Microstructured Macromaterials Based on IPN Microgels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of IPN Microgels
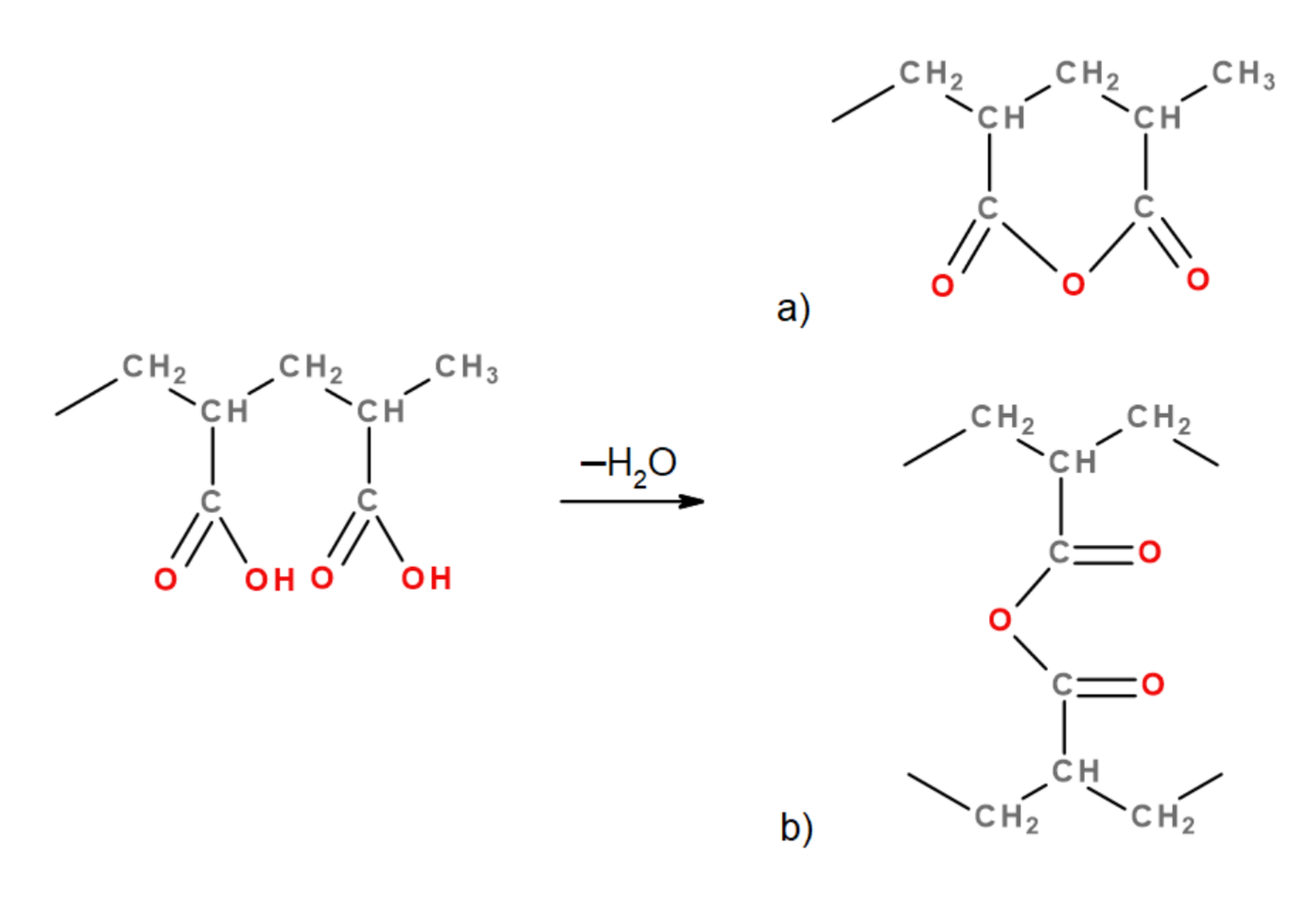
2.3. Macromaterial Preparation
2.4. Instrumental Methods
2.5. Computer Simulations
3. Results and Discussions
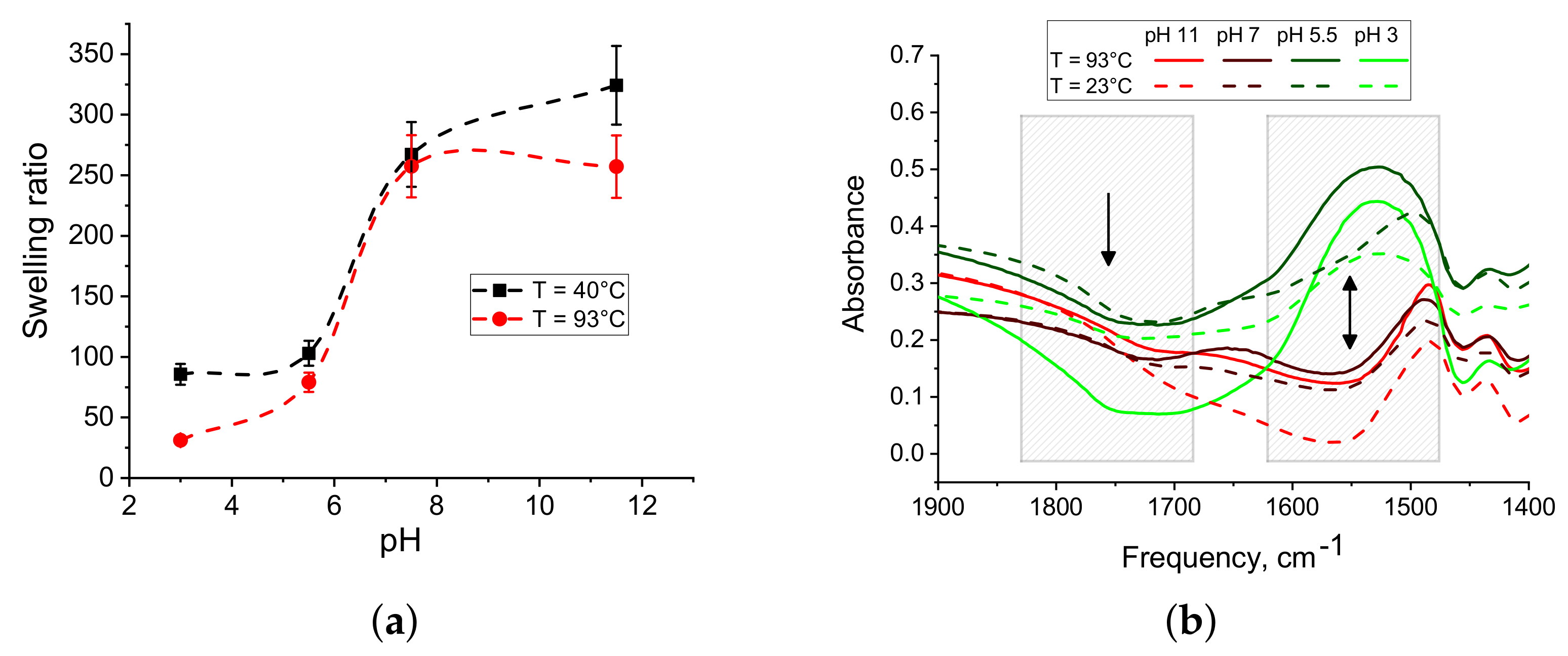
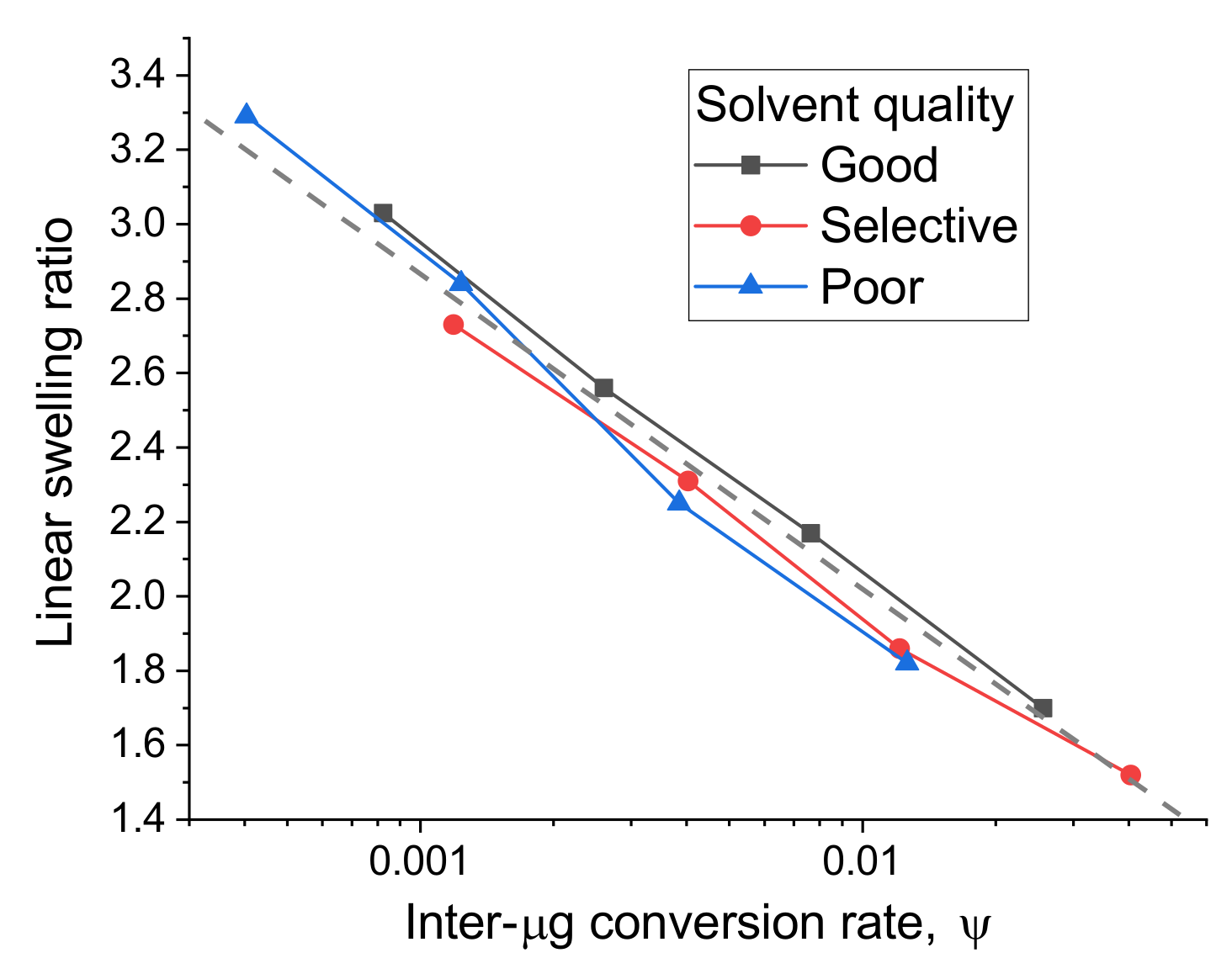
3.1. Experimental Study of Macrostructured Materials from IPN Microgels
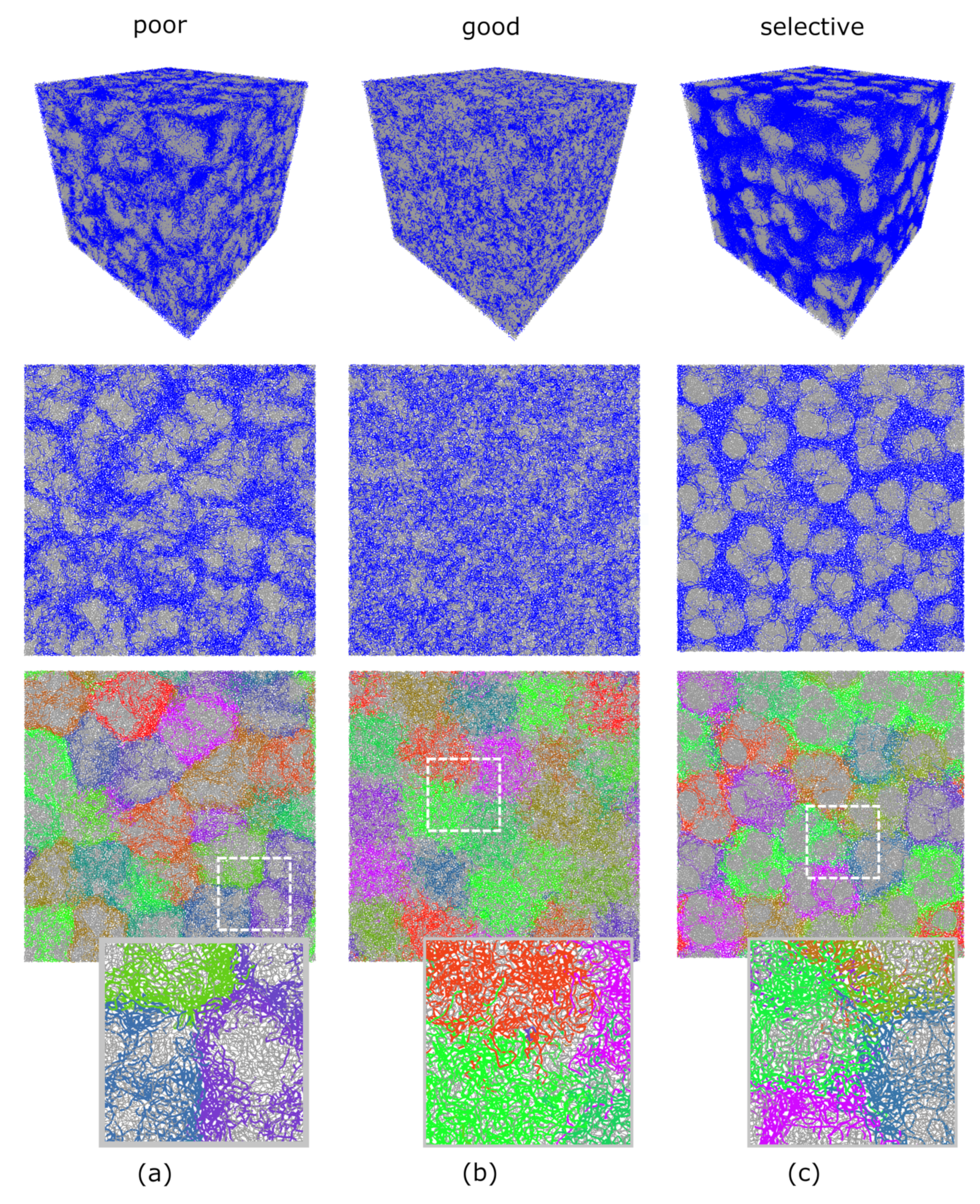
3.2. Computer Simulations of Microstructured Macromaterials Based on IPN Microgels
3.3. Microscopic Structure of Macromaterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khokhlov, A.R.; Starodubtzev, S.G.; Vasilevskaya, V.V. Conformational transitions in polymer gels: Theory and experiment. In Responsive Gels: Volume Transitions I; Dušek, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 123–171. [Google Scholar] [CrossRef]
- Philippova, O.; Khokhlov, A. 1.13-Polymer Gels. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 339–366. [Google Scholar] [CrossRef]
- Hoffman, A.S. Applications of “Smart Polymers” as Biomaterials. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 247–258. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Pich, A.; Richtering, W. Polymer Nanogels and Microgels. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 309–350. [Google Scholar] [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef] [PubMed]
