Potential Impacts of Prunus domestica Based Natural Gum on Physicochemical Properties of Polyaniline for Corrosion Inhibition of Mild and Stainless Steel
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PDG-Grafted Polyaniline (PDG-g-PANI)
2.3. Optimization
2.4. Characterization
2.5. Corrosion Study
3. Results and Discussion
3.1. Solubility
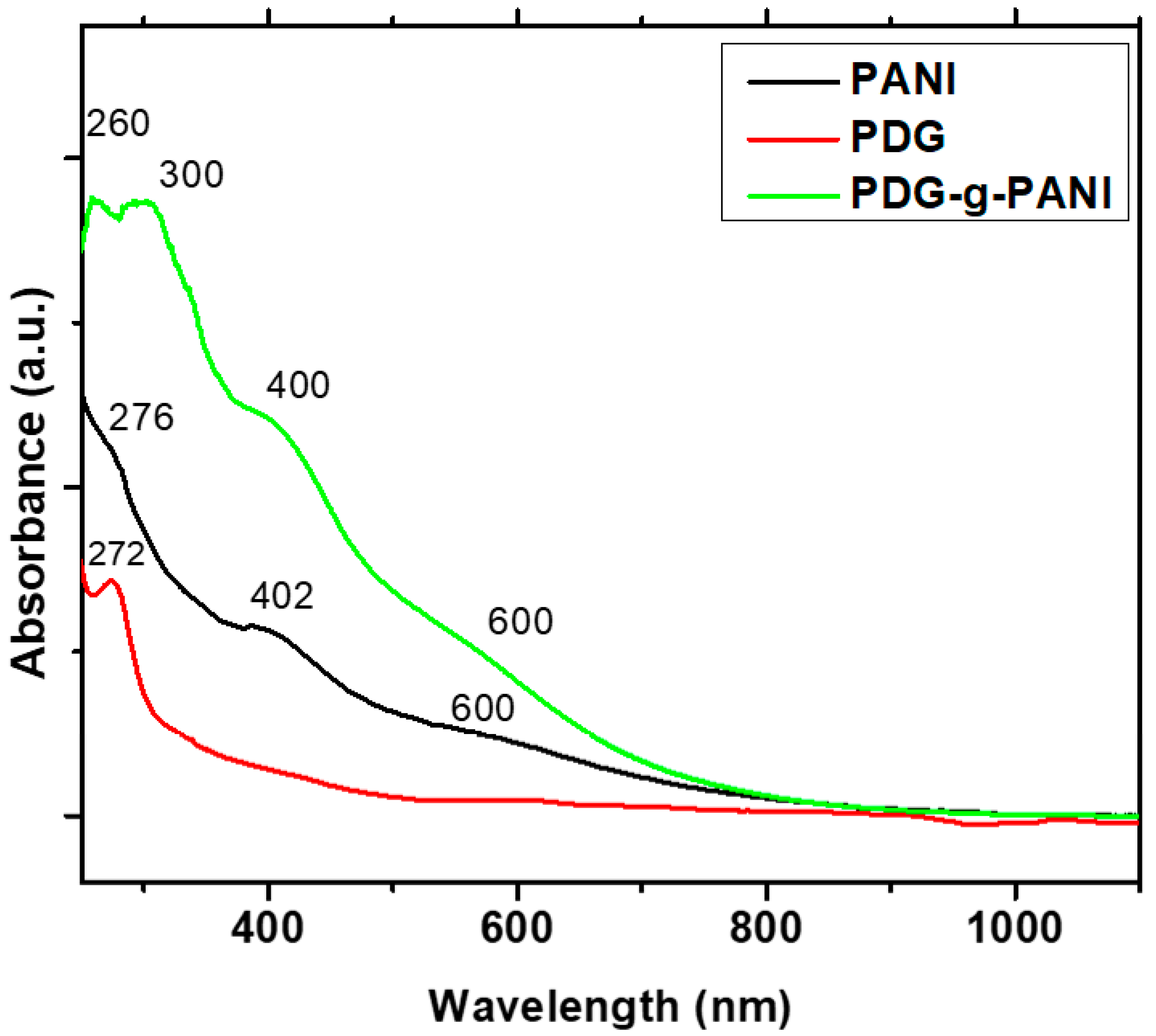
3.2. UV-Vis Spectroscopy
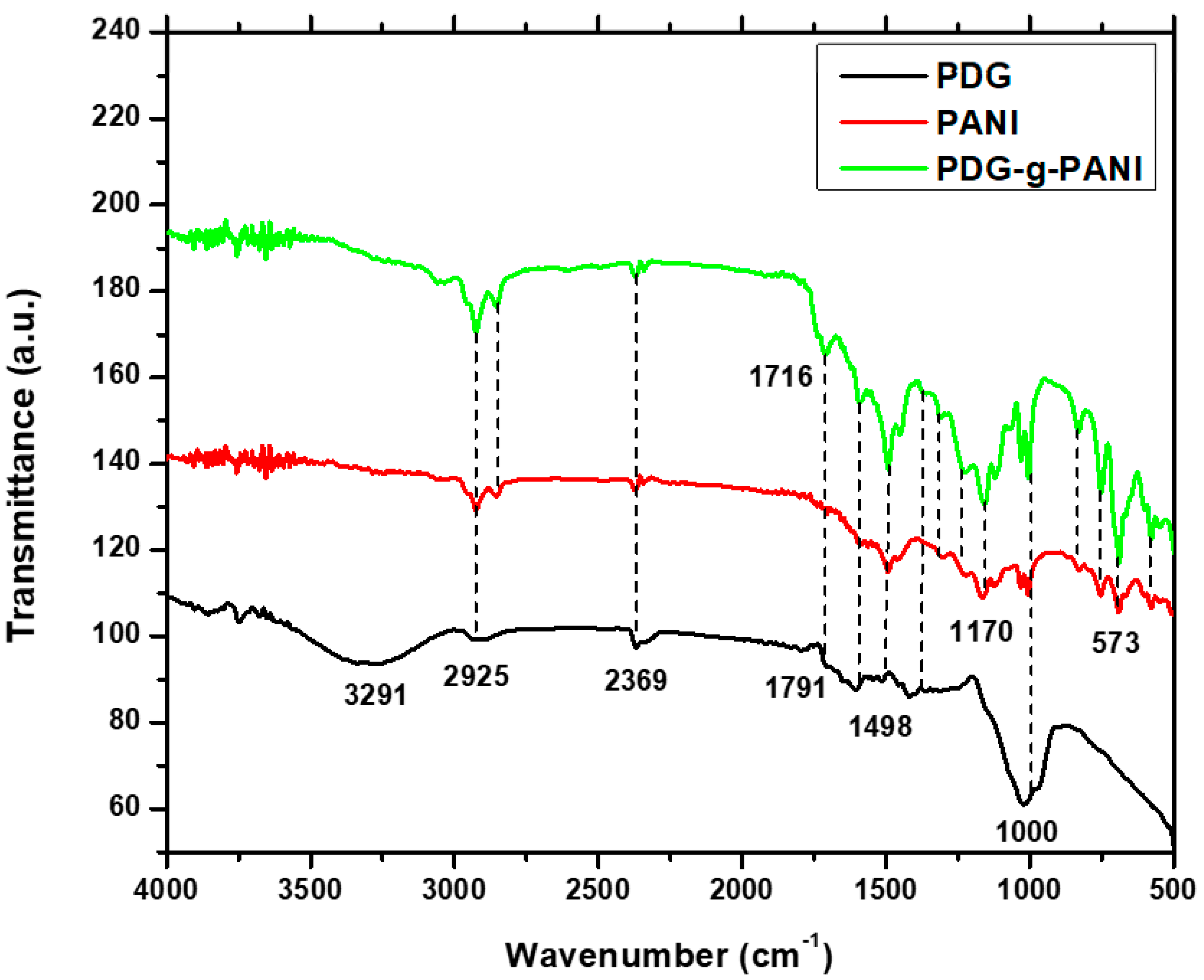
3.3. FTIR Spectroscopy
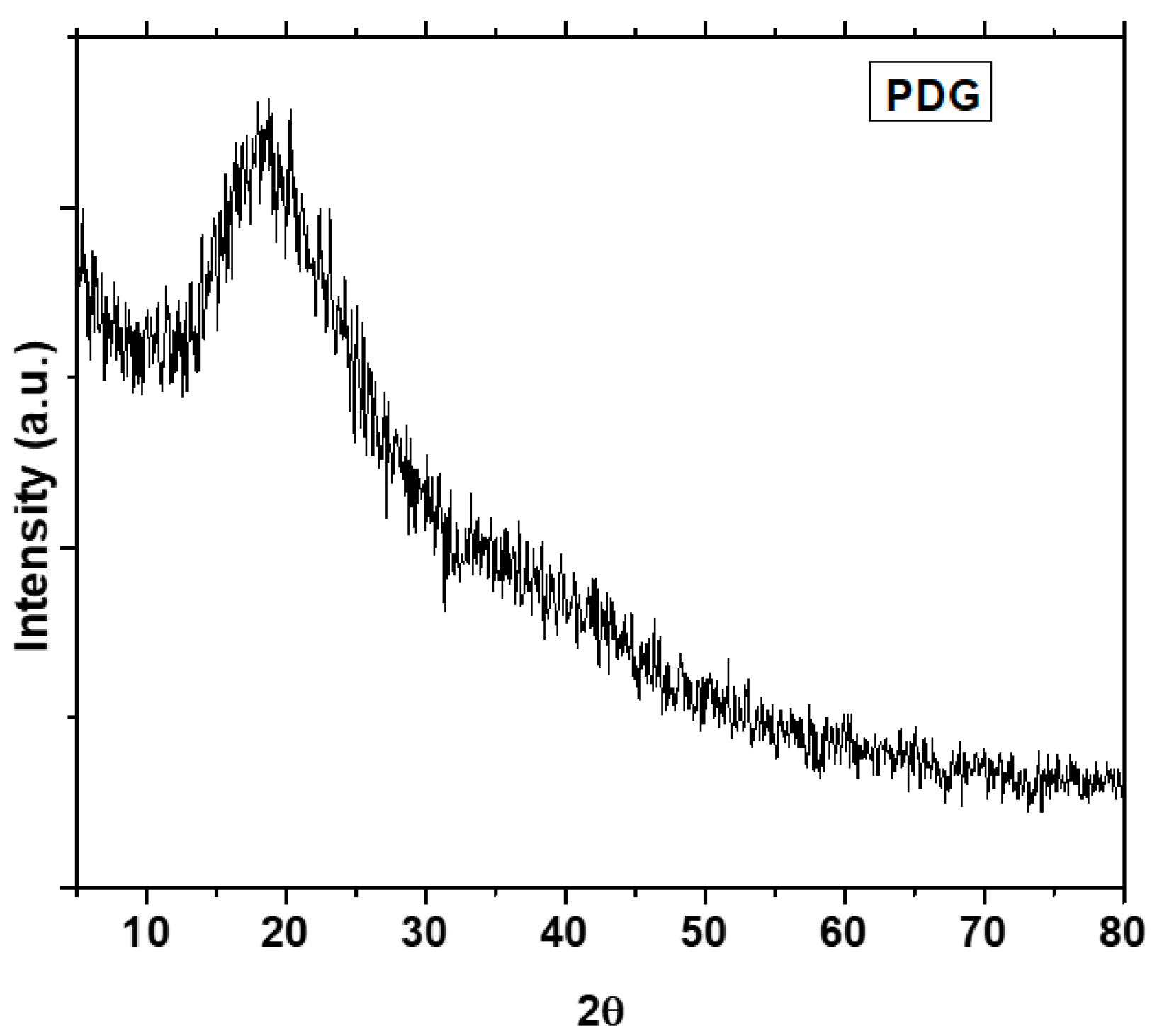
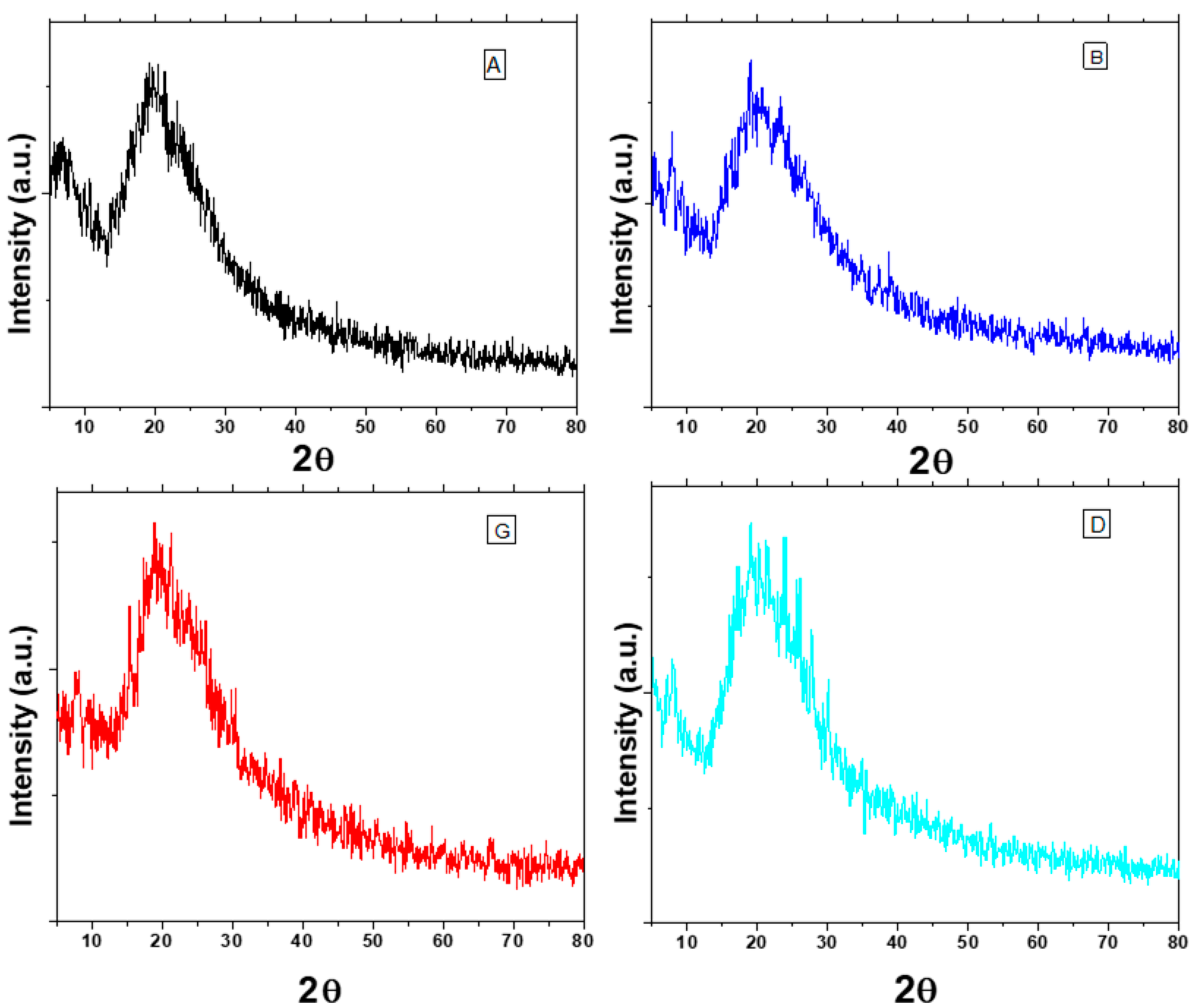
3.4. X-ray Diffraction (PXRD) Analysis
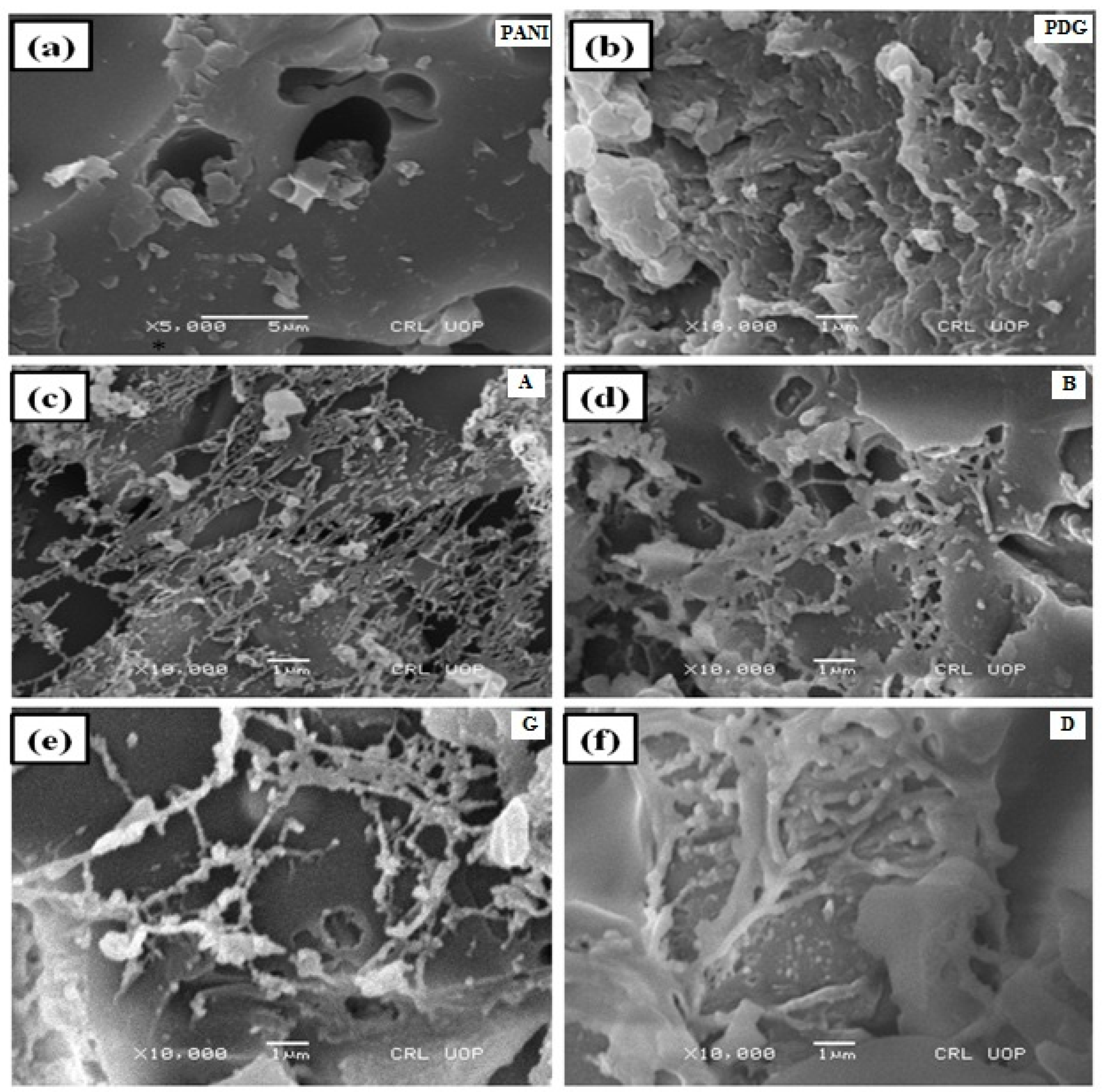
3.5. Scanning Electron Microscopy (SEM)
3.6. Corrosion Study
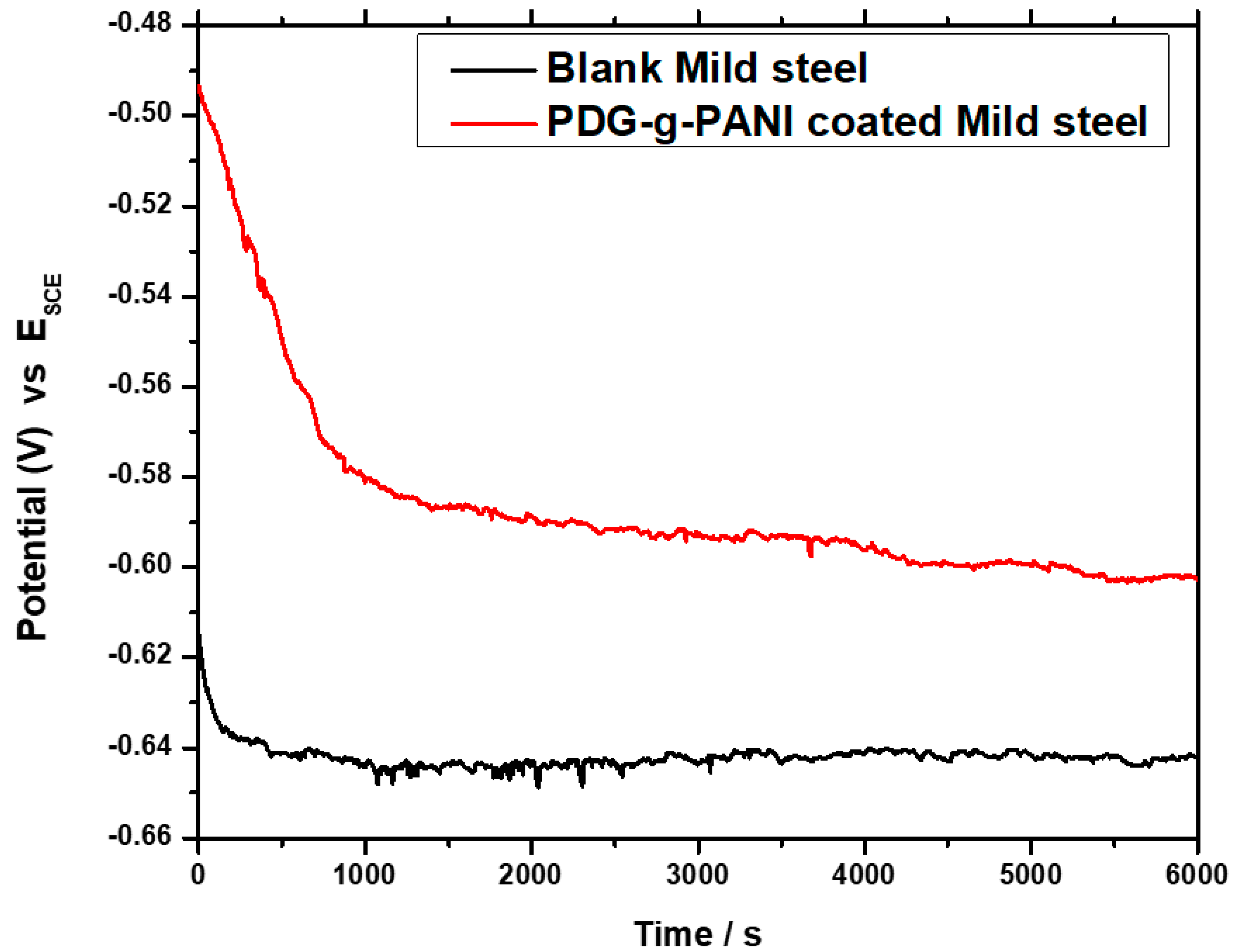
3.6.1. Open Circuit Potential
3.6.2. Electrochemical Impedance Spectroscopy (EIS)
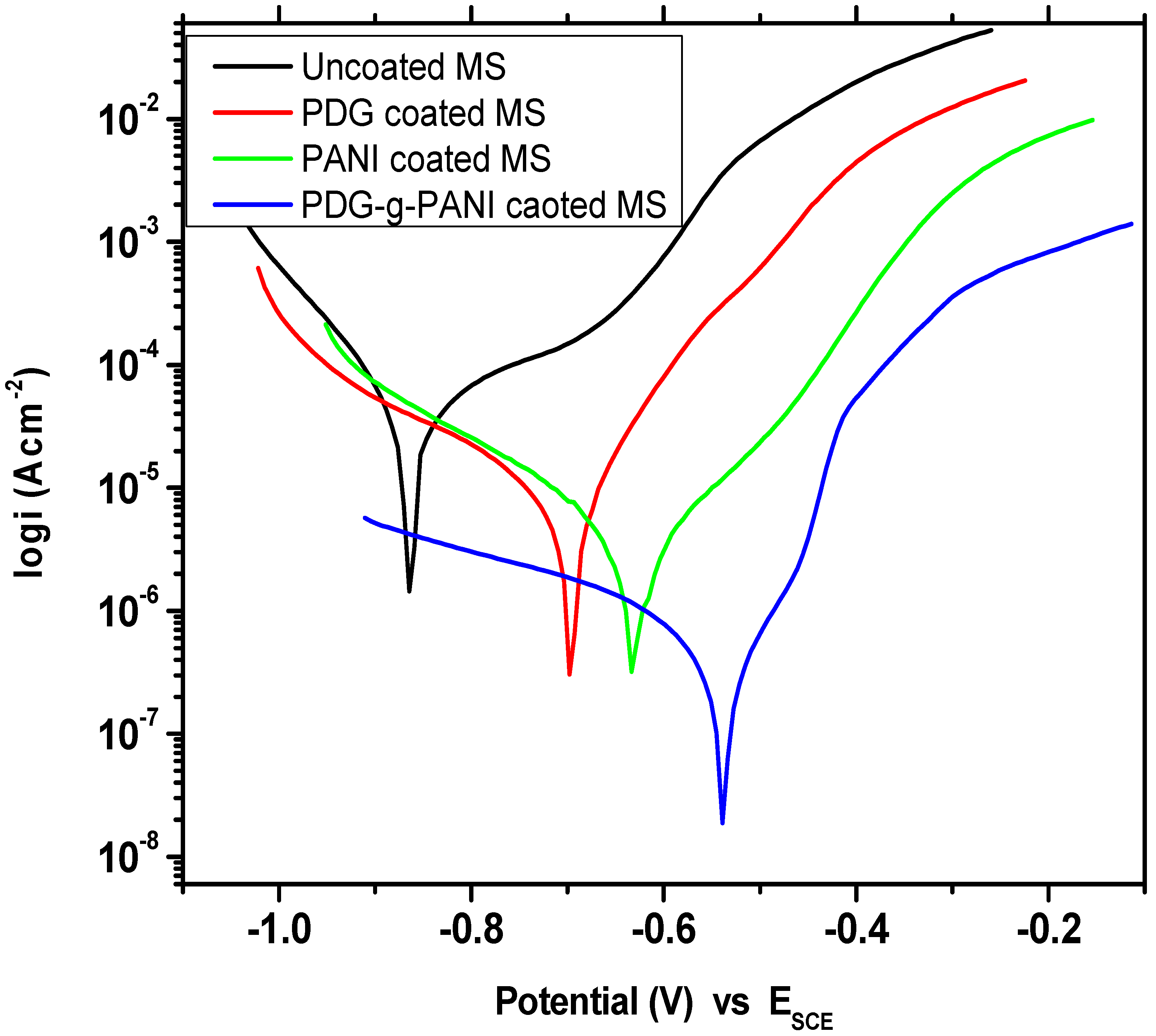
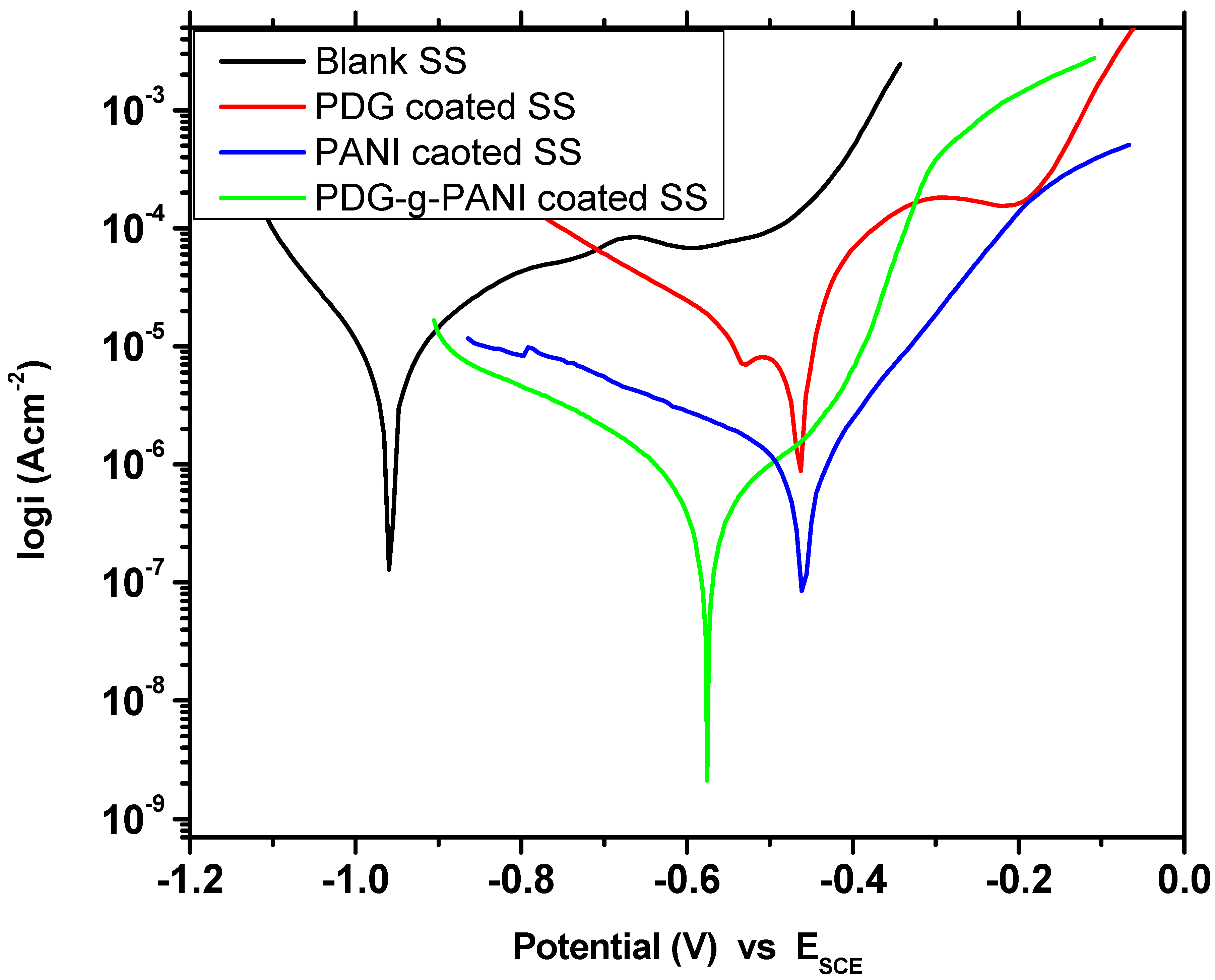
3.6.3. Potentiodynamic Polarization Curve (Tafel Plot)
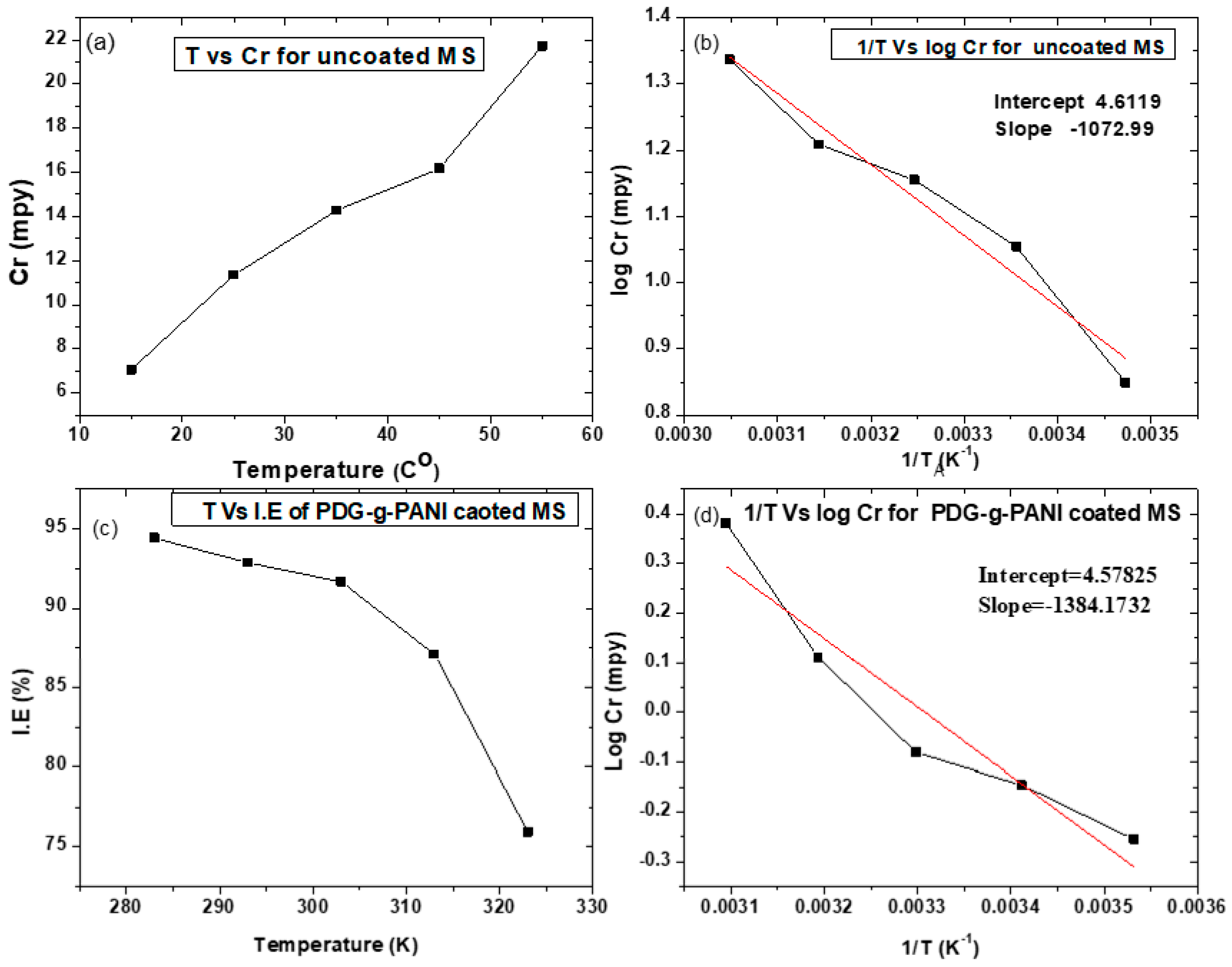
3.6.4. Effect of Temperature
3.6.5. Corrosion Studies in Sulphuric Acid Medium
3.7. Kinetic and Gravimetric Studies
Open Air Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nawaz, M.; Shakoor, R.; Kahraman, R.; Montemor, M. Cerium oxide loaded with Gum Arabic as environmentally friendly anti-corrosion additive for protection of coated steel. Mater. Des. 2020, 198, 109361. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Wang, M.; Yun, H.; Tan, K.; Guo, A.; Ling, J.; Jiang, F.; Shen, X.; Xu, Q. One-step electrochemical synthesis of poly(vinyl pyrrolidone) modified polyaniline coating on stainless steel for high corrosion protection performance. Prog. Org. Coat. 2020, 149, 105908. [Google Scholar] [CrossRef]
