Recyclability of Opaque PET from High Speed Melt Spinning: Determination of the Structures and Properties of Filaments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Materials
2.1.2. Extruded Blends
2.2. Processing Methods
2.2.1. Extrusion
2.2.2. Melt Spinning
- Initial velocity of filament:
- Hencky strain :
- Draw ratio (DR):
- Take-up speed :
2.3. Characterization Methods
2.3.1. TGA
2.3.2. SEM/EDX
2.3.3. DSC
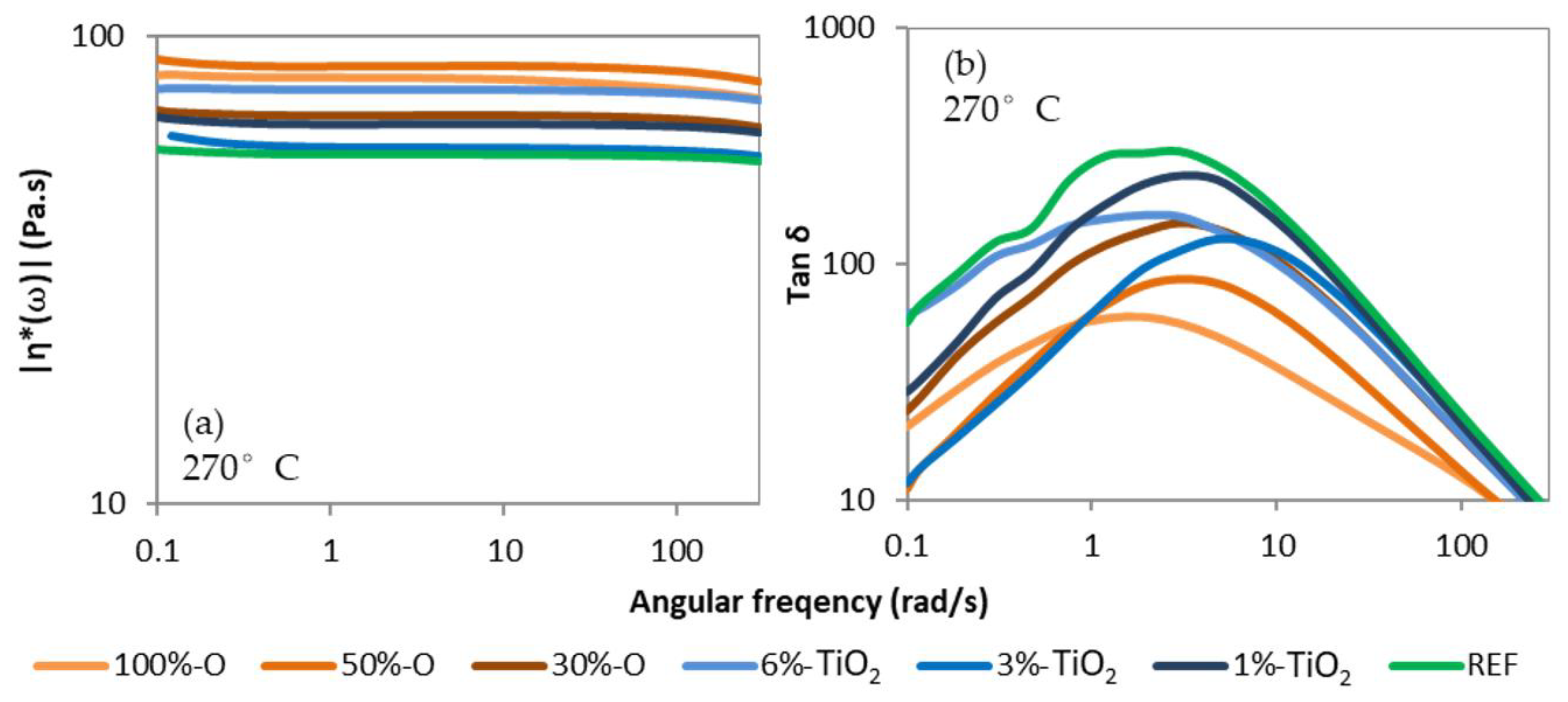
2.3.4. Rheology/Absolute Complex Viscosity
