Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthesis
2.4. Determination of Thiol Content in MβCD-SH
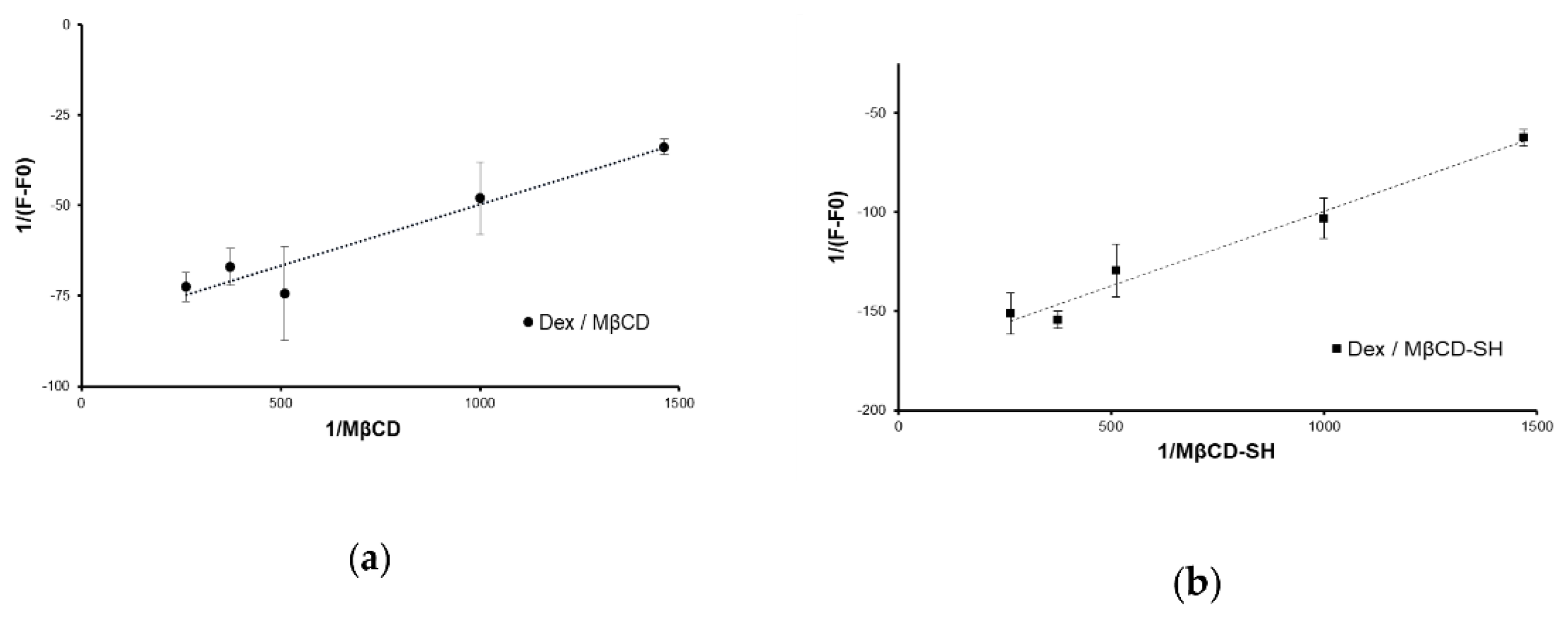
2.5. Determination of the Dex/Cyclodextrins Association Constant
2.6. Determination of Nanometric Aggregates
2.7. Microrheological Tests
2.8. Cell Viability Assay
2.9. Statistical Data Analyses
3. Results and Discussion
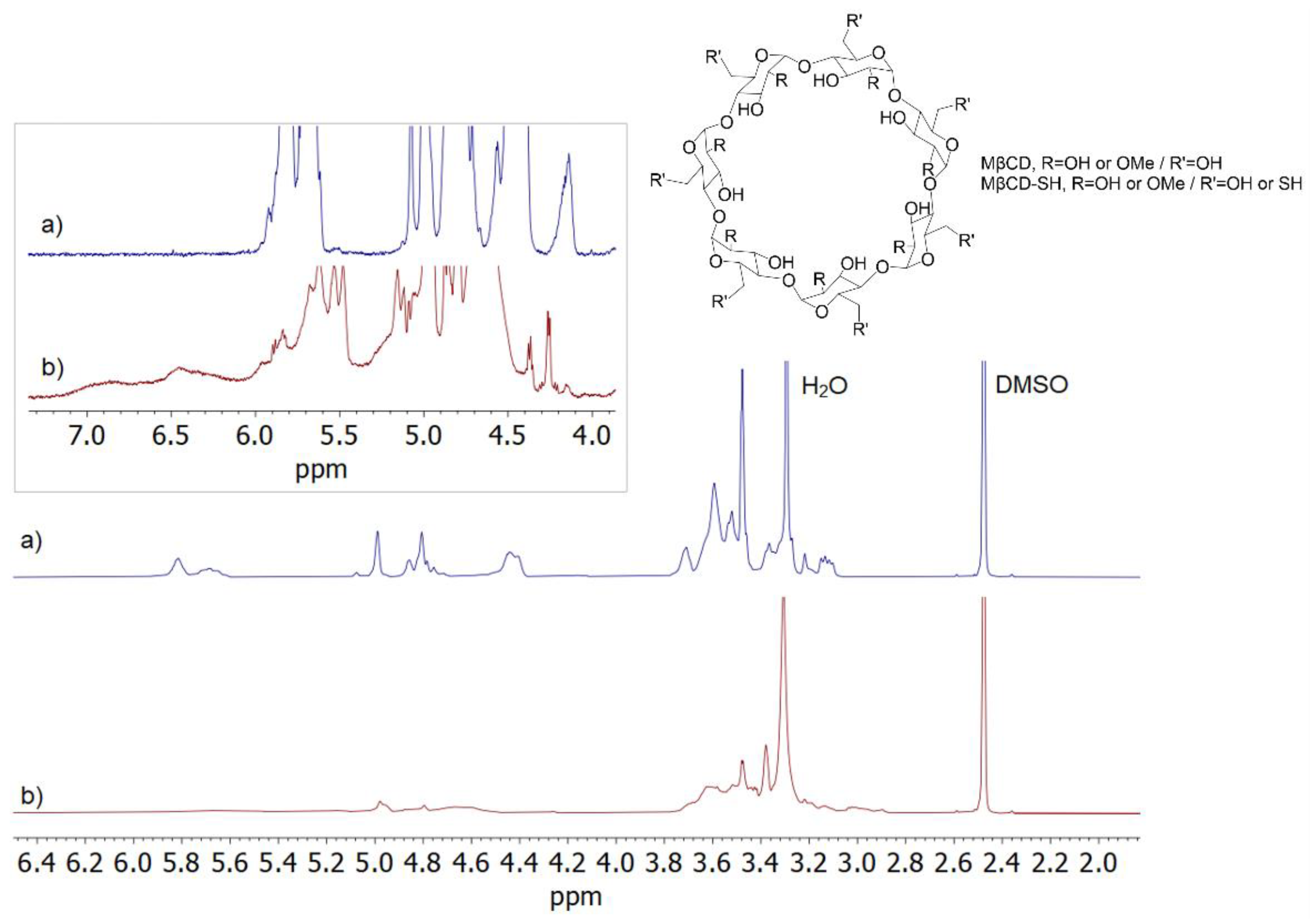
3.1. Synthesis
3.2. NMR Characterization
3.3. Complexation to Dex
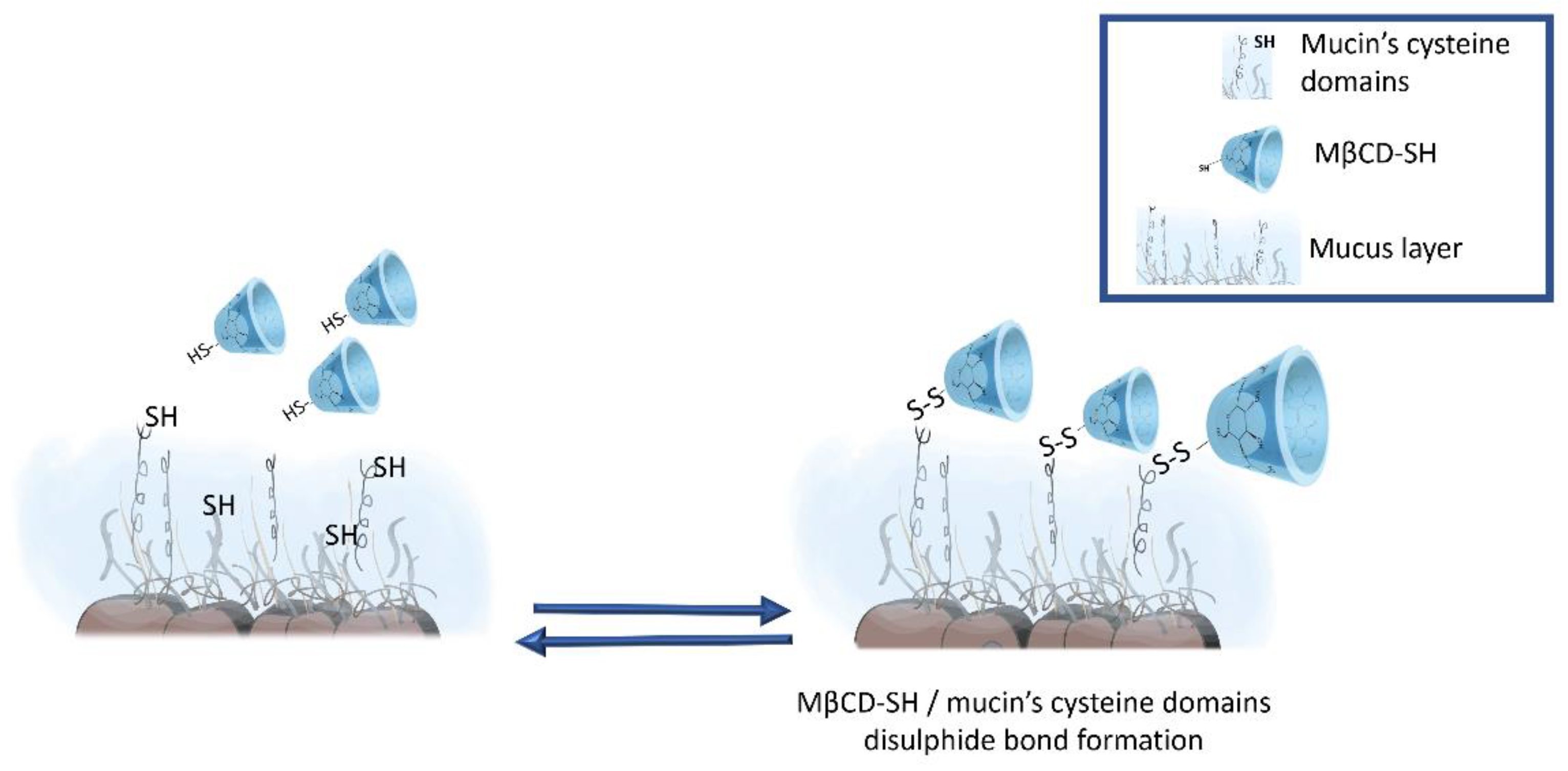
3.4. Microrheological Evaluations
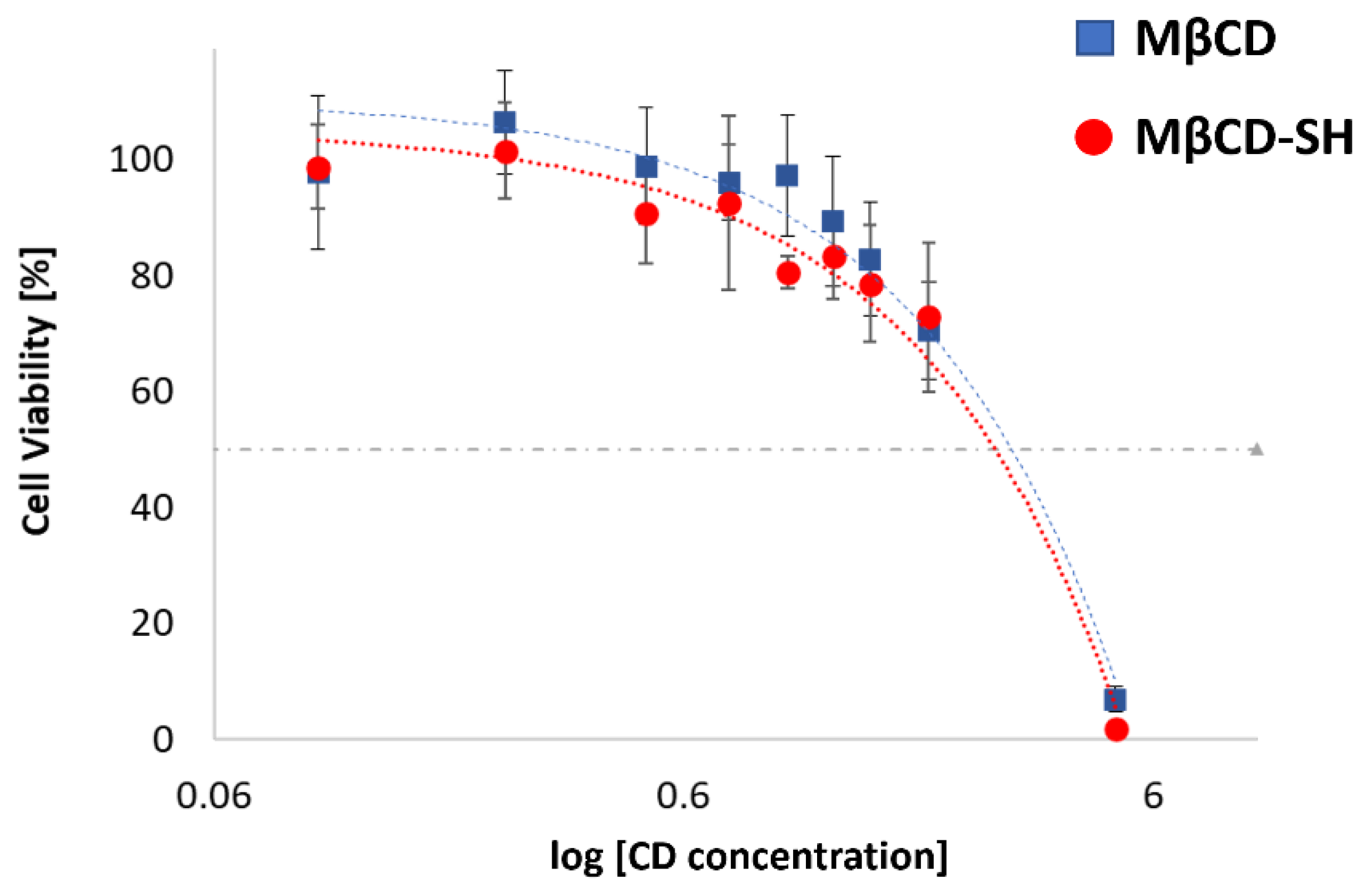
3.5. Cell Viability Evaluations
3.6. Determination of Nanometric Aggregates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.G.; Shao, J. Hybrid Films Prepared from a Combination of Electrospinning and Casting for Offering a Dual-Phase Drug Release. Polymers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef] [PubMed]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnurch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.P.; Chater, P.I.; Wilcox, M.D. The properties of the mucus barrier, a unique gel—How can nanoparticles cross it? Ther. Deliv. 2016, 7, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lu, Q.; Sommerfeld, S.D.; Chan, A.; Menon, N.G.; Schmidt, T.A.; Elisseeff, J.H.; Singh, A. Targeted delivery of hyaluronic acid to the ocular surface by a polymer-peptide conjugate system for dry eye disease. Acta Biomater. 2017, 55, 163–171. [Google Scholar] [CrossRef]
