall biocompatibility of the final device. Moreover, as proof of retained functionality for the anchored ICOS-Fc, the ability to inhibit the motility of human osteosarcoma cells expressing ICOSL (ICOS-Fc surface receptor) has been investigated, in analogy to the studies performed using the free form of the functional molecule [
21].
To the best of the authors’ knowledge, this strategy is not yet reported in the literature and, therefore, represents a valuable contribution to the field of BTE.
2. Materials and Methods
2.1. Materials
Type I collagen was extracted from rat tail (N-COL) by NOVAICOS. ICOS-Fc, ICOSL (Sino-Biological, Inc., Beijing, China) and anti-ICOS-Fc antibody (C398.4A) were produced and provided by NOVAICOS. The following materials were purchased from Sigma Aldrich: glacial acetic acid (AA), N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), 1-Hydroxy-2,5-pyrrolidinedione (NHS), pure ethanol (EtOH), and double distilled water (ddH2O).
2.1.1. Collagen Extraction and Preparation of ESP Solution
Type I collagen was extracted from rat tail tendons (N-COL), using a protocol developed in-house, in line with the one reported by Rajan et al. [
23]. Briefly, the collagen fibres were dissected into small portions and dried under a biological hood. The dried fibres were weighted and transferred in 0.2% acetic acid (0.2%AA) creating a stock solution. The collagen was stirred at 4 °C for 3 days, and then triturated using a standard hand blender, in an ice bath to prevent overheating. The obtained homogenized mixture was centrifuged at 3500 rpm, at 4 °C for 45 min, then filtered and stored at 4 °C. Prior to use, the collagen was aliquoted in 50 mL tubes, and frozen at −20 °C for 24 h. The samples were then lyophilized with a Lyovapor L-200 freeze-dryer (Büchi, Switzerland) under vacuum (<0.1 mbar) for 72 h. 1 g of N-COL was added to 5 mL of a solution of acetic acid (40%
v/
v) in ddH
2O (40%AA), to achieve a final concentration of N-COL 20%
w/
w (named hereafter 20N-COL). The solution was left to stir overnight, at room temperature, to ensure full dissolution of the collagen.
2.1.2. Production of ICOS-Fc Recombinant Molecule
Based on the work of Di Niro et al. [
24], the extracellular portion of human ICOS was cloned as a fusion protein to the human IgG1 Fc region, generating the ICOS-Fc recombinant protein. After stable transfection, cells were able to express and secrete ICOS-Fc in the culture supernatant as a soluble protein. The human ICOS-Fc was harvested and purified from the supernatant via protein G affinity chromatography.
2.2. Assessment of the Structural Integrity of Extracted Collagen before and after ESP
2.2.1. Circular Dichroism Analysis (CD)
The CD analysis was performed on N-COL as provided and after being dissolved in 40%AA (20N-COL). The 20N-COL sample was prepared as in
Section 2.1.1, transferred into a 5 mL Eppendorf, frozen at −20 °C overnight and then lyophilized under vacuum (<0.1 mbar) for 24 h. Then, 5 mg of each sample (N-COL and 20N-COL) was added to 5 mL of ddH
2O under stirring at room temperature, attaining a concentration of 1 mg/mL. To avoid the signal saturation (absorbance higher than 1.0), a calibration step was performed for each sample where collagen was diluted up to a concentration of 0.1 mg/mL. The CD analysis was performed on diluted samples (0.1 mg/mL), using a JASCO J-815 Circular Dichroism Spectropolarimeter, equipped with a Xe arc lamp, to record data in the far-UV spectral range. A total number of 3 scans were recorded for each sample at 50 nm/sec scanning rate and at 20 °C to obtain the final averaged CD spectra, and the data analysed with the Spectra Analysis software, purchased by JASCO. All the tests were performed using a quartz circular cuvette with a path length of 0.1 mm in the 180–260 nm wavelength range. Correction of spectra were performed considering the correspondent solvent medium as baseline (ddH
2O).
