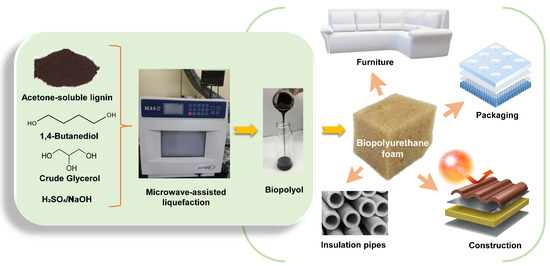
Microwave-Assisted Two-Step Liquefaction of Acetone-Soluble Lignin of Silvergrass Saccharification Residue for Production of Biopolyol and Biopolyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of A High-Lignin Residue from Miscanthus Sacchariflorus
2.3. Microwave-Assisted Two-Step Liquefaction of Acetone-Soluble Lignin
2.4. Determination of Biomass Conversion
2.5. Preparation of Polyurethane Foam
2.6. Characterization of Acetone-Soluble Lignin, Biopolyol, and Polyurethane Foams
3. Results and Discussion
3.1. Composition Analysis of High-Lignin Residue from Miscanthus
3.2. Optimization of Reaction Conditions for Microwave-Assisted Liquefaction of Acetone-Soluble Lignin
3.2.1. Effect of Solvent Blending Ratio
3.2.2. Effect of Biomass Loading
3.2.3. Effect of Catalyst Loading
3.2.4. Effect of Reaction Temperature
3.3. Characterization of Acetone-Soluble Lignin, Biopolyol, and Synthesized Polyurethane Foams
3.3.1. FT-IR Analysis
3.3.2. Thermogravimetric Analysis
3.3.3. Determination of Density and Compressive Strength
3.3.4. Determination of Cell Morphology of Polyurethane Foams by HR-SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Holdren, J.P.; Smith, K.R.; Kjellstrom, T.; Streets, D.; Wang, X.; Fischer, S. Energy, the environment and health. N. Y. United Nations Dev. Programme 2000, 3, 61–110. [Google Scholar]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.; Suely, A.; Boyce, A.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Ong, V.Z.; Wu, T.Y. An application of ultrasonication in lignocellulosic biomass valorisation into bio-energy and bio-based products. Renew. Sustain. Energy Rev. 2020, 132, 109924. [Google Scholar] [CrossRef]
- Cheng, J. Biomass to Renewable Energy Processes; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Strezov, V.; Anawar, H.M. Renewable Energy Systems from Biomass: Efficiency, Innovation and Sustainability; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hasunuma, T.; Ogino, C.; Kondo, A. Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr. Opin. Biotechnol. 2016, 42, 30–39. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Y.; Lee, D.-J. Enzymatic saccharification of lignocellulosic biorefinery: Research focuses. Bioresour. Technol. 2018, 252, 198–215. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Phan, D.-P.; Sarwar, A.; Tran, M.H.; Lee, O.K.; Lee, E.Y. Valorization of industrial lignin to value-added chemicals by chemical depolymerization and biological conversion. Ind. Crops Prod. 2021, 161, 113219. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y. Two-step sequential liquefaction of lignocellulosic biomass by crude glycerol for the production of polyols and polyurethane foams. Bioresour. Technol. 2014, 161, 410–415. [Google Scholar] [CrossRef]
- Hu, S.; Luo, X.; Li, Y. Polyols and polyurethanes from the liquefaction of lignocellulosic biomass. ChemSusChem 2014, 7, 66–72. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Z. Liquefaction of wheat straw and preparation of rigid polyurethane foam from the liquefaction products. J. Appl. Polym. Sci. 2009, 111, 508–516. [Google Scholar] [CrossRef]