- Migone, C.; Mattii, L.; Giannasi, M.; Moscato, S.; Cesari, A.; Zambito, Y.; Piras, A.M. Nanoparticles Based on Quaternary Ammonium Chitosan-methyl-beta-cyclodextrin Conjugate for the Neuropeptide Dalargin Delivery to the Central Nervous System: An In Vitro Study. Pharmaceutics 2020, 13, 5. [Google Scholar] [CrossRef]
- Leichner, C.; Jelkmann, M.; Bernkop-Schnurch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 191–221. [Google Scholar] [CrossRef]
- Cesari, A.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Uccello-Barretta, G.; Balzano, F. Binding and mucoadhesion of sulfurated derivatives of quaternary ammonium-chitosans and their nanoaggregates: An NMR investigation. J. Pharm. Biomed. Anal. 2020, 177, 112852. [Google Scholar] [CrossRef]
- Hussain Asim, M.; Ijaz, M.; Rösch, A.C.; Bernkop-Schnürch, A. Thiolated cyclodextrins: New perspectives for old excipients. Coord. Chem. Rev. 2020, 420, 213433. [Google Scholar] [CrossRef]
- Asim, M.H.; Nazir, I.; Jalil, A.; Matuszczak, B.; Bernkop-Schnürch, A. Tetradeca-thiolated cyclodextrins: Highly mucoadhesive and in-situ gelling oligomers with prolonged mucosal adhesion. Int. J. Pharm. 2020, 577, 119040. [Google Scholar] [CrossRef]