- Peter, A.; Obot, I.B.; Sharma, S.K. Use of natural gums as green corrosion inhibitors: An overview. Int. J. Ind. Chem. 2015, 6, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion Protection of Copper Using Al2O3, TiO2, ZnO, HfO2, and ZrO2 Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Möhwald, H.; Ferreira, M.G.S. Anticorrosion Coatings with Self-Healing Effect Based on Nanocontainers Impregnated with Corrosion Inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Montemor, M. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Nawaz, M.; Yusuf, N.; Habib, S.; Shakoor, R.A.; Ubaid, F.; Ahmad, Z.; Kahraman, R.; Mansour, S.; Gao, W. Development and Properties of Polymeric Nanocomposite Coatings. Polymers 2019, 11, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBerry, D.W. Modification of the Electrochemical and Corrosion Behavior of Stainless Steels with an Electroactive Coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Liou, S.-J.; Lai, C.-Y.; Wu, P.-C.; Tsai, T.-Y. Enhancement of Corrosion Protection Effect in Polyaniline via the Formation of Polyaniline−Clay Nanocomposite Materials. Chem. Mater. 2001, 13, 1131–1136. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Liu, X.; Shi, C.; Wang, Y.; Meng, G.; Zeng, X.; Yang, Y. A study on corrosion protection of different polyaniline coatings for mild steel. Prog. Org. Coat. 2017, 111, 240–247. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Wang, X.; Yuan, S.; Lin, D.; Zhu, Y.; Wang, H. Synthesis of hydrophobic fluoro-substituted polyaniline filler for the long-term anti-corrosion performance enhancement of epoxy coatings. Corros. Sci. 2020, 178, 109094. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Vandghanooni, S.; Eskandani, M. Electrically conductive biomaterials based on natural polysaccharides: Challenges and applications in tissue engineering. Int. J. Biol. Macromol. 2019, 141, 636–662. [Google Scholar] [CrossRef] [PubMed]