- Newsom, J.P.; Payne, K.A.; Krebs, M.D. Microgels: Modular, tunable constructs for tissue regeneration. Acta Biomater. 2019, 88, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Preman, N.K.; Jain, S.; Johnson, R.P. “Smart” Polymer Nanogels as Pharmaceutical Carriers: A Versatile Platform for Programmed Delivery and Diagnostics. ACS Omega 2021, 6, 5075–5090. [Google Scholar] [CrossRef]
- Li, Z.; Ngai, T. CHAPTER 5. Emulsions Stabilized by Soft Microgel Particles. In Particle-Stabilized Emulsions and Colloids; Royal Society of Chemistry: London, UK, 2014; pp. 93–128. Available online: https://pubs.rsc.org/en/content/chapter/bk9781849738811-00093/978-1-78262-014-3 (accessed on 28 March 2021). [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Khan, S.R.; Begum, R. Temperature-responsive hybrid microgels for catalytic applications: A review. Mater. Sci. Technol. 2016, 33, 129–137. [Google Scholar] [CrossRef]
- Ballauff, M.; Lu, Y. “Smart” nanoparticles: Preparation, characterization and applications. Polymer 2007, 48, 1815–1823. [Google Scholar] [CrossRef] [Green Version]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [Green Version]
- Pacholski, C. Two-Dimensional Arrays of Poly(N-Isopropylacrylamide) Microspheres: Formation, Characterization and Application. Z. FüR Phys. Chem. 2015, 229, 283–300. [Google Scholar] [CrossRef]
- Lohaus, T.; de Wit, P.; Kather, M.; Menne, D.; Benes, N.; Pich, A.; Wessling, M. Tunable permeability and selectivity: Heatable inorganic porous hollow fiber membrane with a thermo-responsive microgel coating. J. Membr. Sci. 2017, 539, 451–457. [Google Scholar] [CrossRef]
- Litowczenko, J.; Gapiński, J.; Markiewicz, R.; Woźniak, A.; Wychowaniec, J.K.; Peplińska, B.; Jurga, S.; Patkowski, A. Synthesis, characterization and in vitro cytotoxicity studies of poly-N-isopropyl acrylamide gel nanoparticles and films. Mater. Sci. Eng. C 2021, 118, 111507. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y. PNIPAM microgels for biomedical applications: From dispersed particles to 3D assemblies. Soft Matter 2011, 7, 6375. [Google Scholar] [CrossRef]
- Buratti, E.; Sanzari, I.; Dinelli, F.; Prodromakis, T.; Bertoldo, M. Formation and Stability of Smooth Thin Films with Soft Microgels Made of Poly(N-Isopropylacrylamide) and Poly(Acrylic Acid). Polymers 2020, 12, 2638. [Google Scholar] [CrossRef] [PubMed]
- Cutright, C.; Brotherton, Z.; Alexander, L.; Harris, J.; Shi, K.; Khan, S.; Genzer, J.; Menegatti, S. Packing density, homogeneity, and regularity: Quantitative correlations between topology and thermoresponsive morphology of PNIPAM-co-PAA microgel coatings. Appl. Surf. Sci. 2020, 508, 145129. [Google Scholar] [CrossRef]