- Asim, M.H.; Ijaz, M.; Mahmood, A.; Knoll, P.; Jalil, A.; Arshad, S.; Bernkop-Schnurch, A. Thiolated cyclodextrins: Mucoadhesive and permeation enhancing excipients for ocular drug delivery. Int. J. Pharm. 2021, 599, 120451. [Google Scholar] [CrossRef]
- Asim, M.H.; Nazir, I.; Jalil, A.; Laffleur, F.; Matuszczak, B.; Bernkop-Schnurch, A. Per-6-Thiolated Cyclodextrins: A Novel Type of Permeation Enhancing Excipients for BCS Class IV Drugs. ACS Appl. Mater. Interfaces 2020, 12, 7942–7950. [Google Scholar] [CrossRef] [Green Version]
- Grassiri, B.; Knoll, P.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Bernkop-Schnurch, A. Thiolated Hydroxypropyl-beta-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2612. [Google Scholar] [CrossRef]
- Popovics-Tóth, N.; Tajti, Á.; Hümpfner, E.; Bálint, E. Synthesis of 3,4-Dihydropyrimidin-2(1H)-one-phosphonates by the Microwave-Assisted Biginelli Reaction. Catalysts 2020, 11, 45. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Fabiano, A.; Piras, A.M.; Guazzelli, L.; Storti, B.; Bizzarri, R.; Zambito, Y. Impact of Different Mucoadhesive Polymeric Nanoparticles Loaded in Thermosensitive Hydrogels on Transcorneal Administration of 5-Fluorouracil. Pharmaceutics 2019, 11, 623. [Google Scholar] [CrossRef] [Green Version]