- Zheludkevich, M.L.; Tedim, J.; Freire, C.S.R.; Fernandes, S.C.; Kallip, S.; Lisenkov, A.; Gandini, A.; Ferreira, M.G.S. Self-healing protective coatings with “green” chitosan based pre-layer reservoir of corrosion inhibitor. J. Mater. Chem. 2011, 21, 4805–4812. [Google Scholar] [CrossRef]
- Shen, C.; Alvarez, V.; Koenig, J.D.B.; Luo, J.-L. Gum Arabic as corrosion inhibitor in the oil industry: Experimental and theoretical studies. Corros. Eng. Sci. Technol. 2019, 54, 444–454. [Google Scholar] [CrossRef]
- Singh, A.; Mohamed, H.S.; Singh, S.; Yu, H.; Lin, Y. Corrosion inhibition using guar gum grafted 2-acrylamido-2-methylpropanesulfonic acid (GG-AMPS) in tubular steel joints. Constr. Build. Mater. 2020, 258, 119728. [Google Scholar] [CrossRef]
- Palumbo, G.; Górny, M.; Banaś, J. Corrosion Inhibition of Pipeline Carbon Steel (N80) in CO2-Saturated Chloride (0.5 M of KCl) Solution Using Gum Arabic as a Possible Environmentally Friendly Corrosion Inhibitor for Shale Gas Industry. J. Mater. Eng. Perform. 2019, 28, 6458–6470. [Google Scholar] [CrossRef] [Green Version]
- Eddy, N.O.; Ameh, P.; Gimba, C.E.; Ebenso, E.E. GCMS studies on Anogessus leocarpus (Al) gum and their corrosion inhibition potential for mild steel in 0.1 M HCl. Int. J. Electrochem. Sci. 2011, 6, 5815–5829. [Google Scholar]
- Mobin, M.; Rizvi, M. Inhibitory effect of xanthan gum and synergistic surfactant additives for mild steel corrosion in 1 M HCl. Carbohydr. Polym. 2016, 136, 384–393. [Google Scholar] [CrossRef]
- Aslam, J.; Mobin, M.; Huda; Aslam, A.; Aslam, R. Corrosion inhibition performance of multi-phytoconstituents from Eucalyptus bark extract on mild steel corrosion in 5% HCl solution. Int. J. Environ. Sci. Technol. 2022; published online. [Google Scholar] [CrossRef]
- Anh, H.; Vu, N.; Huyen, L.; Tran, N.; Thu, H.; Bach, L.; Trinh, Q.; Vattikuti, S.P.; Nam, N. Ficus racemosa leaf extract for inhibiting steel corrosion in a hydrochloric acid medium. Alex. Eng. J. 2020, 59, 4449–4462. [Google Scholar] [CrossRef]