2.2.2. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The SDS-PAGE analysis was performed on lyophilized samples of N-COL and 20N-COL (prepared as in
Section 2.1.1). The solutions for SDS-PAGE were prepared through the in-house standard procedure for this assay. Briefly, the control samples, N-COL as extracted (2 mg/mL) and type I collagen from rat tail, Roche (the commercial reference sample, 3 mg/mL), were dissolved in 0.2%AA to ensure preservation of N-COL whilst attaining full dissolution. Solutions reached a final concentration of 2 mg/mL, deemed ideal to obtain a reliable result from the SDS-PAGE analysis. The dissolution of 20N-COL samples proved to be difficult, meaning the aforementioned procedure had to be adapted and optimized. In details, 100 mg of lyophilized 20N-COL were added to 50 mL of 0.2%AA and the solution left stirring overnight, at 4 °C. The resultant solution was homogenized with a hand blender and left stirring for 2 additional days, at 4 °C. To ensure the final solution possessed the amount of protein needed for the analysis, a Bicinchoninic Acid Protein Assay (BCA) was performed. Since the BCA assay demands a fully homogeneous solution, free of undissolved material and possible agglomerates, the solution was re-blended and filtered through a 100 µm strainer. At this stage, the resulting sample was quantified using BCA, revealing a final concentration of 1.7 mg/mL.
For the SDS-PAGE analysis, 2 and 4 μg of each preparation (N-COL, 20N-COL, and Roche collagen) were resuspended into a loading buffer, heated for 5 min at 95 °C, and then loaded into the gel wells to perform the run. To visualize the bands, a Coomassie gel staining solution was prepared by mixing 0.1 Coomassie brilliant blue R-250 (Sigma-Aldrich, Burlington, MA, USA) in a solution of 45% methanol and 10%AA. This solution was added to the protein bands for 30 min, and then replaced by a Coomassie gel de-staining solution consisting in 10%AA and 10% methanol. The de-staining solution was left to develop overnight, until the bands were clearly detectable. Pre-stained protein size markers (Thermo-Fisher, Waltham, MA, USA) were used to estimate the apparent size of the collagen subunits.
2.3. Processing and Functionalization of the Electrospun Collagen Membranes
2.3.1. ESP of 20N-COL
The 20N-COL solution was prepared using the method described previously in
Section 2.1.1, then transferred to a 10 mL syringe and electrospun for 3 h onto a plate collector covered by aluminium foil, at flow rate of 300 µL/h, a voltage of 22 kV and a working distance of 12 cm. The obtained membranes (20N-COL/ESP) were left to air dry overnight, at room temperature.
2.3.2. Crosslinking and Functionalization of the Electrospun Membranes
The electrospun collagen-based scaffolds were cut into 1 × 1 cm squares (n = 3), placed in 12-well plates, and incubated at 4 °C overnight. Then, 10 mM of EDC and 5 mM of NHS were added to pure EtOH (previously incubated at 4 °C) and stirred for 5 min. Then, 2 mL of the resultant crosslinking solution was added to each well, followed by the addition of ICOS-Fc at different concentrations: 50, 75, and 100 µg/mL. Samples were incubated for 8 h, at 4 °C then quickly washed 3 times with EtOH and frozen at −20 °C, overnight. Finally, the meshes were lyophilized under vacuum (<0.1 mbar) for 24 h. Crosslinked and functionalized samples will be referred as 20N-COL/ICOS-Fc. Unfunctionalized crosslinked samples were also prepared as reference and will be addressed as 20N-COL/ESP+CL.
2.4. Assessment of ICOS-Fc Binding and Functionality
ELISA-like assays were performed to measure the amount of residual functional ICOS-Fc in the supernatants recovered after the crosslinking/biofunctionalization reaction (i.e., the unbound ICOS-Fc), allowing to assess indirectly the successful grafting of the biomolecule and the retention of its functionality (as the ability to bind ICOSL). The molecule was added in triplicate at different concentrations, 50, 75, and 100 μg/mL, and samples incubated for 8 h, at 4 °C. The electrospun membranes were then removed and the related supernatant was collected and centrifuged at 13 000 rpm for 15 min to allow the precipitation of residual ICOS-Fc (i.e., the unbound ICOS-Fc). The EtOH was substituted with ddH2O for performing the ELISA-like assays.