- Dodero, A.; Williams, R.; Gagliardi, S.; Vicini, S.; Alloisio, M.; Castellano, M. A micro-rheological and rheological study of biopolymers solutions: Hyaluronic acid. Carbohydr. Polym. 2019, 203, 349–355. [Google Scholar] [CrossRef]
- Cesari, A.; Piras, A.M.; Zambito, Y.; Uccello Barretta, G.; Balzano, F. 2-Methyl-beta-cyclodextrin grafted ammonium chitosan: Synergistic effects of cyclodextrin host and polymer backbone in the interaction with amphiphilic prednisolone phosphate salt as revealed by NMR spectroscopy. Int. J. Pharm. 2020, 587, 119698. [Google Scholar] [CrossRef]
- Cesari, A.; Recchimurzo, A.; Fabiano, A.; Balzano, F.; Rossi, N.; Migone, C.; Uccello-Barretta, G.; Zambito, Y.; Piras, A.M. Improvement of Peptide Affinity and Stability by Complexing to Cyclodextrin-Grafted Ammonium Chitosan. Polymers 2020, 12, 474. [Google Scholar] [CrossRef] [Green Version]
- Piras, A.M.; Fabiano, A.; Chiellini, F.; Zambito, Y. Methyl-beta-cyclodextrin quaternary ammonium chitosan conjugate: Nanoparticles vs macromolecular soluble complex. Int. J. Nanomed. 2018, 13, 2531–2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piras, A.M.; Zambito, Y.; Burgalassi, S.; Monti, D.; Tampucci, S.; Terreni, E.; Fabiano, A.; Balzano, F.; Uccello-Barretta, G.; Chetoni, P. A water-soluble, mucoadhesive quaternary ammonium chitosan-methyl-beta-cyclodextrin conjugate forming inclusion complexes with dexamethasone. J. Mater. Sci. Mater. Med. 2018, 29, 42. [Google Scholar] [CrossRef]