- Rashid, M.; Sabir, S.; Rahim, A.A.; Waware, U. Polyaniline/Palm Oil Blend for Anticorrosion of Mild Steel in Saline Environment. J. Appl. Chem. 2014, 2014, 973653. [Google Scholar] [CrossRef] [Green Version]
- Babaladimath, G.; Vishalakshi, B.; Nandibewoor, S. Electrical conducting Xanthan Gum-graft-polyaniline as corrosion inhibitor for aluminum in hydrochloric acid environment. Mater. Chem. Phys. 2018, 205, 171–179. [Google Scholar] [CrossRef]
- Sharma, A.; Bhushette, P.R.; Annapure, U.S. Purification and physicochemical characterization of Prunus domestica exudate gum polysaccharide. Carbohydr. Polym. Technol. Appl. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Choudhary, S.; Garg, A.; Mondal, K. Relation Between Open Circuit Potential and Polarization Resistance with Rust and Corrosion Monitoring of Mild Steel. J. Mater. Eng. Perform. 2016, 25, 2969–2976. [Google Scholar] [CrossRef]
- Kumar, K.V.; Rao, B.A. Phosphorylated xanthan gum, an environment-friendly, efficient inhibitor for mild steel corrosion in aqueous 200 ppm NaCl. Mater. Today Proc. 2019, 15, 155–165. [Google Scholar] [CrossRef]
- Bilal, S.; Gul, S.; Holze, R.; Shah, A.-U.H.A. An impressive emulsion polymerization route for the synthesis of highly soluble and conducting polyaniline salts. Synth. Met. 2015, 206, 131–144. [Google Scholar] [CrossRef]
- Tiwari, A. Gum Arabic-Graft-Polyaniline: An Electrically Active Redox Biomaterial for Sensor Applications. J. Macromol. Sci. Part A 2007, 44, 735–745. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, V. Microwave-induced synthesis of electrical conducting gum acacia-graft-polyaniline. Carbohydr. Polym. 2008, 74, 427–434. [Google Scholar] [CrossRef]