2.4.1. ELISA-like Assay with ICOSL as Capture
ELISA plates were coated by overnight incubation at 4 °C with 1 µg/mL of human ICOSL-His (Sino-Biological, Beijing, China) in Phosphate Buffer Solution (PBS) 1X, pH 7.4. After washing 5 times with 0.05% Tween-20 in PBS 1X (pH 7.4), non-specific binding was blocked by incubating in the same solution at room temperature for 1 h. Subsequently, samples were added in duplicate and incubated at 37 °C for 2 h and then washed 5 times with horseradish peroxidase (HRP)-conjugated SV5 (Thermo-Fisher) and incubated for 1 h, at room temperature. TMB (tetra-methyl-benzidine) (Merck Life Science, Darmstadt, Germany) (5 times) was added to each well and the reaction stopped by adding H2SO4 2N (Merck Life Science). A plate reader spectrophotometer at 450 nm (Packard SpectraCount, Meriden, CT, USA) was used to analyse all the samples and the results were recorded as optical density (OD).
2.4.2. ELISA-like Assay with Anti-ICOS (Clone C398.A) as Capture
The ELISA assay was also performed with anti-ICOS (clone C398.4A) as capture, which can bind and detect ICOS-Fc also if non-functional, allowing to evidence any potential denaturation occurred during the binding step. To this purpose, ELISA plates were coated with 1 µg/mL of mAb anti-ICOS clone C398.4A, by overnight incubation in PBS 1X, pH 7.4, at 4 °C. The washing and reading steps used were described in the previous
Section 2.4.1.
2.5. Physicochemical Characterization of Electrospun Membranes
The morphology of the 20N-COL/ESP and 20NCOL/ESP+CL was analysed with Field Emission Scanning Electron Microscopy (FESEM) using a ZEISS MERLIN instrument (Carl Zeiss AG, Oberkochen, Germany). The analysis was performed on 3 samples, each one mounted onto an aluminium stub and coated with a 7 nm-thin platinum layer. The fibre and pore diameter were estimated by collecting 5 measurements from each image. Since pores were irregularly shaped, the largest distance between pore edges was considered. The final value was obtained using OriginPro2016 and presented as mean ± standard deviation (SD).
The Attenuated Total Reflection-Fourier Transform Infra-Red spectroscopy (ATR-FTIR) analysis was performed on 5 different of samples, corresponding to different phases of the process: (1) collagen as supplied (N-COL); (2) 20N-COL solution lyophilized (20N-COL); (3) electrospun membranes (20N-COL/ESP); (4) crosslinked without ICOS-Fc (20N-COL/ESP+CL); and (5) crosslinked membranes functionalized with ICOS-Fc (20N-COL/ICOS-Fc). FTIR spectra were obtained in the 4000–650 cm−1 range, and collected with a Bruker Equinox 55 spectrometer, equipped with MCT cryodetector, at a spectral resolution of 4 cm−1 and accumulation of 32 scans, by using the attenuated total reflection (ATR) mode.
The Thermogravimetric Analysis (TGA) analysis was performed with a TGA/SDTA851 (Mettler Toledo, USA) using a heating rate of 10 °C/min, within the temperature range of 23–700 °C, in air. The data were collected with STARe software and treated on OriginPro2016. The measurement of residual free amines was conducted for 20N-COL/ESP, 20N-COL/ESP+CL and 20N-COL/ICOS-Fc (50 μg/mL).
2.6. Measurement of Free Amine Residues with 2,4,6-Trinitrobenzene Sulfonic Acid Assay (TNBS)
Since both ICOS-Fc binding and crosslinking involves the reaction of collagen amine groups, TNBS—a UV-absorbing chromophore—was used to show the changes in the free primary amino groups of collagen, before and after crosslinking and functionalization [
25]. The measurement of residual free amines was conducted for 20N-COL/ESP, 20N-COL/ESP+CL and 20N-COL/ICOS-Fc (50 μg/mL). For the analysis, 1 mL of 4% (
w/
v) sodium bicarbonate (NaHCO
3, pH 8.5) was added to each sample (11 mg), followed by 1 mL of 0.5% (
w/
v) TNBS. The samples were placed in a dynamic shaker at 40 °C and incubated for 3 h under mild agitation. Subsequently, 3 mL of 6 M HCl were added to each sample, followed by 1 h incubation in the dynamic shaker, under mild agitation at 90 °C. Prior to the analysis, the dissolved samples were diluted in ddH
2O (1:20), then transferred into a 5 mL cuvette for the spectrophotometry analysis. Samples were measured in triplicate and a buffer made of TNBS-only solution was used as control. The absorbance was measured in double mode with a UV-Vis-NIR spectrophotometer (Carry 5000 1.12, Agilent, Santa Clara, CA, USA) and the data obtained through the instrument’s software (Scan 3.0). The peak of interest was identified as 346 nm [
26,
27] on the data that was later processed in OriginPro2016.