- Asim, M.H.; Moghadam, A.; Ijaz, M.; Mahmood, A.; Gotz, R.X.; Matuszczak, B.; Bernkop-Schnurch, A. S-protected thiolated cyclodextrins as mucoadhesive oligomers for drug delivery. J. Colloid Interface Sci. 2018, 531, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.C.S.; Ramadevi, K.; Sirisha, K. Effect of β-cyclodextrin on Rheological Properties of some Viscosity Modifiers. Indian J. Pharm. Sci. 2014, 76, 545–548. [Google Scholar] [PubMed]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Messner, M.; Kurkov, S.V.; Brewster, M.E.; Jansook, P.; Loftsson, T. Self-assembly of cyclodextrin complexes: Aggregation of hydrocortisone/cyclodextrin complexes. Int. J. Pharm. 2011, 407, 174–183. [Google Scholar] [CrossRef]
- Messner, M.; Kurkov, S.V.; Jansook, P.; Loftsson, T. Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 2010, 387, 199–208. [Google Scholar] [CrossRef]
- Muankaew, C.; Saokham, P.; Jansook, P.; Loftsson, T. Self-assembly of cyclodextrin complexes: Detection, obstacles and benefits. Pharmazie 2020, 75, 307–312. [Google Scholar]
- Jansook, P.; Kurkov, S.V.; Loftsson, T. Cyclodextrins as solubilizers: Formation of complex aggregates. J. Pharm. Sci. 2010, 99, 719–729. [Google Scholar] [CrossRef]