- Shah, A.-U.A.; Kamran, M.; Bilal, S.; Ullah, R. Cost Effective Chemical Oxidative Synthesis of Soluble and Electroactive Polyaniline Salt and Its Application as Anticorrosive Agent for Steel. Materials 2019, 12, 1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingam, R.; Al-Lohedan, H.; Radhika, T. Synthesis, surface and textural characterization of Ag doped Polyaniline-SiO2 (Pan-Ag/RHA) nanocomposites derived from biomass materials. Dig. J. Nanomater. Biostructures (DJNB) 2016, 11, 731–740. [Google Scholar]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M.; Zaib, S.; Iqbal, J. A multi-target therapeutic potential of Prunus domestica gum stabilized nanoparticles exhibited prospective anticancer, antibacterial, urease-inhibition, anti-inflammatory and analgesic properties. BMC Complement. Altern. Med. 2017, 17, 276. [Google Scholar] [CrossRef] [PubMed]
- Rahim, H.; Khan, M.A.; Badshah, A.; Chishti, K.A.; Khan, S.; Junaid, M.; Patil, M.P.; Gaikwad, N.J. Evaluation of Prunus domestica gum as a novel tablet binder. Braz. J. Pharm. Sci. 2014, 50, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Ullah, R.; Yaseen, S.; Shah, A.-U.A.; Bilal, S.; Kamran, M.; Rahim, M. Anticorrosive polyaniline synthesized using coconut oil as the dispersion medium. Mater. Chem. Phys. 2021, 273, 125071. [Google Scholar] [CrossRef]
- Meka, V.S.; Nali, S.R.; Songa, A.S.; Kolapalli, V.R.M. Characterization and In Vitro Drug Release Studies of a Natural Polysaccharide Terminalia catappa Gum (Badam Gum). AAPS PharmSciTech 2012, 13, 1451–1464. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, J.; Chen, Y.; Lu, H.; Zhuang, J. Electrochemical polymerization of polyaniline doped with Cu2+ as the electrode material for electrochemical supercapacitors. RSC Adv. 2013, 4, 5547–5552. [Google Scholar] [CrossRef]