- Tran, M.H.; Lee, E.Y. Green preparation of bioplastics based on degradation and chemical modification of lignin residue. J. Wood Chem. Technol. 2018, 38, 460–478. [Google Scholar] [CrossRef]
- Kim, K.H.; Yu, J.-H.; Lee, E.Y. Crude glycerol-mediated liquefaction of saccharification residues of sunflower stalks for production of lignin biopolyols. J. Ind. Eng. Chem. 2016, 38, 175–180. [Google Scholar] [CrossRef]
- Lee, Y.; Tran, M.H.; Lee, E.Y. Acid–base-catalyzed two-step liquefaction of empty fruit bunch lignin residue for preparation of biopolyol and high-performance biopolyurethanes. Wood Sci. Technol. 2021, 55, 315–330. [Google Scholar] [CrossRef]
- Kržan, A.; Kunaver, M. Microwave heating in wood liquefaction. J. Appl. Polym. Sci. 2006, 101, 1051–1056. [Google Scholar] [CrossRef]
- Ouyang, X.; Zhu, G.; Huang, X.; Qiu, X. Microwave assisted liquefaction of wheat straw alkali lignin for the production of monophenolic compounds. J. Energy Chem. 2015, 24, 72–76. [Google Scholar] [CrossRef]
- Xiao, W.; Han, L.; Zhao, Y. Comparative study of conventional and microwave-assisted liquefaction of corn stover in ethylene glycol. Ind. Crops Prod. 2011, 34, 1602–1606. [Google Scholar] [CrossRef]
- Dos Santos, R.; Bordado, J.; Mateus, M. Microwave-assisted liquefaction of cork—from an industrial waste to sustainable chemicals. Ind. Eng. Manag. 2015, 4, 173. [Google Scholar]
- Xu, J.; Jiang, J.; Hse, C.; Shupe, T.F. Renewable chemical feedstocks from integrated liquefaction processing of lignocellulosic materials using microwave energy. Green Chem. 2012, 14, 2821–2830. [Google Scholar] [CrossRef]
- Gosz, K.; Kosmela, P.; Hejna, A.; Gajowiec, G.; Piszczyk, Ł. Biopolyols obtained via microwave-assisted liquefaction of lignin: Structure, rheological, physical and thermal properties. Wood Sci. Technol. 2018, 52, 599–617. [Google Scholar] [CrossRef] [Green Version]
- Joo, J.C.; Oh, Y.H.; Yu, J.H.; Hyun, S.M.; Khang, T.U.; Kang, K.H.; Song, B.K.; Park, K.; Oh, M.-K.; Lee, S.Y. Production of 5-aminovaleric acid in recombinant Corynebacterium glutamicum strains from a Miscanthus hydrolysate solution prepared by a newly developed Miscanthus hydrolysis process. Bioresour. Technol. 2017, 245, 1692–1700. [Google Scholar] [CrossRef]
- Sluiter, A.; Sluiter, J. Determination of Starch in Solid Biomass Samples by HPLC: Laboratory Analytical Procedure (LAP): Issue Date, 07/17/2005; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Lee, Y.; Lee, E.Y. Liquefaction of red pine wood, pinus densiflora, biomass using peg-400-Blended crude glycerol for biopolyol and biopolyurethane production. J. Wood Chem. Technol. 2016, 36, 353–364. [Google Scholar] [CrossRef]
- Jung, J.Y.; Yu, J.-H.; Lee, E.Y. Completely Bio-based Polyol Production from Sunflower Stalk Saccharification Lignin Residue via Solvothermal Liquefaction Using Biobutanediol Solvent and Application to Biopolyurethane Synthesis. J. Polym. Environ. 2018, 26, 3493–3501. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.-Z. A novel method of utilizing the biomass resource: Rapid liquefaction of wheat straw and preparation of biodegradable polyurethane foam (PUF). J. Chin. Inst. Chem. Eng. 2007, 38, 95–102. [Google Scholar] [CrossRef]
