Rapid Prototyping of a Nanoparticle Concentrator Using a Hydrogel Molding Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sample Dispersion
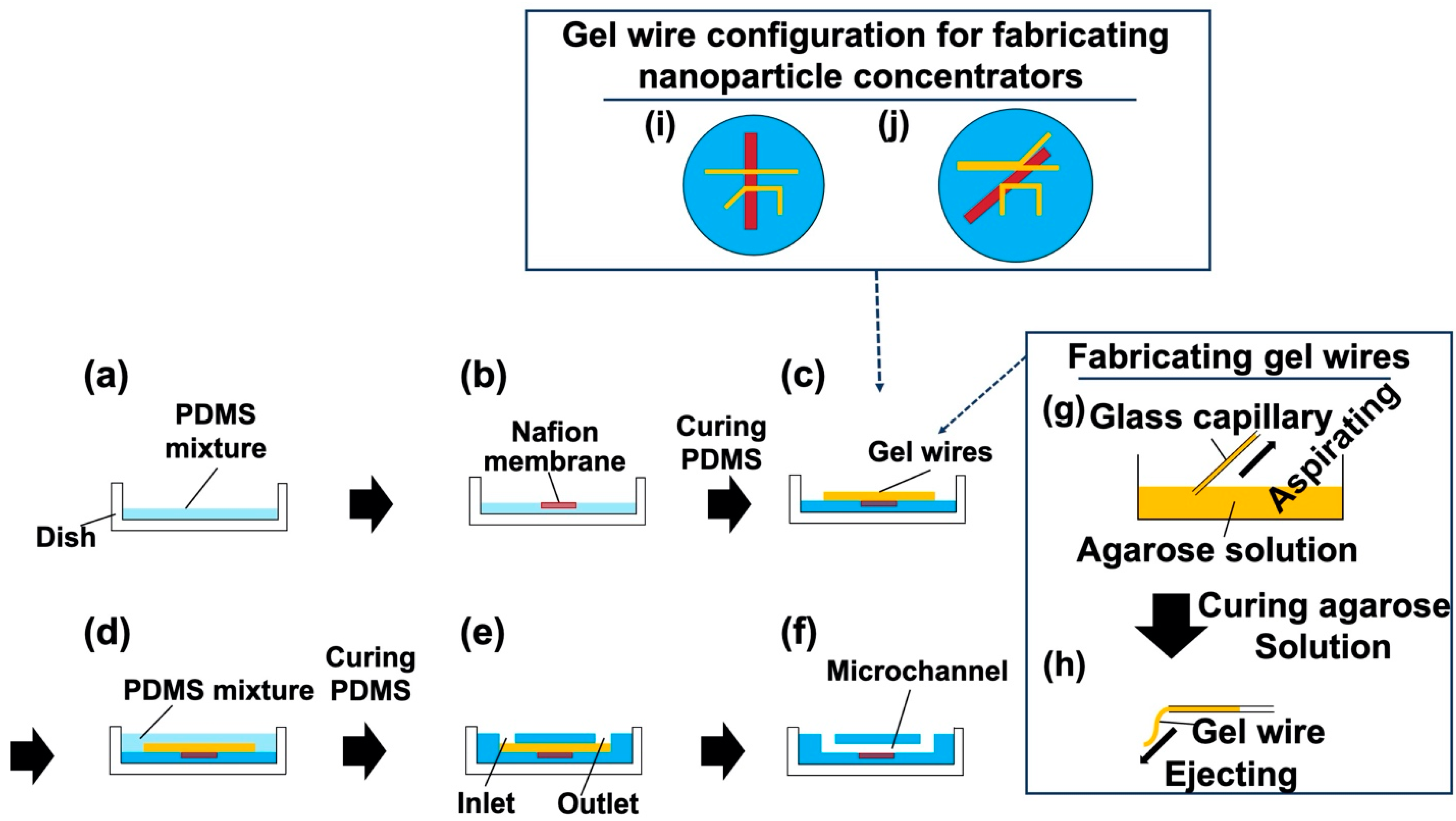
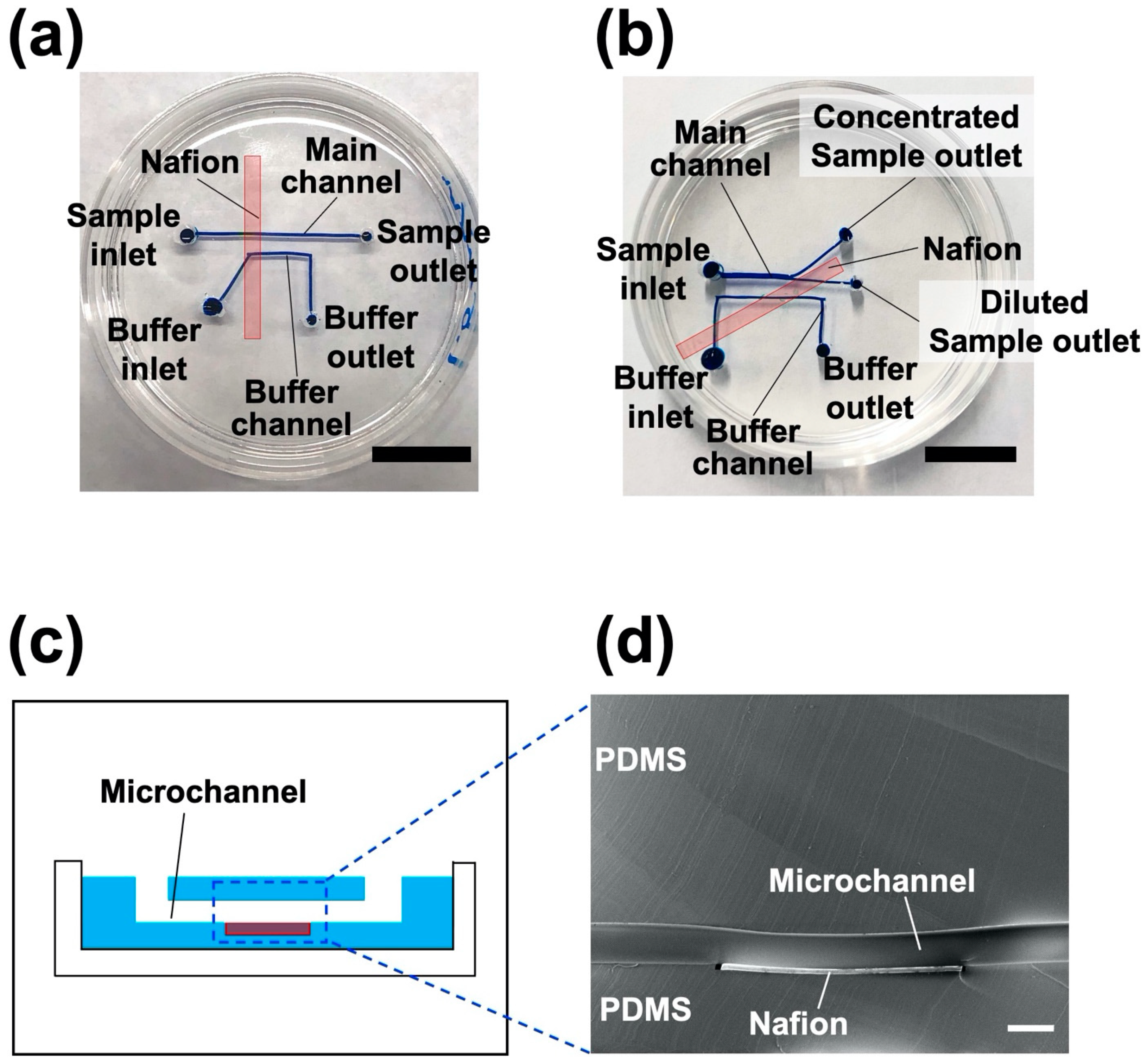
2.2. Device Fabrication Using a Hydrogel Molding Method
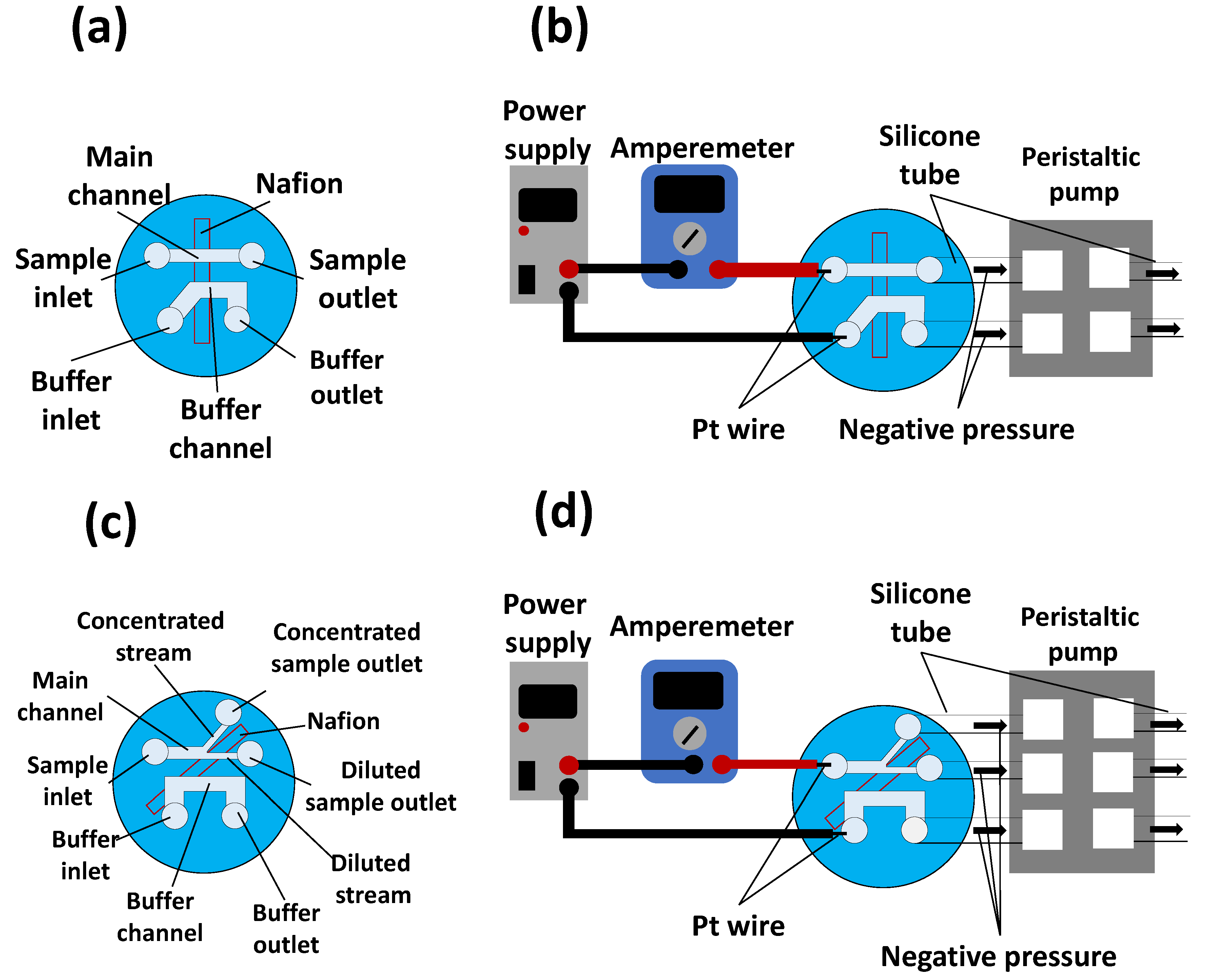
2.3. Generation of Ion Concentration Polarization
2.4. Characterization
3. Results and Discussions
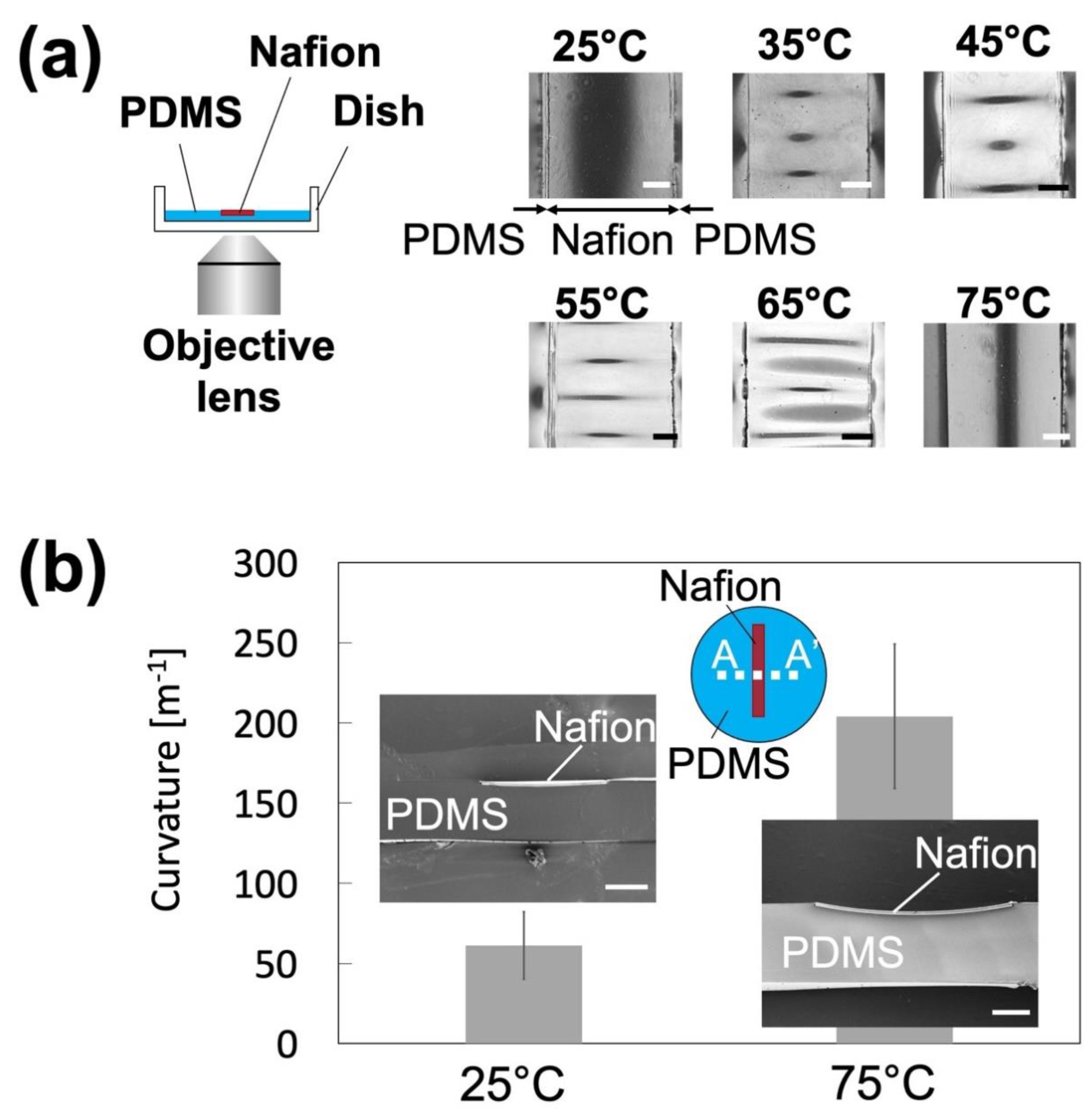
3.1. Fabricated Nanoparticle Concentrator
3.2. A Nanoparticle Concentrator with a Straight Channel
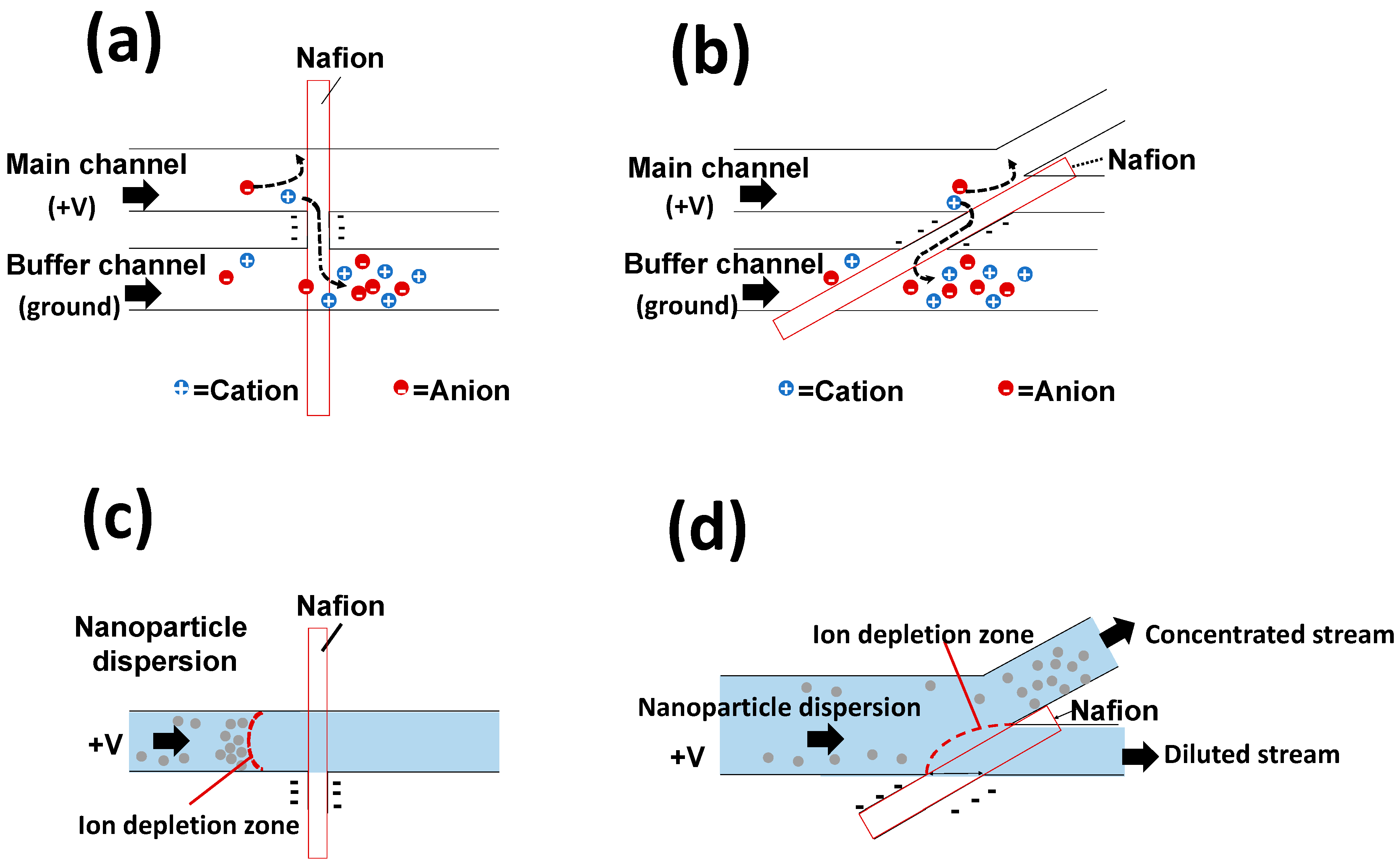
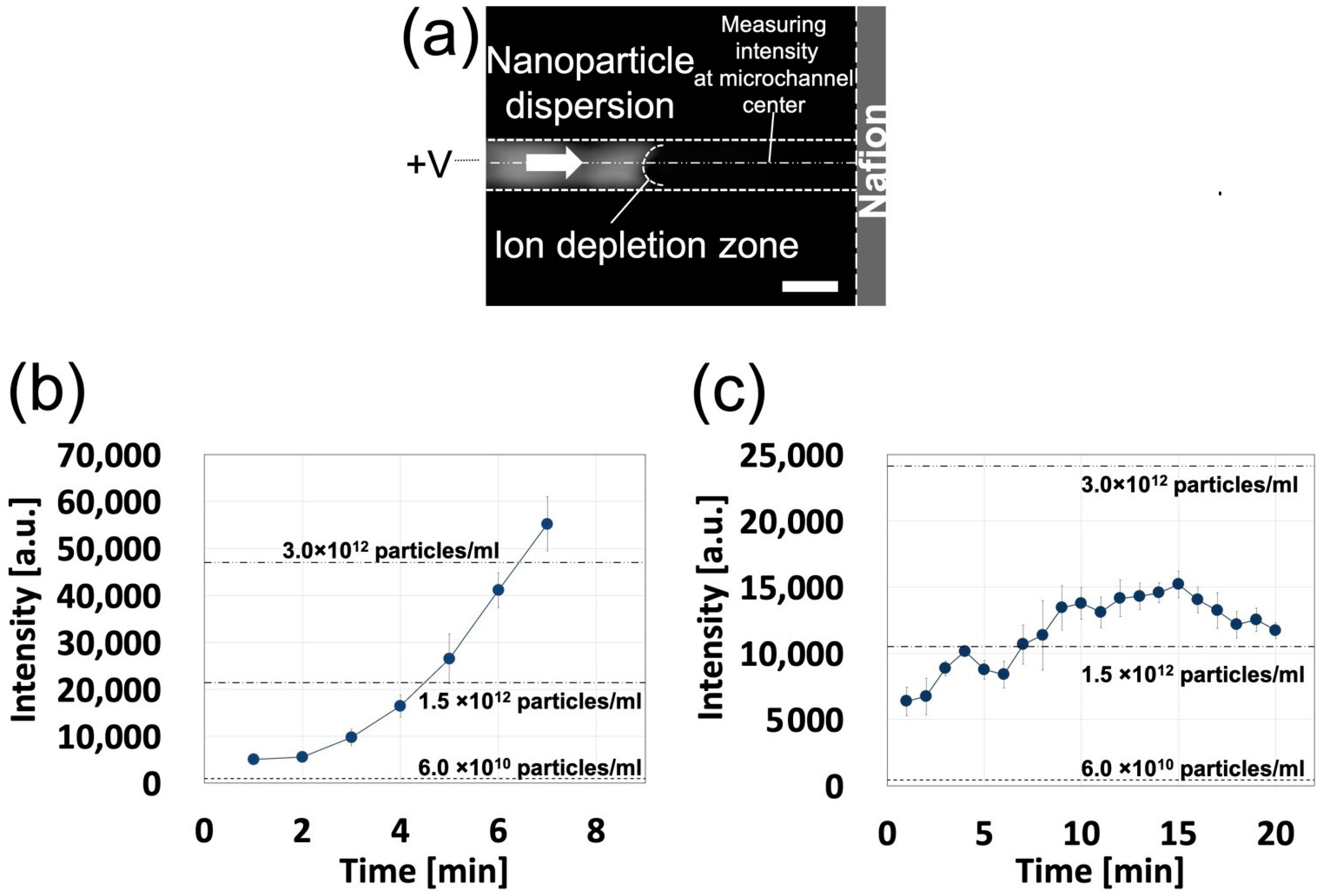
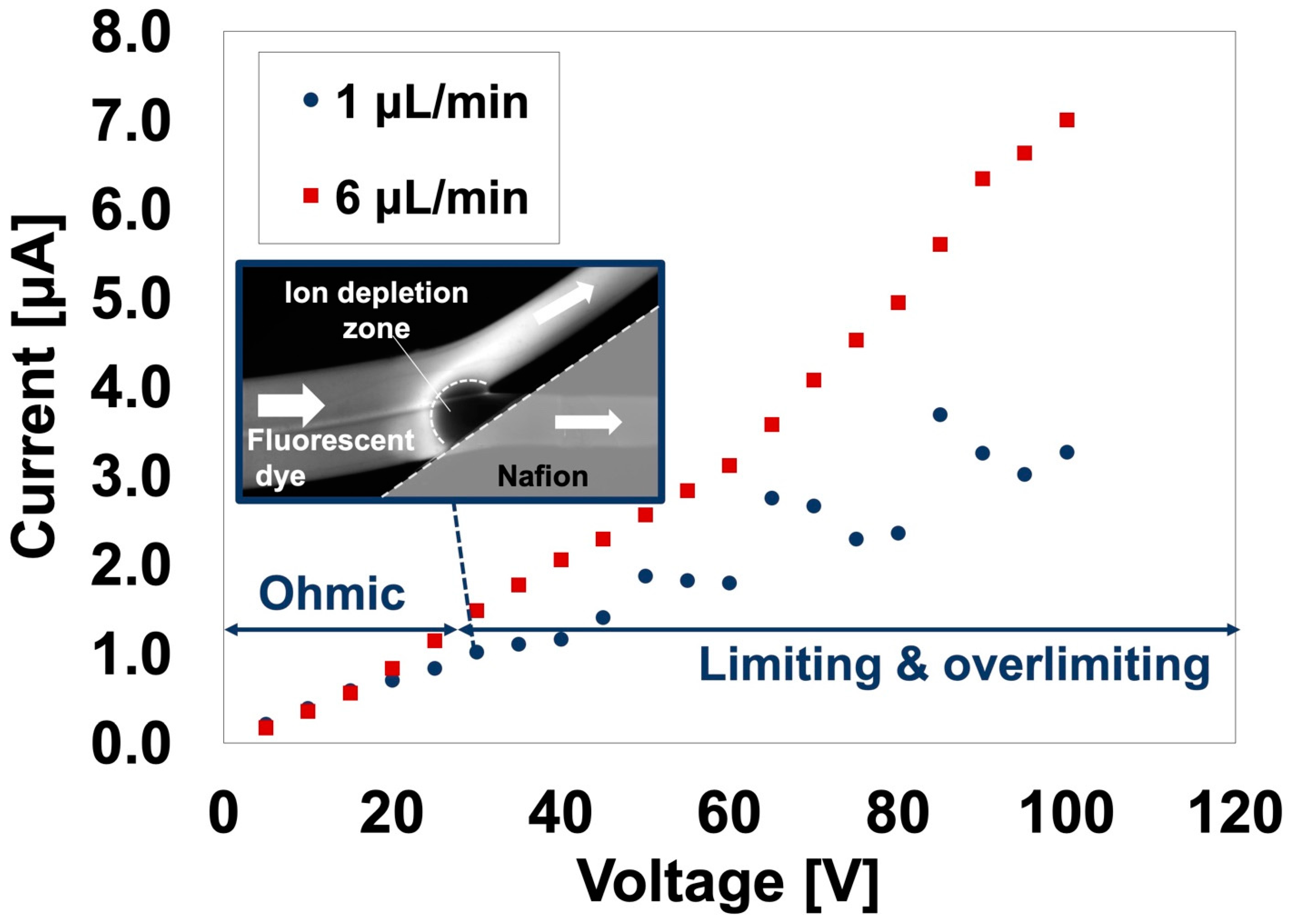
3.2.1. Device Validation for Ion Concentration Polarization
3.2.2. Nanoparticle Concentration
3.3. A Nanoparticle Concentrator with a Branched Channel
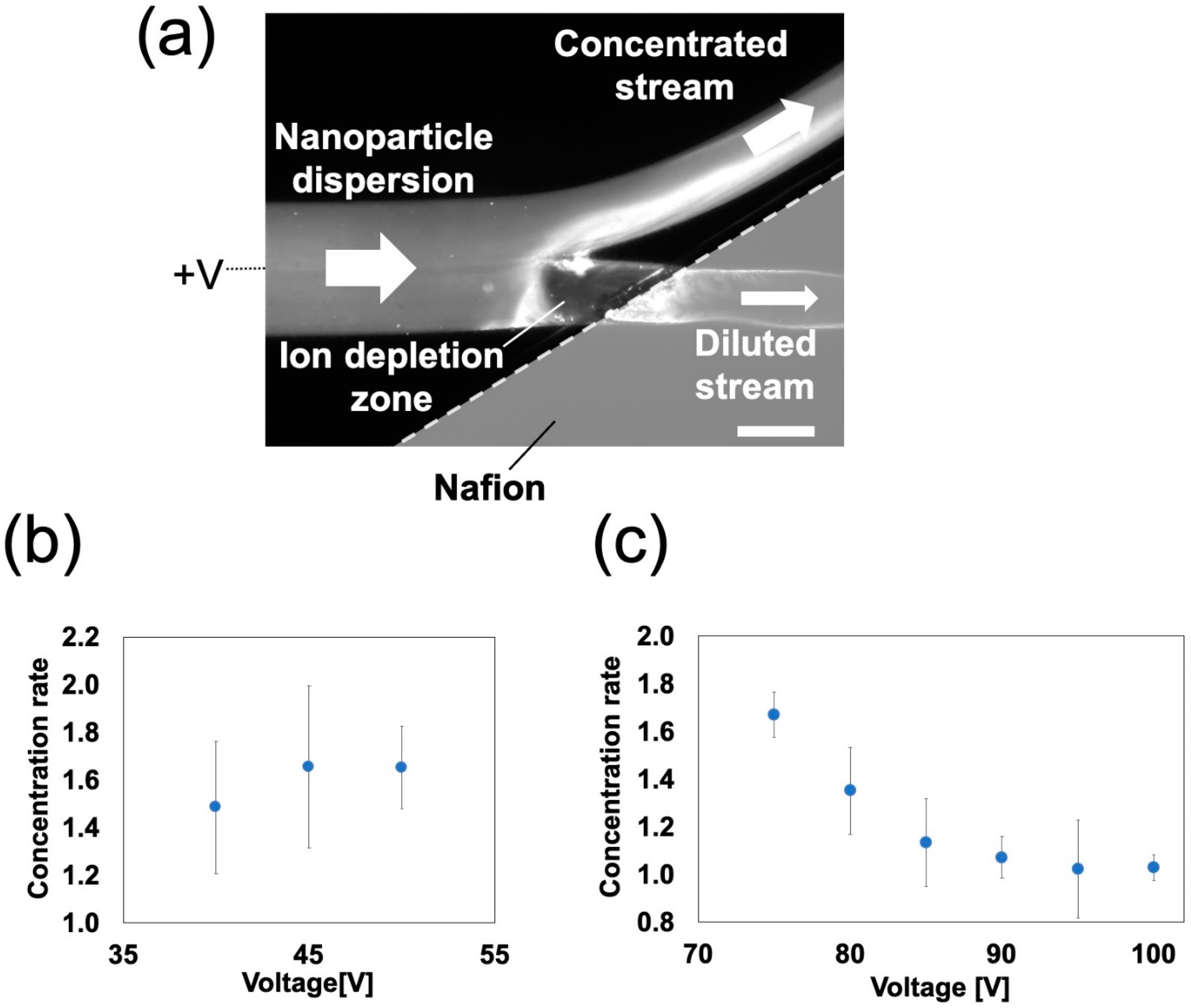