- Pandey, S.; Ramontja, J. Rapid, facile microwave-assisted synthesis of xanthan gum grafted polyaniline for chemical sensor. Int. J. Biol. Macromol. 2016, 89, 89–98. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, P.; Lu, Z.; Chen, S.; Wang, L. Synthesis and corrosion protection of Nb doped TiO2 nanopowders modified polyaniline coating on 316 stainless steel bipolar plates for proton-exchange membrane fuel cells. Prog. Org. Coat. 2019, 137, 105327. [Google Scholar] [CrossRef]
- Mahato, N.; Cho, M.H. Graphene integrated polyaniline nanostructured composite coating for protecting steels from corrosion: Synthesis, characterization, and protection mechanism of the coating material in acidic environment. Constr. Build. Mater. 2016, 115, 618–633. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Mobin, M.; Ahmad, I.; Basik, M.; Murmu, M.; Banerjee, P. Experimental and theoretical assessment of almond gum as an economically and environmentally viable corrosion inhibitor for mild steel in 1 M HCl. Sustain. Chem. Pharm. 2020, 18, 100337. [Google Scholar] [CrossRef]
- Pakiet, M.; Kowalczyk, I.H.; Garcia, R.L.; Akid, R.; Brycki, B.E. Influence of different counterions on gemini surfactants with polyamine platform as corrosion inhibitors for stainless steel AISI 304 in 3 M HCl. J. Mol. Liq. 2018, 268, 824–831. [Google Scholar] [CrossRef] [Green Version]
- Pokhmurs’kyi, V.; Kornii, S.; Karpenko, O.; Khlopyk, O.; Korets’ka, N. Inhibition of the Corrosion of Carbon Steel by Xanthan Biopolymer. Mater. Sci. 2020, 55, 522–528. [Google Scholar] [CrossRef]
- Ahmad, N.; MacDiarmid, A.G. Inhibition of corrosion of steels with the exploitation of conducting polymers. Synth. Met. 1996, 78, 103–110. [Google Scholar] [CrossRef]
- Ansari, R.; Keivani, M.B. Polyaniline Conducting Electroactive Polymers Thermal and Environmental Stability Studies. E-J. Chem. 2006, 3, 202–217. [Google Scholar] [CrossRef] [Green Version]