- Sanzari, I.; Buratti, E.; Huang, R.; Tusan, C.G.; Dinelli, F.; Evans, N.D.; Prodromakis, T.; Bertoldo, M. Poly(N-isopropylacrylamide) based thin microgel films for use in cell culture applications. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Chester, D.; Kathard, R.; Nortey, J.; Nellenbach, K.; Brown, A.C. Viscoelastic properties of microgel thin films control fibroblast modes of migration and pro-fibrotic responses. Biomaterials 2018, 185, 371–382. [Google Scholar] [CrossRef]
- Keal, L.; Lapeyre, V.; Ravaine, V.; Schmitt, V.; Monteux, C. Drainage dynamics of thin liquid foam films containing soft PNiPAM microgels: Influence of the cross-linking density and concentration. Soft Matter 2017, 13, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Hellweg, T.; von Klitzing, R. Packing Density Control in P(NIPAM-co-AAc) Microgel Monolayers: Effect of Surface Charge, pH, and Preparation Technique. Langmuir 2008, 24, 12595–12602. [Google Scholar] [CrossRef]
- Bell, D.; Ludwanowski, S.; Lüken, A.; Sarikaya, B.; Walther, A.; Wessling, M. Hydrogel membranes made from crosslinked microgel multilayers with tunable density. J. Membr. Sci. 2021, 620, 118912. [Google Scholar] [CrossRef]
