Structure and Properties of Octenyl Succinic Anhydride-Modified High-Amylose Japonica Rice Starches
Abstract
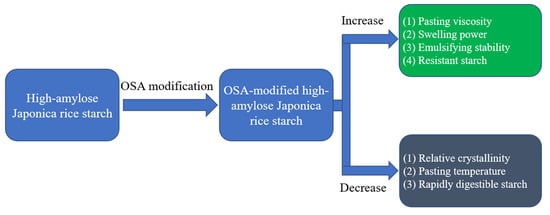
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Starch
2.3. Preparation of OSA-Modified Starch
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5. Scanning Electron Microscopy (SEM)
2.6. Determination of Particle Size Distribution
2.7. X-ray Diffraction (XRD) Analysis
2.8. Pasting Property Measurement
2.9. Swelling Power Determination
2.10. Emulsifying Stability Measurements
2.11. In Vitro Digestibility Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Degrees of Substitution (DS) of OSA-Modified Japonica Rice Starches
3.2. Chemical Structures of OSA-Modified Japonica Rice Starches Determined by FTIR Spectroscopy
3.3. Morphologies and Particle Size Distributions of OSA-Modified Japonica Rice Starches
3.4. Crystalline Structures of OSA-Modified Japonica Rice Starches
3.5. Pasting Properties of OSA-Modified Japonica Rice Starches
3.6. Swelling Powers of OSA-Modified Japonica Rice Starches
3.7. Emulsifying Stabilities of OSA-Modified Japonica Rice Starches
3.8. In Vitro Digestiblities of OSA-Modified Japonica Rice Starches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Song, M.; Liu, L.; Hong, J.; Guan, E.; Bian, K.; Zheng, X. Effect of heat-moisture treatment on the structure and physicochemical properties of ball mill damaged starches from different botanical sources. Int. J. Biol. Macromol. 2020, 156, 403–410. [Google Scholar] [CrossRef]
- Prompiputtanapon, K.; Sorndech, W.; Tongta, S. Surface modification of tapioca starch by using the chemical and enzymatic method. Starch-Stärke 2020, 72, 1900133. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Z.; Xu, B.; Shi, Y.-C. Distribution of octenylsuccinate substituents within a single granule of modified waxy maize starch determined by Raman microspectroscopy. Carbohydr. Polym. 2019, 216, 282–286. [Google Scholar] [CrossRef]
- Li, G.; Xu, X.; Zhu, F. Physicochemical properties of dodecenyl succinic anhydride (DDSA) modified quinoa starch. Food Chem. 2019, 300, 125201. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yao, T.; Xu, Y.; Corke, H.; Sui, Z. Pasting, thermal and rheological properties of octenylsuccinylate modified starches from diverse small granule starches differing in amylose content. J. Cereal Sci. 2020, 95, 103030. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Bello-Perez, L.A.; Castillo-Rodriguez, V.M.; Agama-Acevedo, E.; Alvarez-Ramirez, J. In vitro digestibility characteristics of octenyl succinic acid (OSA) modified starch with different amylose content. Food Chem. 2019, 304, 125434. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Q.; Li, Z.; Fu, D.; Dong, Z. Effects of amylose content on the paste properties and emulsification of octenyl succinic starch esters. Starch-Stärke 2013, 65, 112–122. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Jiang, S.W.; Zheng, Z.; Cao, X.M.; Pan, L.J. Preparation and properties of OSA-modified taro starches and their application for stabilizing pickering emulsions. Int. J. Biol. Macromol. 2019, 137, 277–285. [Google Scholar] [CrossRef]
- Qin, C.; Guo, Y.; Wu, J.; Wang, L.; Zhang, Y. Comparative population genomic analysis provides insights into breeding of modern Indica rice in China. Gene 2020, 768, 145303. [Google Scholar] [CrossRef]
- Sahoo, S.; Saha, B.; Awasthi, J.P.; Omisun, T.; Borgohain, P.; Hussain, S.; Panigrahi, J.; Panda, S.K. Physiological introspection into differential drought tolerance in rice cultivars of north east India. Acta Physiol. Plant. 2019, 41, 53. [Google Scholar] [CrossRef]
- Kato, K.; Suzuki, Y.; Hosaka, Y.; Takahashi, R.; Kodama, I.; Sato, K.; Kawamoto, T.; Kumamaru, T.; Fujita, N. Effect of high temperature on starch biosynthetic enzymes and starch structure in japonica rice cultivar ‘Akitakomachi’ (Oryza sativa L.) endosperm and palatability of cooked rice. J. Cereal Sci. 2019, 87, 209–214. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, C.-S.; Jeong, J.-U.; Reinke, R.F.; Jeong, J.-M. Marker-assisted breeding for improvement of anaerobic germination in japonica rice (Oryza sativa). Plant Breed. 2019, 138, 810–819. [Google Scholar] [CrossRef] [Green Version]