3.3.1. Device Validation for Ion Concentration Polarization
3.3.2. Nanoparticle Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target. Ther. 2020, 5, 144. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.C.; Wu, H.J.; Ugaz, V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef]
- Chen, D.; Du, H.; Tay, C. Rapid Concentration of Nanoparticles with DC Dielectrophoresis in Focused Electric Fields. Nanoscale Res. Lett. 2009, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Boettcher, M.; Schmidt, S.; Latz, A.; Jaeger, M.S.; Stuke, M.; Duschl, C. Filtration at the microfluidic level: Enrichment of nanoparticles by tunable filters. J. Phys. Condens. Matter 2011, 23, 324101. [Google Scholar] [CrossRef]
- Mogi, K.; Hayashida, K.E.I.; Honda, A.; Yamamoto, T. Development of Virus Concentration Device by Controlling Ion Depletion Zone for Ultrasensitive Virus Sensing. Electron. Commun. Jpn. 2017, 100, 56–63. [Google Scholar] [CrossRef]
- Mogi, K.; Hayashida, K.; Yamamoto, T. Damage-less handling of exosomes using an ion-depletion zone in a microchannel. Anal. Sci. 2018, 34, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.; Lee, H.; Kang, K.H.; Lim, G. Ion concentration polarization-based continuous separation device using electrical repulsion in the depletion region (vol 3, 3483, 2013). Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef] [Green Version]
- Pu, Q.; Yun, J.; Temkin, H.; Liu, S. Ion-Enrichment and Ion-Depletion Effect of Nanochannel Structures. Nano Lett. 2004, 4, 1099–1103. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Stevens, A.L.; Han, J. Million-fold Preconcentration of Proteins and Peptides by Nanofluidic Filter. Anal. Chem. 2005, 77, 4293–4299. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, Y.