2.7. Biological Assessment of ICOS-Fc Functionalized Collagen Scaffolds
The electrospun membranes for the following biological assays were prepared by ESP the 20N-COL solution onto round cover slips (15 mm diameter) for 15 min, using the process parameters described in
Section 2.3.1. After ESP, samples were frozen at −20 °C and lyophilized. Subsequently, they were placed in a 24-well plate, immersed in 1 mL of crosslinking solution containing ICOS-Fc at a concentration of 50 µg/mL, then incubated for 1 h at 4 °C. The incubation time was established according to the indications provided by Ribeiro et al. which suggest it should match the amount of collagen present in each membrane [
15]. Prior to cell seeding, samples were gradually rehydrated in EtOH/ddH
2O at the following concentration (
v/
v): 100% EtOH, 90%, 70%, 60%, 50%, 40%, 30%, 15%, 10%, and 100% ddH
2O.
2.7.1. Cells for Biocompatibility and Migration Assays
Human osteosarcoma cell line U2OS (ICOSL positive) was obtained from the American Type Culture Collection (Manassas, VA, USA) and grown as a monolayer in DMEM (Gibco, Life Technologies, Carlsbad, CA, USA) while human osteosarcoma cell line HOS (ICOSL negative) was obtained from Sigma-Aldrich and cultured in MEM (Gibco) + 1% non-essential amino acids (Sigma-Aldrich). All media were supplemented with 10% Fetal Bovine Serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco), and cells maintained at 37 °C in a 5% CO2 humidified atmosphere.
2.7.2. Cytocompatibility of 20N-COL and 20N-COL/ICOS-Fc Membranes
Sterilisation of 20N-COL/ESP+CL and 20N-COL/ICOS-Fc samples were performed under UV-light for 30 min. Before seeding, samples were incubated in DMEM for 30 min. U2OS cells were then seeded onto the samples, plating 30 × 10
3, 7.5 × 10
3, and 1.5 × 10
3 cells in 1 mL/well for 3–5–7 days, respectively, in complete DMEM medium (Gibco) and the samples incubated at 37 °C, in a 5% CO
2 humidified atmosphere. At each time point, viable cells were evaluated by adding XTT [2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carbox-anilide)] reagent (Trevigen, Helgerman CT, Gaithersburg, MD, USA) for 3 h at 37 °C. A plate reader spectrophotometer at 490 nm (Packard SpectraCount) was used to read all samples and cell viability was calculated according to the following formula:
2.7.3. Assessment of Cell Motility
Prior to cell seeding, samples were gradually rehydrated in EtOH/ddH
2O using protocol described in
Section 2.6. The cells were seeded on the collagen membranes for 30 min at 37 ° C, then, detached, counted, and used for the migration assay (2 × 10
3 cells in 50 μL/well). To perform the Boyden chamber migration assay (BD Biosciences, Milan, Italy) cells were plated onto the apical side of 50 μg/mL Matrigel-coated filters (8.2 mm diameter and 0.5 μm pore size; Neuro Probe, Inc.; BIOMAP snc, Milan, Italy) with the addition of serum-free medium. In parallel, medium containing 20% FBS was placed in the basolateral chamber as a chemoattractant and after 6 h, cells on the apical side were wiped off with Q-tips. Methanol and crystal-violet were subsequently used to stain the cells present at the bottom of the filter and cell count was carried out with an inverted microscope. The data have been expressed as percentages and reported as mean ± SEM (n = 5) of the percentage of migration versus control migration. Five independent experiments were performed.