2.3.5. Intrinsic Viscosity
2.3.6. Melt Strength
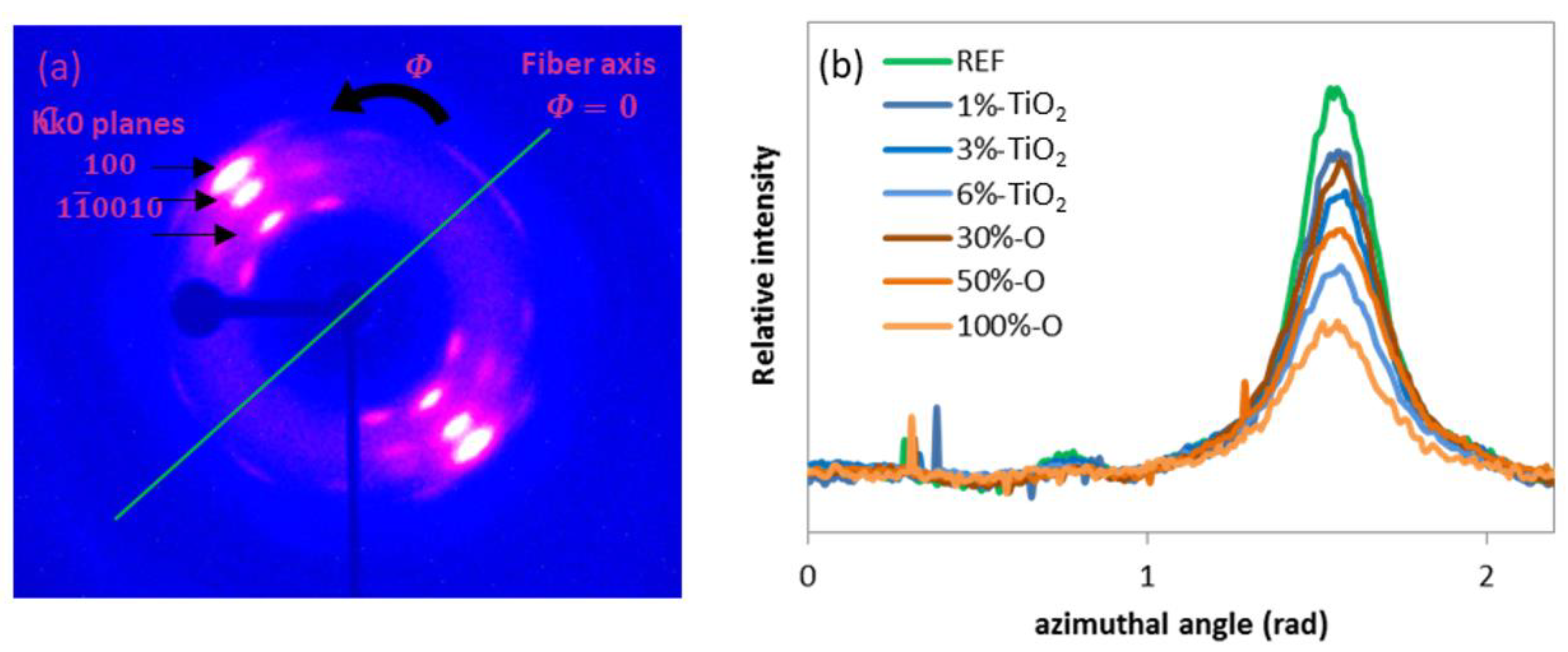
2.3.7. WAXS
2.3.8. Stress-Strain Curves and Tenacity
3. Results and Discussion
3.1. Raw Materials and Extruded Samples
3.2. PET Melt Spinning Filaments
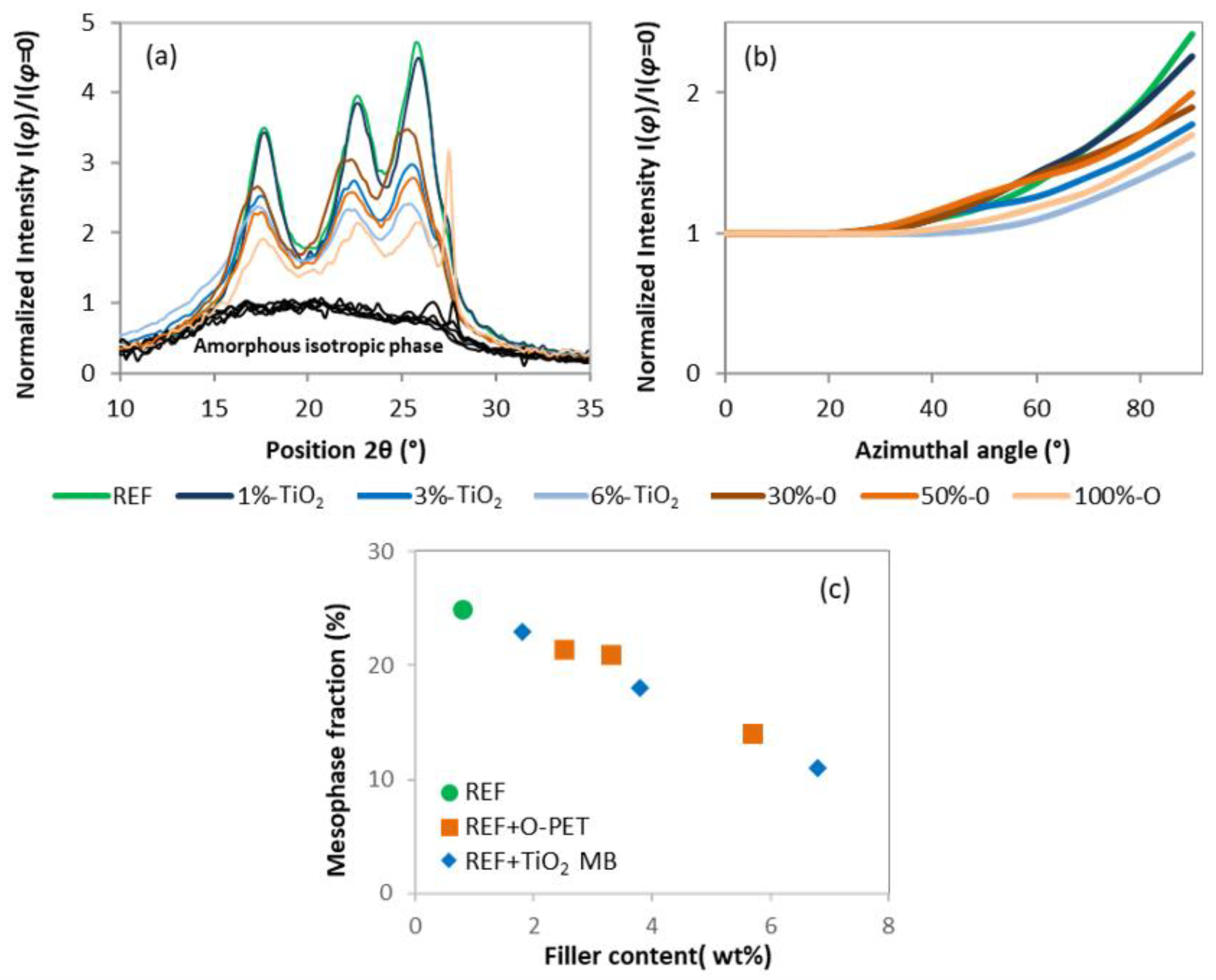
3.2.1. Crystallinity
3.2.2. Molecular Orientation
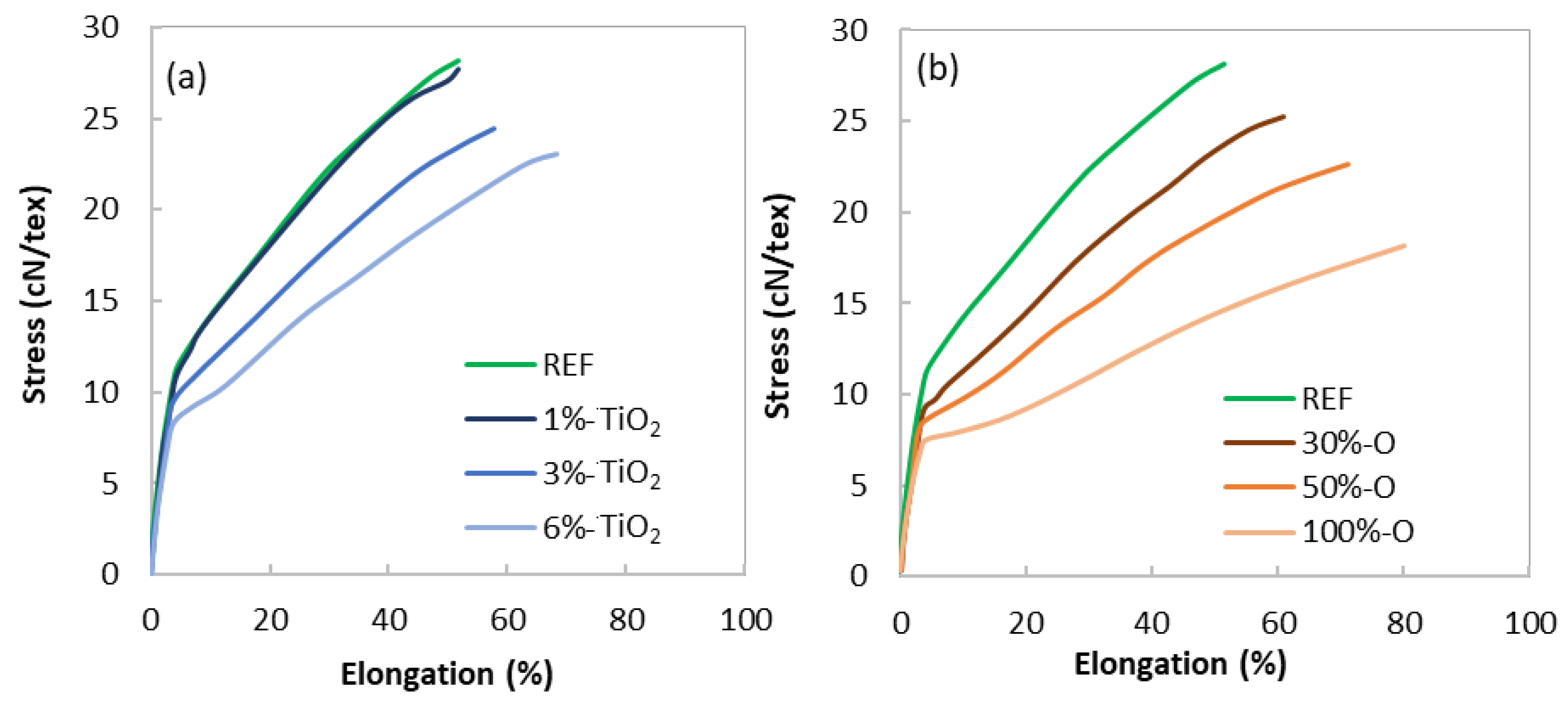
3.2.3. Mechanical Properties
4. Conclusions
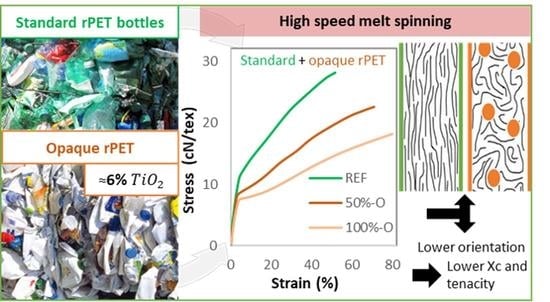
- The degree of crystallinity, along with the crystalline orientation, is lower for opaque PET compared to standard rPET, from 40% to 30% and 0.83 to 0.61, respectively.
- Mesophase fraction seems to decrease from 25% to 15% due to a reduced molecular orientation during high-speed spinning.
- The tenacity of the melt-spun filaments decreases with the addition of opaque PET, from 29 cN/tex without opaque PET to 19 cN/tex at 100% opaque PET. The formulation containing TiO2 from the commercial masterbatch and no opaque PET follows the same results but to a lesser extent, with a tenacity of 23 cN/tex at 6.8% of filler (6% of TiO2).
- The more TiO2 is added, the lower the crystallinity, molecular orientation, and tenacity.
- Opaque PET filaments have lower properties than standard rPET filled with TiO2 from the masterbatch at the same filler content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PlasticsEurope.Plastics-the-Facts 2019. Available online: http://www.plasticseurope.org/Document/plastics-the-facts2019.aspx?FolID=2 (accessed on 11 December 2021).