- Valente, A.J.; Carvalho, R.A.; Soderman, O. Do Cyclodextrins Aggregate in Water? Insights from NMR Experiments. Langmuir 2015, 31, 6314–6320. [Google Scholar] [CrossRef]
- Loftsson, T.; Saokham, P.; Sa Couto, A.R. Self-association of cyclodextrins and cyclodextrin complexes in aqueous solutions. Int. J. Pharm. 2019, 560, 228–234. [Google Scholar] [CrossRef]
- Mazmanian, K.; Sargsyan, K.; Grauffel, C.; Dudev, T.; Lim, C. Preferred Hydrogen-Bonding Partners of Cysteine: Implications for Regulating Cys Functions. J. Phys. Chem. B 2016, 120, 10288–10296. [Google Scholar] [CrossRef]
- Biswal, H.S.; Shirhatti, P.R.; Wategaonkar, S. O−H···O versus O−H···S Hydrogen Bonding I: Experimental and Computational Studies on the p-Cresol·H2O and p-Cresol·H2S Complexes. J. Phys. Chem. A 2009, 113, 5633–5643. [Google Scholar] [CrossRef]
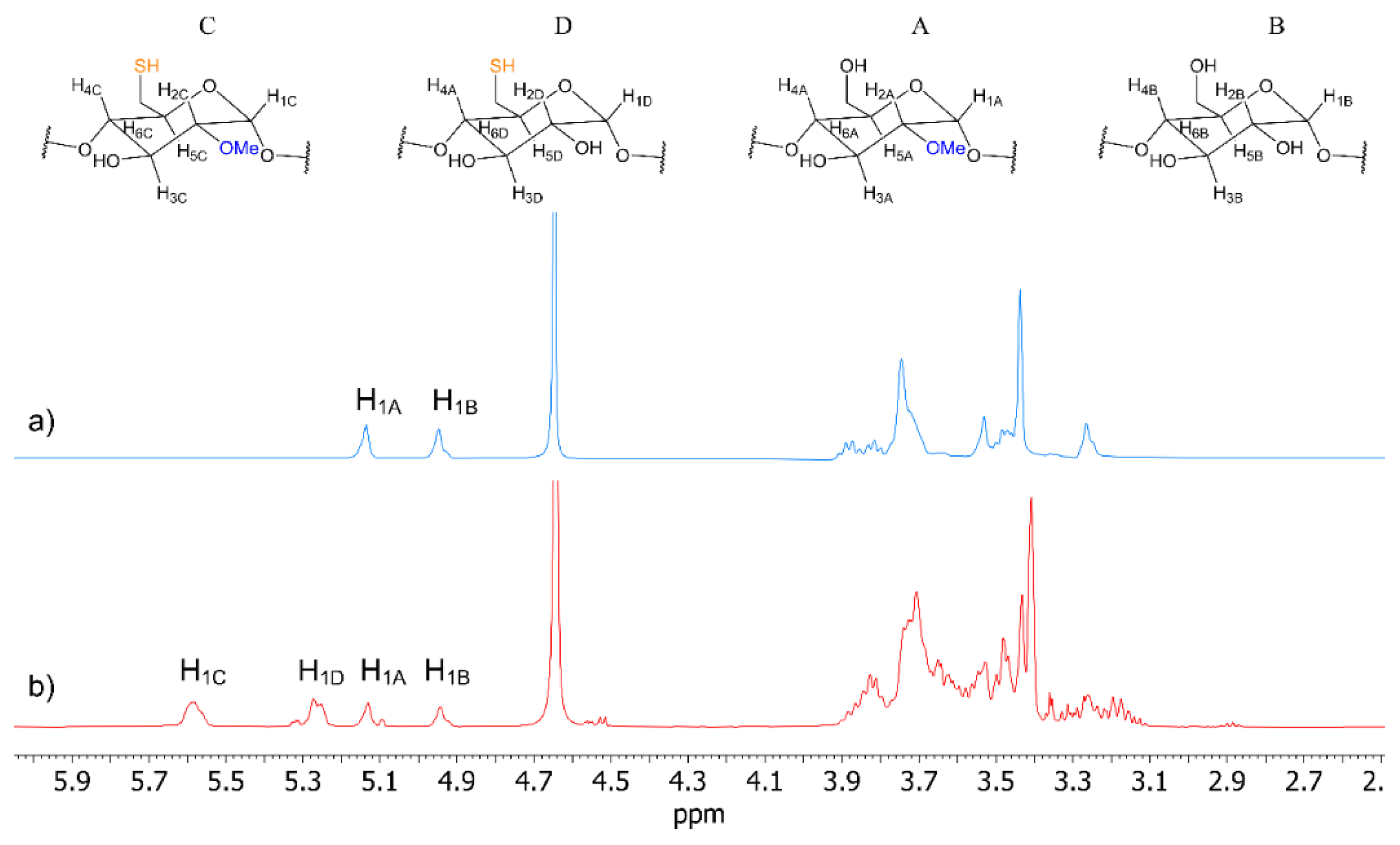

| MβCD-SH | MβCD | β-CD | |||||
|---|---|---|---|---|---|---|---|
| Unit C | Unit D | Unit A | Unit B | Unit A | Unit B | ||
| H1 | 5.58 | 5.26 | 5.13 | 4.94 | 5.13 | 4.94 | 4.94 |
| H2 | 3.18 | 3.47 | 3.26 | 3.53 | 3.26 | 3.53 | 3.52 |
| H3 | 3.83 | 3.81 | 3.88 | 3.81 | 3.88 | 3.81 | 3.84 |
| H4 | 3.55 | 3.60 | 3.48 | 3.46 | 3.48 | 3.45 | 3.46 |
| H5 | 3.63 | 3.71 | 3.69 | 3.72 | 3.70 | 3.73 | 3.74 |
| H6/6′ | 3.63 | 3.71 | 3.73 | 3.73 | 3.74 | 3.74 | 3.75 |
| MeO | 3.40 | - | 3.43 | - | 3.44 | - | - |
| Sample | Complex Viscosity (cP) | G′ (Pa) | G′′ (Pa) |