- Shahini, M.; Ramezanzadeh, B.; Mohammadloo, H.E. Recent advances in biopolymers/carbohydrate polymers as effective corrosion inhibitive macro-molecules: A review study from experimental and theoretical views. J. Mol. Liq. 2020, 325, 115110. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Giacomelli, F.C.; Spinelli, A. Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Li, W.-H.; He, Q.; Zhang, S.-T.; Pei, C.-L.; Hou, B.-R. Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J. Appl. Electrochem. 2007, 38, 289–295. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Adindu, C.B.; Enenebeaku, C.K.; Ogukwe, C.E.; Chidiebere, M.A.; Oguzie, K.L. Natural Products for Materials Protection: Mechanism of Corrosion Inhibition of Mild Steel by Acid Extracts of Piper guineense. J. Phys. Chem. C 2012, 116, 13603–13615. [Google Scholar] [CrossRef]





| Sample Name | Rct Ohm | Rs Ohm | CPE F | Inhibition Efficiency % |
|---|---|---|---|---|
| Uncoated MS | 4.046 | 68.90 | 64.94 × 10−6 | - |
| PDG-g-PANI-coated MS | 208.9 | 78.70 | 3.696 × 10−6 | 98.06 |
| Sample Name | Beta A V/Decade | Beta C V/Decade | Icorr μA/cm2 | Ecorr mV | Corrosion Rate (m/Year) | Inhibition Efficiency % |
|---|---|---|---|---|---|---|
| Uncoated MS | 0.170 | 0.089 | 21.90 | −866 | 10.00 | - |
| PDG | 0.135 | 0.297 | 12.50 | −697 | 5.68 | 43.2 |
| PANI | 0.127 | 0.189 | 3.09 | −630 | 1.410 | 85.89 |
| PDG-g-PANI | 0.119 | 0.407 | 0.691 | −538 | 0.315 | 96.84 |
| Sample Name | Beta A V/Decade | Beta C V/Decade | Icorr μAcm−2 | Ecorr mV | Corrosion Rate (m/Year) | Inhibition Efficiency % |
|---|---|---|---|---|---|---|
| Uncoated SS | 0.444 | 0.163 | 11.90 | −960 | 5.421 | - |
| PDG | 0.184 | 0.265 | 8.70 | −467 | 3.976 | 26.89 |
| PANI | 0.146 | 0.456 | 1.56 | −459 | 0.714 | 86.89 |
| PDG-g-PANI | 0.109 | 0.180 | 0.232 | −573 | 0.105 | 98.05 |
| Sample Name | (KJ) |
|---|---|
| MS | 20.54 |
| PDG-g-PANI-coated MS | 26.50 |
| Sample Name | Beta A V/Decade | Beta C V/Decade | Icorr μAcm−2 | Ecorr mV | Corrosion Rate (m/Year) | Inhibition Efficiency % |
|---|---|---|---|---|---|---|