- Cai, T.; Wang, G.; Thompson, S.; Marquez, M.; Hu, Z. Photonic Hydrogels with Poly(ethylene glycol) Derivative Colloidal Spheres as Building Blocks. Macromolecules 2008, 41, 9508–9512. [Google Scholar] [CrossRef]
- Serpe, M.; Kim, J.; Lyon, L. Colloidal Hydrogel Microlenses. Adv. Mater. 2004, 16, 184–187. [Google Scholar] [CrossRef]
- Kim, J.; Nayak, S.; Lyon, L.A. Bioresponsive Hydrogel Microlenses. J. Am. Chem. Soc. 2005, 127, 9588–9592. [Google Scholar] [CrossRef]
- Keskin, D.; Mergel, O.; van der Mei, H.C.; Busscher, H.J.; van Rijn, P. Inhibiting Bacterial Adhesion by Mechanically Modulated Microgel Coatings. Biomacromolecules 2018, 20, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigolaeva, L.V.; Gladyr, S.Y.; Gelissen, A.P.H.; Mergel, O.; Pergushov, D.V.; Kurochkin, I.N.; Plamper, F.A.; Richtering, W. Dual-Stimuli-Sensitive Microgels as a Tool for Stimulated Spongelike Adsorption of Biomaterials for Biosensor Applications. Biomacromolecules 2014, 15, 3735–3745. [Google Scholar] [CrossRef] [PubMed]
- Kozhunova, E.Y.; Vyshivannaya, O.V.; Nasimova, I.R. “Smart” IPN microgels with different network structures: Self-crosslinked vs conventionally crosslinked. Polymer 2019, 176, 127–134. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Rosi, B.; Bertoldo, M.; Buratti, E.; Casciardi, S.; Sennato, S.; Ruzicka, B. Study of network composition in interpenetrating polymer networks of poly(N isopropylacrylamide) microgels: The role of poly(acrylic acid). J. Colloid Interface Sci. 2019, 545, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Hu, Z. Synthesis and Light Scattering Study of Microgels with Interpenetrating Polymer Networks. Langmuir 2004, 20, 2094–2098. [Google Scholar] [CrossRef]
- Nasimova, I.R.; Vyshivannaya, O.V.; Gallaymov, M.O.; Kozhunova, E.Y. Thermo- and pH-Sensitive Microgels Based on Interpenetrating Networks as Components for Creating Polymeric Materials. Polym. Sci. Ser. A 2019, 61, 773–779. [Google Scholar] [CrossRef]
- Rudyak, V.Y.; Kozhunova, E.Y.; Chertovich, A.V. Simulation of interpenetrating networks microgel synthesis. Soft Matter 2020, 16, 4858–4865. [Google Scholar] [CrossRef] [PubMed]
- Rudyak, V.Y.; Kozhunova, E.Y.; Chertovich, A.V. Towards the realistic computer model of precipitation polymerization microgels. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comp. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- McGaugh, M.C.; Kottle, S. The thermal degradation of poly(acrylic acid). J. Polym. Sci. Part B Polym. Lett. 1967, 5, 817–820. [Google Scholar] [CrossRef]