-A.; Han, J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: Theory, fabrication, and applications. Chem. Soc. Rev. 2010, 39, 912–922. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Ko, S.H.; Kang, K.H.; Han, J. Direct seawater desalination by ion concentration polarization. Nat. Nanotechnol. 2010, 5, 297–301. [Google Scholar] [CrossRef]
- Li, M.; Anand, R.K. Recent advancements in ion concentration polarization. Analyst 2016, 141, 3496–3510. [Google Scholar] [CrossRef] [Green Version]
- Berzina, B.; Anand, R.K. Tutorial review: Enrichment and separation of neutral and charged species by ion concentration polarization focusing. Anal. Chim. Acta 2020, 1128, 149–173. [Google Scholar] [CrossRef]
- Kwak, R.; Kim, S.J.; Han, J. Continuous-Flow Biomolecule and Cell Concentrator by Ion Concentration Polarization. Anal. Chem. 2011, 83, 7348–7355. [Google Scholar] [CrossRef] [PubMed]
- Son, S.Y.; Lee, S.; Lee, H.; Kim, S.J. Engineered nanofluidic preconcentration devices by ion concentration polarization. Biochip J. 2016, 10, 251–261. [Google Scholar] [CrossRef]
- Ko, S.H.; Song, Y.-A.; Kim, S.J.; Kim, M.; Han, J.; Kang, K.H. Nanofluidic preconcentration device in a straight microchannel using ion concentration polarization. Lab Chip 2012, 12, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, W.; Lee, H.; Kim, S. Stabilization of Ion Concentration Polarization Layer using Micro Fin Structure for High-Throughput Applications. Nanoscale 2017, 9. [Google Scholar] [CrossRef]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.N.; Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Yuan, X.; Renaud, L.; Audry, M.-C.; Kleimann, P. Electrokinetic Biomolecule Preconcentration Using Xurography-Based Micro-Nano-Micro Fluidic Devices. Anal. Chem. 2015, 87. [Google Scholar] [CrossRef]