- 2018 Fact Sheet. 2020. Available online: https://www.epa.gov/sites/default/files/2020-11/documents/2018_ff_fact_sheet.pdf (accessed on 18 January 2022).
- Sarioğlu, E.; Kaynak, H.K. PET Bottle Recycling for Sustainable Textiles. In Polyester—Production, Characterization and Innovative Applications; Nurhan Onar Camlibel, Ed.; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/58300 (accessed on 23 May 2022). [CrossRef] [Green Version]
- Ziabicki Orientation Mechanisms in the Development of High-Performance Fibers. Prog. Colloid Polym. Sci. 1993, 92, 1–7.
- Ziabicki, A.; Jarecki, L.; Sorrentino, A. The Role of Flow-Induced Crystallisation in Melt Spinning. E-Polymers 2004, 4, 823–836. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Kothary, V.B. Manufactured Fibre Technology; Springer Science & Business Media: Dordrecht, The Netherlands, 1997; ISBN 978-94-010-6473-6. [Google Scholar]
- White, J.L.; Cakmak, M. Orientation Development and Crystallization in Melt Spinning of Fibers. Adv. Polym. Technol. 1986, 6, 295–337. [Google Scholar] [CrossRef]
- Ziabicki, A. Effects of Molecular Weight Spinning and Mechanical Properties of High-Performance Poly (Ethylene Terephthalate) Fibers Correlation of Molecular Weight. Polish Acad. Sci. 1996, 66, 705–712. [Google Scholar]
- Chen, K.; Liu, Y.; Ji, H.; Zhang, Y.; Song, M.; Jiang, Q.; Zhang, Y.; Wang, H. The Evaluation of Structure and Properties of High-Strength Polyester Industrial Fibers with Different Polycondensation Processes. J. Text. Inst. 2021, 112, 727–732. [Google Scholar] [CrossRef]
- Taniguchi, A.; Cakmak, M. The Suppression of Strain Induced Crystallization in PET through Sub Micron TiO2 Particle Incorporation. Polymer 2004, 45, 6647–6654. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Wang, Y.; Gan, X.; Wang, N. Preparation and Properties of Opaque Polyethylene Terephthalate/TiO2 Filaments. Medziagotyra 2021, 27, 325–329. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, J.; Realff, M.L.; Kumar, S.; Schiraldi, D.A. Processing, Structure, and Properties of Fibers from Polyester/Carbon Nanofiber Composites. Compos. Sci. Technol. 2003, 63, 1617–1628. [Google Scholar] [CrossRef]
- Litchfield, D.W.; Baird, D.G. The Role of Nanoclay in the Generation of Poly(Ethylene Terephthalate) Fibers with Improved Modulus and Tenacity. Polymer 2008, 49, 5027–5036. [Google Scholar] [CrossRef]
- Jang, K.H.; Kim, B.C.; Hahm, W.G.; Kikutani, T. High-Speed Melt Spinning of Nanoparticle-Filled High Molecular Weight Polyethylene Terephthalate. Int. Polym. Process. 2008, 23, 370–376. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Zheng, J.; Yao, X.; Liu, W.; Cui, P.; Li, Y. Relationship between Microstructure and Tensile Properties of PET/Silica Nanocomposite Fibers. J. Macromol. Sci. Part B Phys. 2008, 47, 368–377. [Google Scholar] [CrossRef]
- Zoller, P.; Bolli, P. Pressure-Volume-Temperature Relationships of Solid and Molten Poly(Ethylene Terephthalate). J. Macromol. Sci. Part B 1980, 18, 555–568. [Google Scholar] [CrossRef]
- Tuminello, W.H. Determining Molecular Weight Distributions from the Rheological Properties of Polymer Melts. In Proceedings of the 71st The Society of Rheology Meeting, Madison, WI, USA, 17–21 October 1999. [Google Scholar]
- Solomon, O.F.; Ciutǎ, I.Z. Ciutta Determination de La Viscosite Intrinseque de Solutions de Polymeres Par Une Simple Determination de La Viscosite. J. Appl. Polym. Sci 1962, 6, 683–686. [Google Scholar] [CrossRef]