| Uncoated MS | 0.159 | 0.209 | 1480 | −508 | 675.3 | - |
| PDG-g-PANI-coated MS | 0.077 | 0.200 | 4.680 | −376 | 2.139 | 99.68 |
| Sample Name | Beta A V/Decade | Beta C V/Decade | Icorr μAcm−2 | Ecorr mV | Corrosion Rate (m/Year) | Inhibition Efficiency % |
|---|---|---|---|---|---|---|
| Uncoated SS | 0.138 | 0.257 | 4720 | −491 | 2157 | - |
| PDG-g-PANI | 0.092 | 0.203 | 111 | −487 | 50.84 | 97.64 |
| Sample Name | Weight before Immersion (g) | Weight after Immersion (g) | Weight Loss (g) | Corrosion Rate (m/Year) | Inhibition Efficiency % |
|---|---|---|---|---|---|
| Uncoated MS | 62.8049 | 62.7979 | 0.07 | 2.33 | - |
| PDG-g-PANI-coated MS | 61.2450 | 61.2445 | 0.005 | 0.16 | 93.13 |
| Sample Name | Weight before Exposure (g) | Weight after Exposure (g) | Weight Loss (g) | Corrosion Rate (m/Year) | Inhibition Efficiency % |
|---|---|---|---|---|---|
| Uncoated MS | 48.8160 | 48.8028 | 0.0132 | 0.1 | - |
| PDG-g-PANI | 49.2429 | 49.2425 | 0.0004 | 0.0031 | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamran, M.; Shah, A.u.H.A.; Rahman, G.; Bilal, S. Potential Impacts of Prunus domestica Based Natural Gum on Physicochemical Properties of Polyaniline for Corrosion Inhibition of Mild and Stainless Steel. Polymers 2022, 14, 3116. https://doi.org/10.3390/polym14153116
Kamran M, Shah AuHA, Rahman G, Bilal S. Potential Impacts of Prunus domestica Based Natural Gum on Physicochemical Properties of Polyaniline for Corrosion Inhibition of Mild and Stainless Steel. Polymers. 2022; 14(15):3116. https://doi.org/10.3390/polym14153116
Chicago/Turabian StyleKamran, Muhammad, Anwar ul Haq Ali Shah, Gul Rahman, and Salma Bilal. 2022. "Potential Impacts of Prunus domestica Based Natural Gum on Physicochemical Properties of Polyaniline for Corrosion Inhibition of Mild and Stainless Steel" Polymers 14, no. 15: 3116. https://doi.org/10.3390/polym14153116