- Hirama, H.; Odera, T.; Torii, T.; Moriguchi, H. A lithography-free procedure for fabricating three-dimensional microchannels using hydrogel molds. Biomed. Microdevices 2012, 14, 689–697. [Google Scholar] [CrossRef]
- Odera, T.; Hirama, H.; Kuroda, J.; Moriguchi, H.; Torii, T. Droplet formation behavior in a microfluidic device fabricated by hydrogel molding. Microfluid. Nanofluid. 2014, 17, 469–476. [Google Scholar] [CrossRef]
- Sugiura, Y.; Hirama, H.; Torii, T. Fabrication of Microfluidic Valves Using a Hydrogel Molding Method. Sci. Rep. 2015, 5, 13375. [Google Scholar] [CrossRef] [Green Version]
- Hirama, H.; Sugiura, Y.; Komazaki, Y.; Torii, T. Robotic Fabrication of Microchannels for Microfluidic Analysis by Hydrogel Molding. Chem. Lett. 2019, 48, 971–974. [Google Scholar] [CrossRef]
- Wei, Y.; Li, S.H.; Zhang, X.F.; Fu, Y.J.; Chen, K.J. Smart Devices Based on the Soft Actuator with Nafion-Polypropylene-PDMS/Graphite Multilayer Structure. Appl. Sci. 2020, 10, 1829. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, G.H.; Woo, H.; An, T.; Lim, G. Fabrication of a Novel Nanofluidic Device Featuring ZnO Nanochannels. ACS Omega 2020, 5, 3144–3150. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.P.; Liu, W. Length-dependent instability of shear electroconvective flow: From electroconvective instability to Rayleigh-Benard instability. J. Appl. Phys. 2018, 124, 12. [Google Scholar] [CrossRef]
- Cho, I.; Sung, G.Y.; Kim, S.J. Overlimiting current through ion concentration polarization layer: Hydrodynamic convection effects. Nanoscale 2014, 6, 4620–4626. [Google Scholar] [CrossRef]
- Yossifon, G.; Chang, H.-C. Selection of Nonequilibrium Overlimiting Currents: Universal Depletion Layer Formation Dynamics and Vortex Instability. Phys. Rev. Lett. 2008, 101, 254501. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Ko, S.H.; Kwak, R.; Posner, J.D.; Kang, K.H.; Han, J. Multi-vortical flow inducing electrokinetic instability in ion concentration polarization layer. Nanoscale 2012, 4, 7406–7410. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirama, H.; Otahara, R.; Mogi, K.; Hayase, M.; Torii, T.; Mekaru, H. Rapid Prototyping of a Nanoparticle Concentrator Using a Hydrogel Molding Method. Polymers 2021, 13, 1069. https://doi.org/10.3390/polym13071069
Hirama H, Otahara R, Mogi K, Hayase M, Torii T, Mekaru H. Rapid Prototyping of a Nanoparticle Concentrator Using a Hydrogel Molding Method. Polymers. 2021; 13(7):1069. https://doi.org/10.3390/polym13071069
Chicago/Turabian StyleHirama, Hirotada, Ryutaro Otahara, Katsuo Mogi, Masanori Hayase, Toru Torii, and Harutaka Mekaru. 2021. "Rapid Prototyping of a Nanoparticle Concentrator Using a Hydrogel Molding Method" Polymers 13, no. 7: 1069. https://doi.org/10.3390/polym13071069