4. Discussion
Collagen, as one of the main constituents of bone, is a common choice as a base material for the development of scaffolds aiming at BTE. The combination of ESP with collagen can boost the degree of biomimicry of a device as it allows the creation of structures that are similar to the ECM, at both compositional and architectural level [
6].
In this work, an aqueous solution of acetic acid was used as mild solvent for the dissolution of collagen in order to avoid the protein denaturation and allow the electrospinning process [
16,
42]. To investigate the effects of the solubilization and ESP process on collagen’s structural integrity, CD and SDS-PAGE analysis were performed (
Figure 2), both confirming the presence of the triple helical structure. At the molecular level, the triple helix of collagen type I is composed of three identical α-chains (two α
1 chains and one α
2), each one composed of a repeating amino acid sequence of glycine-X-Y, where typically X is a proline and Y is an hydroxyproline [
28,
29]. The three α chains then assemble into a triple helix by coiling around each other in a rope-like fashion, forming tropocollagen. In SDS-PAGE analysis, these chains are represented in an electrophoretic profile as bands. The analysis performed on N-COL, 20N-COL, and the commercial reference Roche (
Figure 2A) shows the α
1 chains at 180 kDa and the α
2 at 130 kDa. The representation of α
1 and α
2-chains together, or two α
1-chains, is seen by the presence of the β-band, a dimer, which is seen at 250 kDa. Finally, the confirmation that the three α-chains are together, possibly arranged as a triple helix, is given by the γ-band, which was present for all samples. The data from SDS-PAGE was further confirmed by the CD analysis (
Figure 2B), where the strong positive peak at 220 nm indicates the presence of a triple helix conformation [
30]. Altogether these results show that the used solvent system allowed to preserve the protein structure ensuring the full retention of its bioactivity.
ATR-FTIR spectroscopy provided further insights on collagen structure upon each process step. The collected spectra (
Figure 6) show that all samples presented the expected amide bands, with exception of 20N-COL/ESP which did not present the amide III signal. This was associated with a slight loss of molecular structure [
36], specifically to β-sheet secondary structures, associated with a band at 1239 cm
−1 [
34]. The loss of this band can be ascribed to the fast solvent evaporation and fibre assembly occurring during ESP, which together with the dissolution in the solvent, can affect to some extent the structural conformation of the protein [
36]. Similar results are reported in the studies by Sizeland et al. [
10], where the results of the ATR-FTIR of electrospun membranes suggested that the alteration occurred, although the SDS-PAGE of their membranes evidenced that the α chains were intact. Their study concluded that the protein was not degraded, but the triple helices did not redevelop after ESP. The spectrum registered after crosslinking shows that the amide III band reappears, highlighting the effect of crosslinking in promoting the reconstitution and stabilization of the collagen molecular structure.
Despite the positive effects in terms of collagen stability, crosslinking treatments are often associated with loss of morphology (e.g., fibre merging and increase in fibre diameter). Therefore, minimizing the post-processing steps and optimizing the crosslinking strategy is key to ensure that the desired microstructure is preserved. In this work, this was achieved by combining the crosslinking and functionalization in a
one-step reaction, by the simultaneous activation via EDC/NHS of the carboxylic groups exposed both by collagen and by ICOS-Fc molecule [
17]. The successful outcome of the functionalization step was confirmed by ELISA-like assays which showed a reduction in ICOS-Fc in the supernatant collected from the crosslinking reaction (
Figure 4). This was seen for all the three concentrations tested (50, 75, and 100 μg/mL), however, due to the high costs of ICOS-Fc (200 EUR/mg), in this instance experiments were performed with 50 μg/mL, to attain a functionalization that is both efficient and easily translated commercially. The UV-Vis analysis (
Figure 5), performed on the samples exposed to 50 μg/mL of ICOS-Fc, confirmed the obtained results for crosslinking and functionalization through a significant decrease in the amount of free amines after crosslinking, and significantly lower after the grafting with ICOS-Fc.
To further investigate how crosslinking improved collagen’s stability, a TGA analysis was performed (
Figure 7), showing that the process led to the increase in the denaturation temperature of ca 10 °C for crosslinked membranes. UV-Vis and TGA results clearly evidenced a high degree of collagen crosslinking, which is expected to provide enhanced biodegradation kinetics and bioactivity (angiogenesis [
43], osteogenesis [
44]).