- He, G.Q.; Song, X.Y.; Ruan, H.; Chen, F. Octenyl succinic anhydride modified early indica rice starches differing in amylose content. J. Agric. Food Chem. 2006, 54, 2775–2779. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Pei, Y.; Zhu, W.; Fu, D.; Ren, H. Particle-stabilizers modified from indica rice starches differing in amylose content. Food Chem. 2014, 153, 74–80. [Google Scholar] [CrossRef]
- Bajaj, R.; Singh, N.; Kaur, A. Properties of octenyl succinic anhydride (OSA) modified starches and their application in low fat mayonnaise. Int. J. Biol. Macromol. 2019, 131, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Winuprasith, T.; Suphantharika, M. Design and synthesis of modified and resistant starch-based oil-in-water emulsions. Food Hydrocolloid. 2019, 89, 153–162. [Google Scholar] [CrossRef]
- No, J.; Mun, S.; Shin, M. Properties and digestibility of octenyl succinic anhydride-modified japonica-type waxy and non-waxy rice starches. Molecules 2019, 24, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Li, P.; Yu, J.; Guo, P.; Wang, S. Multi-scale structures and functional properties of starches from Indica hybrid, Japonica and waxy rice. Int. J. Biol. Macromol. 2017, 102, 136–143. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Q.; Zhang, L.; Wei, C. Evaluation of the Molecular Structural Parameters of Normal Rice Starch and Their Relationships with Its Thermal and Digestion Properties. Molecules 2017, 22, 1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, K.; Gu, X.; Lu, W.; Lu, D. Effects of weak-light stress during grain filling on the physicochemical properties of normal maize starch. Carbohydr. Polym. 2018, 202, 47–55. [Google Scholar] [CrossRef]
- Song, X.Y.; He, G.Q.; Ruan, H.; Chen, Q.H. Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch-Stärke 2006, 58, 109–117. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yu, J.; Wang, S. A comparative study of annealing of waxy, normal and high-amylose maize starches: The role of amylose molecules. Food Chem. 2014, 164, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, M.; Lin, G.; Yang, Y.; Xiong, F. Structural development and physicochemical properties of starch in caryopsis of super rice with different types of panicle. BMC Plant Biol. 2019, 19, 482. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Copeland, L. New insights into loss of swelling power and pasting profiles of acid hydrolyzed starch granules. Starch-Stärke 2012, 67, 538–544. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.; Singh, H. Physical properties and biological fate of OSA-modified-starch-stabilized emulsions containing beta-carotene: Effect of calcium and pH. Food Hydrocolloid. 2018, 77, 549–556. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, J.; Zhang, J.; Liu, R. Changes in starch structures and in vitro digestion characteristics during maize (Zea mays L.) germination. Food Sci. Nutr. 2020, 8, 1700–1708. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Mei, J.Q.; Chen, B.; Chen, H.Q. Digestibility, physicochemical and structural properties of octenyl succinic anhydride-modified cassava starches with different degree of substitution. Food Chem. 2017, 229, 136. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Shi, Y.C. Structure and preparation of octenyl succinic esters of granular starch, microporous starch and soluble maltodextrin. Carbohydr. Polym. 2011, 83, 520–527. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Bello-Perez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effect of amylose content in morphological, functional and emulsification properties of OSA modified corn starch. Food Hydrocolloid. 2019, 97, 105212. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, J.; Hong, Y.; Liu, G.; Zheng, J.; Gu, Z.; Zhang, P. Impact of amylose content on starch physicochemical properties in transgenic sweet potato. Carbohydr. Polym. 2015, 122, 417–427. [Google Scholar] [CrossRef]