|---|---|---|---|
| Mucin | 0.573 ± 0.032 | 1.18 ± 0.20 | 6.32 ± 0.24 |
| Mucin + MβCD | 0.491 ± 0.043 * | 1.08 ± 0.56 | 4.04 ± 0.53 ** |
| Mucin + MβCD-SH | 0.786 ± 0.08 * | 1.24 ± 0.36 | 7.78 ± 0.27 ** |
| Compound | Concentration (% w/v) | Aggregates Size ± Standard Deviation (nm) |
|---|---|---|
| MβCD | 0.30 ÷ 12.50 | not detectable aggregates |
| MβCD-SH | 12.50 | 2.94 ± 0.08 |
| MβCD-SH | 5.00 | 2.86 ± 0.29 |
| MβCD-SH | 0.30 | 2.54 ± 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassiri, B.; Cesari, A.; Balzano, F.; Migone, C.; Kali, G.; Bernkop-Schnürch, A.; Uccello-Barretta, G.; Zambito, Y.; Piras, A.M. Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization. Polymers 2022, 14, 3170. https://doi.org/10.3390/polym14153170
Grassiri B, Cesari A, Balzano F, Migone C, Kali G, Bernkop-Schnürch A, Uccello-Barretta G, Zambito Y, Piras AM. Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization. Polymers. 2022; 14(15):3170. https://doi.org/10.3390/polym14153170
Chicago/Turabian StyleGrassiri, Brunella, Andrea Cesari, Federica Balzano, Chiara Migone, Gergely Kali, Andreas Bernkop-Schnürch, Gloria Uccello-Barretta, Ylenia Zambito, and Anna Maria Piras. 2022. "Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization" Polymers 14, no. 15: 3170. https://doi.org/10.3390/polym14153170