- Cogswell, F.N. Measuring the Extensional Rheology of Polymer Melts. Trans. Soc. Rheol. 1972, 16, 383–403. [Google Scholar] [CrossRef]
- Münstedt, H. Extensional Rheology and Processing of Polymeric Materials. Int. Polym. Process. 2018, 33, 594–618. [Google Scholar] [CrossRef]
- Härth, M.; Kaschta, J.; Schubert, D.W. Shear and Elongational Flow Properties of Long-Chain Branched Poly(Ethylene Terephthalates) and Correlations to Their Molecular Structure. Macromolecules 2014, 47, 4471–4478. [Google Scholar] [CrossRef]
- Aho, J. Rheological Characterization of Polymer Melts in Shear and Extension: Measurement Reliability and Data for Practical Processing. Ph.D. Thesis, Department of Materials Science, Tampere University of Technology, Hervanta, Finland, 2011. [Google Scholar]
- Laun, H.M.; Schuch, H. Transient Elongational Viscosities and Drawability of Polymer Melts. J. Rheol. 1989, 33, 119–175. [Google Scholar] [CrossRef]
- Gupta, V.B.; Kumar, S. Determination of Crystallite Orientation in Polyethylene Terephthalate Fibres. Text. Res. J. Publ. Text. Res. Inst. Inc. Text. Found. 1979, 49, 405–406. [Google Scholar]
- Wu, G.; Jiang, J.D.; Tucker, P.A.; Cuculo, J.A. Oriented Noncrystalline Structure in PET Fibers Prepared with Threadline Modification Process. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2035–2047. [Google Scholar] [CrossRef]
- Todorov, L.V. Multiscale Morphology Evolution of PET and Its Nanocomposites under Deformation. Ph.D. Thesis, University of Minho, Braga, Portugal, 2010. [Google Scholar]
- Ziabicki, A. Processes of Fiber Formation; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Fu, Y.; Busing, W.R.; Jin, Y.; Affholter, K.A.; Wunderlich, B. Structure Analysis of the Noncrystalline Material in Poly(Ethylene Terephthalate) Fibers. Macromol. Chem. Phys. 1994, 195, 803–822. [Google Scholar] [CrossRef]
- Perret, E.; Hufenus, R. Insights into Strain-Induced Solid Mesophases in Melt-Spun Polymer Fibers. Polymer 2021, 229, 124010. [Google Scholar] [CrossRef]
- Prevorsek, D.C.; Kwon, Y.D.; Sharma, R.K. Structure and Properties of Nylon 6 and PET Fibres: The Effects of Crystallite Dimensions. J. Mater. Sci. 1977, 12, 2310–2328. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A General-Purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Perret, E.; Braun, O.; Sharma, K.; Tritsch, S.; Muff, R.; Hufenus, R. High-Resolution 2D Raman Mapping of Mono- and Bicomponent Filament Cross-Sections. Polymer 2021, 229, 124011. [Google Scholar] [CrossRef]
- Tanaka, M.; Young, R.J. Review Polarised Raman Spectroscopy for the Study of Molecular Orientation Distributions in Polymers. J. Mater. Sci. 2006, 41, 963–991. [Google Scholar] [CrossRef]
- Kim, E.S.; Oh, H.J.; Kim, H.J.; Kim, C.G.; Park, S.Y.; Jeong, Y.G.; Hahm, W.G. Effect of Polycondensation Catalyst on Fiber Structure Development in High-Speed Melt Spinning of Poly (Ethylene Terephthalate). Polymers 2019, 11, 1931. [Google Scholar] [CrossRef] [Green Version]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.H.; Baidins, A.; Marganski, R.E. TiO2 Pigment Technology: A Review. Prog. Org. Coatings 1992, 20, 105–138. [Google Scholar] [CrossRef]
- Christopher, W. Macosko Rheology: Principles, Measurements and Applications; VCH Publishers Inc.: New York, NY, USA, 1994; p. 86. ISBN 1560815795. [Google Scholar]