The crosslinked membranes remained intact for 7 days during the cytotoxicity assay, confirming that crosslinking reaction provided a significant improvement in the overall stability and that the electrospun membranes would be able to support cell migration and proliferation.
The produced electrospun collagen fibrous membranes are expected to provide multiple biologicals and topological cues that can modulate cell adhesion, proliferation and/or differentiation and aims to provide an effective alternative strategy to the more common combination with growth factors and cells to enhance and support the process of bone formation [
45,
46]. In this study, with the aim to widen the exerted biological functions, the collagen-based scaffolds have been further biofunctionalized with ICOS-Fc molecule [
17,
21,
22]. The target of the followed approach is the combination in a single multifunctional platform of several abilities to support osteoblast growth and function, whilst temporarily inducing the inhibition of osteoclasts activity through the peculiar biological properties of ICOS-Fc. The resulting multifunctional device is expected to actively contribute to the process of bone regeneration, which is key when the physiological remodelling mechanism is delayed or even altered, as in the case of elderly people or patients affected by osteoporosis.
The biological assays demonstrated that the electrospun membranes are fully cytocompatible, confirming that neither the electrospinning process nor the chemicals of the cross-linking/functionalization step have altered the overall biocompatibility.
Since the effect of free ICOS-Fc on cell migratory activity was previously reported by the authors [
22] the retention of this essential biological effect was also assessed for ICOS-Fc grafted on collagen membranes by using the Boyden chamber migration assay, both with ICOSL-positive and ICOSL-negative cell lines, as a
proof of concept of the efficacy of the developed approach. The results of this assay evidenced a 40% inhibition of U2OS (ICOSL-positive cells) migratory activity after only 30 min of contact between cells and ICOS-Fc functionalized membranes. This finding fully confirmed the retention of the inhibitory effect of ICOS-Fc anchored to electrospun membranes, in analogy to its free form [
21,
22] and when grafted onto the surface of bioactive glass particles [
17]. ICOSL-negative cells (HOS) were used as a control, and exhibited no changes in their migration behaviour, attesting once more the specificity of the inhibitory effect derived from the ICOS:ICOSL binding at the collagen surface.
Further in vitro studies will aim at exploring the action of the functionalized membranes on osteoclast-precursor cells to evaluate the inhibitory effect on cell differentiation and the downregulation of osteoclast differentiation genes.
5. Conclusions
The work presented reports the successful development of one step strategy to achieve simultaneously the crosslinking and biofunctionalization of electrospun collagen membranes. The ICOS-Fc molecule, chosen for its ability to reversibly inhibit osteoclast function by binding its ligand ICOSL, has been effectively grafted on the collagen membranes to impart multifunctional biological effects and thus promoting the remodelling process of bone tissue in compromised clinical situations.
The stability of the ICOS-Fc binding was assessed by an in house-developed ELISA-like assay, revealing a functionalization yield higher than 95%, with a full retention of functionality by the grafted biomolecules.
After 7 days in culture, cells showed high viability regardless of the presence of ICOS-Fc, suggesting that neither the crosslinking nor the functionalization have altered the cytocompatibility of the electrospun collagen scaffolds. The contact of U2OS, chosen as ICOSL-positive cells, with the collagen membranes exposing ICOS-Fc at their surface resulted in the inhibition of cell migration, confirming once more the successful binding to the collagen fibres and the retention of ICOS-Fc biological properties (proof of efficacy). In contrast, the ICOSL-negative cell line (HOS) did not show inhibitory effects, confirming the specificity of the ICOS-Fc effect that was mediated by binding to ICOSL.
By inhibiting the migration of ICOSL-positive cells, e.g., osteoclasts, the developed membranes can play an active role on bone remodelling, placing this strategy in the category of future therapies based on cell behaviour modulation to achieve improved regeneration.
The results of this study highlight the high potential of the developed multifunctional platform (i.e., osteoconductive, pro-osteogenic, anti-clastogenic) for treating delayed bone healing and pave the way to further in vivo studies to implement minimally invasive clinical solutions (e.g., injection via cannulated instruments at the fracture site).