- Arndt, K.F.; Richter, A.; Ludwig, S.; Zimmermann, J.; Kressler, J.; Kuckling, D.; Adler, H.J. Poly(vinyl alcohol)/poly(acrylic acid) hydrogels: FT-IR spectroscopic characterization of crosslinking reaction and work at transition point. Acta Polym. 1999, 50, 383–390. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Szalla, A.; Kamińska, A. Study of poly(acrylic acid)—poly(vinylpyrrolidone) complexes and their photostability. Polymer 2001, 42, 6057–6069. [Google Scholar] [CrossRef]
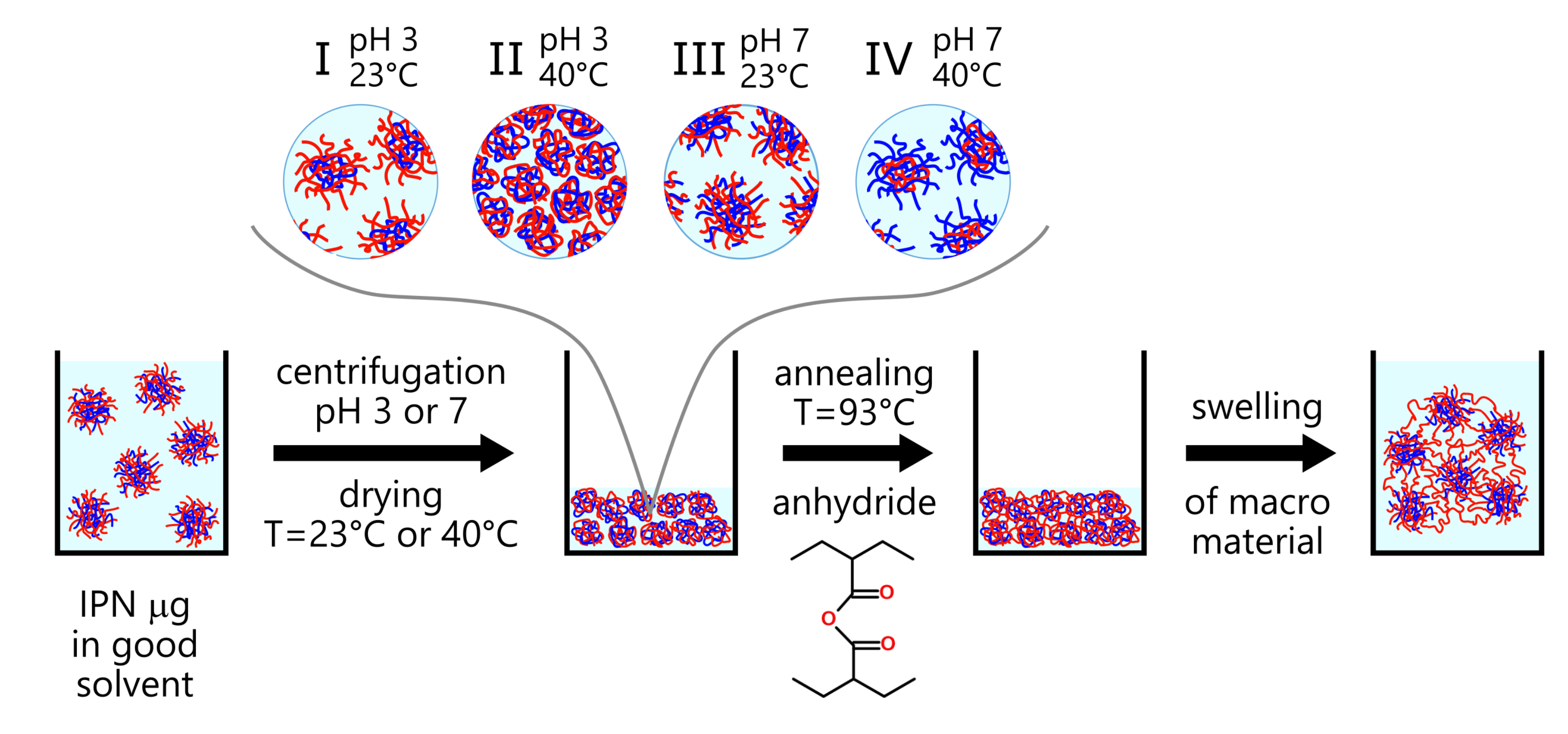



| pH 3, T = 23C | pH 7, T = 23C | pH 3, T = 40C | pH 7, T = 40C | |
|---|---|---|---|---|
| / | 2.2 ± 0.2 | 162 ± 16 | 13.2 ± 1.0 | 144 ± 14 |
| / | 0.93 ± 0.09 | 0.93 ± 0.09 | 0.30 ± 0.03 | 0.90 ± 0.09 |
| / | 5.5 ± 0.5 | 20.6 ± 2 | 11.2 ± 1.0 | 1.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasimova, I.R.; Rudyak, V.Y.; Doroganov, A.P.; Kharitonova, E.P.; Kozhunova, E.Y. Microstructured Macromaterials Based on IPN Microgels. Polymers 2021, 13, 1078. https://doi.org/10.3390/polym13071078
Nasimova IR, Rudyak VY, Doroganov AP, Kharitonova EP, Kozhunova EY. Microstructured Macromaterials Based on IPN Microgels. Polymers. 2021; 13(7):1078. https://doi.org/10.3390/polym13071078
Chicago/Turabian StyleNasimova, Irina Rashitovna, Vladimir Yurievich Rudyak, Anton Pavlovich Doroganov, Elena Petrovna Kharitonova, and Elena Yurievna Kozhunova. 2021. "Microstructured Macromaterials Based on IPN Microgels" Polymers 13, no. 7: 1078. https://doi.org/10.3390/polym13071078