- Jo, Y.J.; Ly, H.V.; Kim, J.; Kim, S.-S.; Lee, E. Preparation of biopolyol by liquefaction of palm kernel cake using PEG# 400 blended glycerol. J. Ind. Eng. Chem. 2015, 29, 304–313. [Google Scholar]
- Kim, K.H.; Jo, Y.J.; Lee, C.G.; Lee, E. Solvothermal liquefaction of microalgal Tetraselmis sp. biomass to prepare biopolyols by using PEG# 400-blended glycerol. Algal Res. 2015, 12, 539–544. [Google Scholar]
- Cole, K.C.; Van Gheluwe, P.; Hébrard, M.J.; Leroux, J. Flexible polyurethane foam. I. FTIR analysis of residual isocyanate. J. Appl. Polym. Sci. 1987, 34, 395–407. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bionerg. 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Luo, X.; Xiao, Y.; Wu, Q.; Zeng, J. Development of high-performance biodegradable rigid polyurethane foams using all bioresource-based polyols: Lignin and soy oil-derived polyols. Int. J. Biol. Macromol. 2018, 115, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Prociak, A.; Szczepkowski, L.; Ryszkowska, J.; Kurańska, M.; Auguścik, M.; Malewska, E.; Gloc, M.; Michałowski, S. Influence of chemical structure of petrochemical polyol on properties of bio-polyurethane foams. J. Polym. Environ. 2019, 27, 2360–2368. [Google Scholar] [CrossRef] [Green Version]














| Component | Composition (g/100 g Dry Biomass) | ||
|---|---|---|---|
| Feedstock | Pretreatment Solid | Saccharification Residue | |
| Cellulose | 37.4 ± 0.0 | 57.2 ± 0.5 | 12.4 ± 0.0 |
| Hemicelluloses | 26.5 ± 0.1 | 4.0 ± 0.1 | 1.9 ± 0.0 |
| Klason lignin | 25.1 ± 0.0 | 28.3 ± 0.9 | 39.2 ± 0.2 |
| Water extractives | 9.7 ± 0.4 (2.2 g glucose and others) | 1.9 ± 0.2 (0.1 g glucose, 1.3 g xylose, 0.1 g arabinose, 0.3 g acetic acid) | 6.8 ± 0.1 (2.3 g glucose, 0.4 g xylose, 0.1 g arabinose, 0.7 g protein, 1.2 g phenolics, 2.1 g unknown) |
| Ethanol extractives | 1.4 ± 0.0 | Not measured | 31.6 ± 1.3 |
| Ash | 3.1 ± 0.0 | 1.9 ± 0.1 | 5.7 ± 0.1 |
| Sample | Temperature at 5% Weight Loss (Td5) | Temperature at 10% Weight Loss (Td10) | Char Yield (%) |
|---|---|---|---|
| Acetone-soluble lignin | 215.62 | 262.50 | 34.54 |
| Biopolyol | 164.06 | 181.25 | 9.09 |
| PUF0 | 287.50 | 306.25 | 10.91 |
| PUF1 | 265.62 | 292.19 | 6.36 |
| PUF2 | 266.12 | 293.05 | 25.54 |
| PUF3 | 253.12 | 281.25 | 20.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, M.H.; Yu, J.-H.; Lee, E.Y. Microwave-Assisted Two-Step Liquefaction of Acetone-Soluble Lignin of Silvergrass Saccharification Residue for Production of Biopolyol and Biopolyurethane. Polymers 2021, 13, 1491. https://doi.org/10.3390/polym13091491
Tran MH, Yu J-H, Lee EY. Microwave-Assisted Two-Step Liquefaction of Acetone-Soluble Lignin of Silvergrass Saccharification Residue for Production of Biopolyol and Biopolyurethane. Polymers. 2021; 13(9):1491. https://doi.org/10.3390/polym13091491
Chicago/Turabian StyleTran, My Ha, Ju-Hyun Yu, and Eun Yeol Lee. 2021. "Microwave-Assisted Two-Step Liquefaction of Acetone-Soluble Lignin of Silvergrass Saccharification Residue for Production of Biopolyol and Biopolyurethane" Polymers 13, no. 9: 1491. https://doi.org/10.3390/polym13091491