- Yang, Z.; Xin, C.; Mughal, W.; Li, X.; He, Y. High-Melt-Elasticity Poly(Ethylene Terephthalate) Produced by Reactive Extrusion with a Multi-Functional Epoxide for Foaming. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Beyreuther, R.; Vogel, R. Spinnability of Polymer Melts - A Complex Problem in Basic Research. Int. Polym. Process. 1996, 11, 154–158. [Google Scholar] [CrossRef]
- Doufas, A.K.; McHugh, A.J.; Miller, C. Simulation of Melt Spinning Including Flow-Induced Crystallization Part I. Model Development and Predictions. J. Nonnewton. Fluid Mech. 2000, 92, 27–66. [Google Scholar] [CrossRef]
- Beyreuther, R.; Brünig, H. Dynamics of Fibre Formation and Processing; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Garg, S.K. Critical Stress for Crystallization in the Threadline during High-speed Spinning of Poly(Ethylene Terephthalate). Appl. Polym. Sci. 1984, 29, 2111–2116. [Google Scholar] [CrossRef]





| Name | Standard rPET | Opaque rPET |
|---|---|---|
| composition | rPET transparent flakes | Opaque rPET flakes |
| Filler content (wt %) | 0.8 | 5.7 |
| Intrinsic viscosity (IV) 1 (dL/g) | 0.82 | 0.73 |
| 1 (Pa.s) at 280 °C | 175 | 175 |
| Melting temperature (°C) | 246 | 252 |
| Crystallization temperature (°C) at 10 °C/min | 192 | 202 |
| Name | REF | 30%-O | 50%-O | 100%-O |
|---|---|---|---|---|
| Composition | standard rPET | standard rPET + 30% opaque PET mix | standard rPET + 50% opaque PET mix | 100% opaque PET mix |
| filler content (wt %) | 0.8 | 2.5 | 3.3 | 5.7 |
| (Pa.s) at 270 °C | 56 | 67 | 86 | 81 |
| Intrinsic viscosity (IV) (dL/g) | 0.56 | 0.56 | 0.55 | 0.55 |
| Name | REF | 1%-Ti | 3%-Ti | 6%-Ti |
|---|---|---|---|---|
| composition | standard rPET | |||
| 50/50 masterbatch | 0 | 2 | 6 | 12 |
| filler content wt % | 0.8 | 1.8 | 3.8 | 6.8 |
| (Pa.s) at 270 °C | 56 | 65 | 57 | 77 |
| Intrinsic viscosity (IV) (dL/g) | 0.56 | 0.56 | 0.53 | 0.52 |
| Name | REF | 1%-Ti02 | 3%-Ti02 | 6%-Ti02 | 30%-O | 50%-O | 100%-O |
|---|---|---|---|---|---|---|---|
| composition | Standard rPET | Std-rPET + 30% opaque rPET | Std-rPET + 50% opaque rPET | 100% opaque rPET | |||
| Filler content (wt %) | 0.8 ± 0.2 | 1.8 ± 0.2 | 3.8 ± 0.3 | 6.8 ± 0.5 | 2.5 ± 0.2 | 3.3 ± 0.3 | 5.7 ± 0.3 |
| Degree of crystallinity (%) | 40.3 ± 1 | 40.0 ± 1 | 37.1 ± 1 | 35.4 ± 1 | 39 ± 1 | 36.3 ± 1 | 29.9 ± 2 |
| Orientation factor | 0.83 | 0.80 | 0.76 | 0.74 | 0.82 | 0.80 | 0.61 |
| Mesophase fraction (%) | 25 | 23 | 18 | 11 | 21 | 21 | 14 |
| Tenacity (cN/tex) | 29 ± 1 | 28 ± 2 | 24.5 ± 2 | 23 ± 1 | 26.5 ± 1 | 23 ± 1 | 19 ± 1 |
| Deformation at break (%) | 58 ± 5 | 55 ± 10 | 59 ± 6 | 68 ± 4 | 67 ± 7 | 73 ± 5 | 89 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odet, F.; Ylla, N.; Fulchiron, R.; Cassagnau, P. Recyclability of Opaque PET from High Speed Melt Spinning: Determination of the Structures and Properties of Filaments. Polymers 2022, 14, 2235. https://doi.org/10.3390/polym14112235
Odet F, Ylla N, Fulchiron R, Cassagnau P. Recyclability of Opaque PET from High Speed Melt Spinning: Determination of the Structures and Properties of Filaments. Polymers. 2022; 14(11):2235. https://doi.org/10.3390/polym14112235
Chicago/Turabian StyleOdet, Félix, Noëllie Ylla, René Fulchiron, and Philippe Cassagnau. 2022. "Recyclability of Opaque PET from High Speed Melt Spinning: Determination of the Structures and Properties of Filaments" Polymers 14, no. 11: 2235. https://doi.org/10.3390/polym14112235