- Raina, C.S.; Singh, S.; Bawa, A.S.; Saxena, D.C. A comparative study of Indian rice starches using different modification model solutions. LWT—Food Sci. Technol. 2007, 40, 885–892. [Google Scholar] [CrossRef]
- Park, I.-M.; Ibáñez, A.M.; Zhong, F.; Shoemaker, C.F. Gelatinization and pasting properties of waxy and non-waxy rice starches. Starch-Stärke 2007, 59, 388–396. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Effect of octenylsuccinylation on physicochemical and functional properties of waxy maize and amaranth starches. Carbohydr. Polym. 2007, 68, 447–456. [Google Scholar] [CrossRef]
- Punia, S.; Siroha, A.K.; Sandhu, K.S.; Kaur, M. Rheological and pasting behavior of OSA modified mungbean starches and its utilization in cake formulation as fat replacer. Int. J. Biol. Macromol. 2019, 128, 230–236. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, T.Z.; Wang, X.; Reimer, M.; Isaak, C.; Ai, Y. Behaviors of starches evaluated at high heating temperatures using a new model of rapid visco analyzer—RVA 4800. Food Hydrocolloid. 2019, 94, 217–228. [Google Scholar] [CrossRef]
- You, S.-Y.; Lim, S.-T.; Lee, J.H.; Chung, H.-J. Impact of molecular and crystalline structures on in vitro digestibility of waxy rice starches. Carbohydr. Polym. 2014, 112, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Comparative study of rs4 type resistant starches derived from cassava and potato starches via octenyl succinylation. Starch-Stärke 2017, 69, 1600264. [Google Scholar] [CrossRef]





| PT (°C) | Ptime (min) | PV (cp) | TRV (cp) | BDV (cp) | FV (cp) | SBV (cp) | |
|---|---|---|---|---|---|---|---|
| Normal-ST | 74.4 ± 0.7 d | 4.35 ± 0.02 c | 3220 ± 45 b | 1146 ± 71 b | 2074 ± 111 b | 2242 ± 126 b | 1095 ± 68 b |
| OSA-NormalST | 64.4 ± 0.1 a | 3.66 ± 0.01 a | 5516 ± 123 d | 3014 ± 77 e | 2502 ± 46 c | 5288 ± 144 e | 2274 ± 79 c |
| HighAmyST | 85.8 ± 0.8 e | 5.40 ± 0.03 e | 1275 ± 21 a | 1052 ± 7 a | 223 ± 22 a | 1785 ± 64 a | 733 ± 58 a |
| OSA-HighAmyST-1 | 67.5 ± 0.5 b | 3.83 ± 0.04 b | 5373 ± 93 c | 1995 ± 47 c | 3378 ± 51 d | 4547 ± 36 c | 2552 ± 14 e |
| OSA-HighAmyST-2 | 69.7 ± 1.7 c | 4.44 ± 0.08 d | 5730 ± 27 e | 2367 ± 25 d | 3363 ± 33 d | 4784 ± 16 d | 2418 ± 35 d |
| Swelling Powers (g/g) | ||||
|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | |
| NormalST | 2.00 ± 0.04 a | 2.68 ± 0.01 a | 11.54 ± 0.48 b | 16.17 ± 0.09 b |
| OSA-NormalST | 6.42 ± 0.23 c | 17.28 ± 0.30 d | 22.33 ± 0.07 e | 23.47 ± 0.48 d |
| HighAmyST | 2.13 ± 0.04 a | 2.31 ± 0.01 a | 4.32 ± 0.06 a | 7.47 ± 0.40 a |
| OSA-HighAmyST-1 | 4.40 ± 0.01 b | 11.29 ± 0.16 c | 17.24 ± 0.05 c | 22.32 ± 0.36 c |
| OSA-HighAmyST-2 | 6.23 ± 0.23 c | 10.54 ± 0.45 b | 18.42 ± 0.19 d | 22.08 ± 0.31 c |
| Creaming Index (%) of Emulsion | ||
|---|---|---|
| Freshly Prepared Emulsion | Emulsion Stored for 48 h | |
| NormalST | 34.1 ± 2.9 b | 38.9 ± 1.0 c |
| OSA-NormalST | 24.3 ± 1.0 a | 28.9 ± 0.5 a |
| HighAmyST | 34.5 ± 1.7 b | 37.9 ± 1.7 c |
| OSA-HighAmyST-1 | 34.2 ± 2.0 b | 35.2 ± 0.3 b |
| OSA-HighAmyST-2 | 27.9 ± 2.4 a | 30.3 ± 0.7 a |
| RDS (%) | SDS (%) | RS (%) | |
|---|---|---|---|
| NormalST | 84.04 ± 0.42 e | 11.09 ± 0.17 b | 4.87 ± 0.24 a |
| OSA-NormalST | 67.64 ± 1.77 b | 7.05 ± 1.08 a | 25.30 ± 0.69 d |
| HighAmyST | 78.74 ± 0.17 d | 9.56 ± 0.37 b | 11.70 ± 0.42 b |
| OSA-HighAmyST-1 | 71.32 ± 1.15 c | 6.59 ± 1.51 a | 22.08 ± 0.36 c |
| OSA-HighAmyST-2 | 53.24 ± 0.05 a | 11.31 ± 0.36 b | 35.45 ± 0.42 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Cheng, B.; Li, J.; Shu, Z.; Wang, P.; Zeng, X. Structure and Properties of Octenyl Succinic Anhydride-Modified High-Amylose Japonica Rice Starches. Polymers 2021, 13, 1325. https://doi.org/10.3390/polym13081325
Zhang W, Cheng B, Li J, Shu Z, Wang P, Zeng X. Structure and Properties of Octenyl Succinic Anhydride-Modified High-Amylose Japonica Rice Starches. Polymers. 2021; 13(8):1325. https://doi.org/10.3390/polym13081325
Chicago/Turabian StyleZhang, Wei, Bei Cheng, Jiahui Li, Zaixi Shu, Pingping Wang, and Xuefeng Zeng. 2021. "Structure and Properties of Octenyl Succinic Anhydride-Modified High-Amylose Japonica Rice Starches" Polymers 13, no. 8: 1325. https://doi.org/10.3390/polym13081325