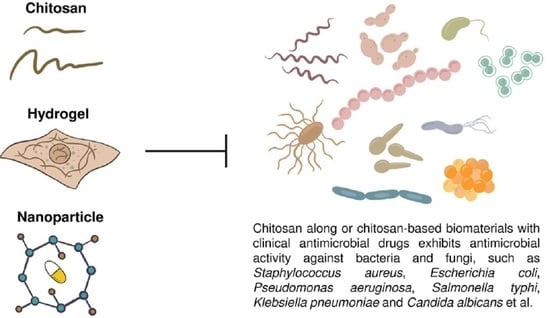
Antimicrobial Actions and Applications of Chitosan
Abstract
:1. Introduction
2. Antimicrobial Actions of Chitosan
2.1. Antimicrobial Activity against Bacteria
2.2. Antimicrobial Activity Against Fungi
3. Factors Influencing the Antimicrobial Activity of Chitosan
3.1. pH
3.2. Molecular Weight
3.3. DDA
3.4. Derivatives
4. Genetic Responses of Chitosan-Treated Bacteria and Fungi
4.1. Bacterial Responses
4.2. Fungal Responses
5. Problems Associated with Chitosan
6. Applications of Chitosan-Based Nanoparticles and Films in Combination with Clinical Drugs against Microbes
6.1. Nanoparticles
6.2. Films
6.3. Implants
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hudson, S.M.; Smith, C. Polysaccharides: Chitin and chitosan: Chemistry and technology of their use as structural materials. In Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998; pp. 96–118. [Google Scholar]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Tolaimate, A.; Rhazi, M.; Alagui, A.; Desbrieres, J.; Rinaudo, M. Valorization of waste products from fishing industry by production of chitin and chitosan. Phys. Chem. News 2008, 42, 120–127. [Google Scholar]
- Nishioka, Y.; Kyotani, S.; Masui, H.; Okamura, M.; Miyazaki, M.; Okazaki, K.; Ohnishi, S.; Yamamoto, Y.; Ito, K. Preparation and release characteristics of cisplatin albumin microspheres containing chitin and treated with chitosan. Chem. Pharm. Bull. 1989, 37, 3074–3077. [Google Scholar] [CrossRef] [Green Version]
- Antonino, R.S.C.M.D.; Fook, B.R.P.L.; Lima, V.A.D.; Rached, R.I.D.; Lima, E.P.N.; Lima, R.J.D.; Covas, C.A.P.; Fook, M.V.L. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- Abebe, L.S.; Chen, X.; Sobsey, M.D. Chitosan coagulation to improve microbial and turbidity removal by ceramic water filtration for household drinking water treatment. Int. J. Environ. Res. Public Health 2016, 13, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altaher, H. The use of chitosan as a coagulant in the pre-treatment of turbid sea water. J. Hazard. Mater. 2012, 233–234, 97–102. [Google Scholar] [CrossRef]
- Ayad, M.; Salahuddin, N.; Fayed, A.; Bastakoti, B.P.; Suzuki, N.; Yamauchi, Y. Chemical design of a smart chitosan-polypyrrole-magnetite nanocomposite toward efficient water treatment. Phys. Chem. Chem. Phys. 2014, 16, 21812–21819. [Google Scholar] [CrossRef] [PubMed]
- Fabris, R.; Chow, C.W.; Drikas, M. Evaluation of chitosan as a natural coagulant for drinking water treatment. Water Sci. Technol. 2010, 61, 2119–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Luo, C.; Li, X.; Duan, H.; Wang, X. Preparation of magnetic ionic liquid/chitosan/graphene oxide composite and application for water treatment. Int. J. Biol. Macromol. 2014, 66, 172–178. [Google Scholar] [CrossRef]
- Liaw, B.S.; Chang, T.T.; Chang, H.K.; Liu, W.K.; Chen, P.Y. Fish scale-extracted hydroxyapatite/chitosan composite scaffolds fabricated by freeze casting—An innovative strategy for water treatment. J. Hazard. Mater. 2020, 382, 121082. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.E.; Alsabagh, A.M.; Nasr, S.A.; Zaki, M.M. Multifunctional nanocomposites of chitosan, silver nanoparticles, copper nanoparticles and carbon nanotubes for water treatment: Antimicrobial characteristics. Int. J. Biol. Macromol. 2017, 97, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Sarmiento-Sanchez, J.I.; Ruelas-Leyva, J.P.; Crini, G.; Hermosillo-Ochoa, E.; Gutierrez-Montes, J.A. Environment-friendly approach toward the treatment of raw agricultural wastewater and river water via flocculation using chitosan and bean straw flour as bioflocculants. ACS Omega 2020, 5, 3943–3951. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Takeshita, A.; Oda, N.; Ukita, M.; Imai, T.; Higuchi, T. On-site treatment of turbid river water using chitosan, a natural organic polymer coagulant. Water Sci. Technol. 2006, 53, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, C.H. Ultrasound treatment of frozen crayfish with chitosan Nano-composite water-retaining agent: Influence on cryopreservation and storage qualities. Food Res. Int. 2019, 126, 108670. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Chen, Y.Z.; Yuan, S.J.; Sheng, G.P.; Yu, H.Q. Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment. Water Res. 2009, 43, 5267–5275. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Velazquez, G.; Savant, V.; Torres, J.A. Surimi wash water treatment for protein recovery: Effect of chitosan-alginate complex concentration and treatment time on protein adsorption. Bioresour. Technol. 2005, 96, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhang, W.; Cui, J.; He, L.; Wang, J.; Yan, C.; Kou, Y.; Li, J. Facile fabrication of magnetic phosphorylated chitosan for the removal of Co(II) in water treatment: Separation properties and adsorption mechanisms. Environ. Sci. Pollut. Res. Int. 2020, 27, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Cota-Arriola, O.; Cortez-Rocha, M.O.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: Development of new strategies for microbial control in agriculture. J. Sci. Food Agric. 2013, 93, 1525–1536. [Google Scholar] [CrossRef]
- Doares, S.H.; Syrovets, T.; Weiler, E.W.; Ryan, C.A. Oligogalacturonides and chitosan activate plant defensive fenes through the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 4095–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.Z.; Li, X.C.; Wang, X.D.; Zhai, Q.L.; Zhan, Y.G. Chitosan activates defense responses and triterpenoid production in cell suspension cultures of Betula platyphylla Suk. Afr. J. Biotechnol. 2010, 9, 2816–2820. [Google Scholar]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.C.; Meng, Q.S.; Zeng, H.H.; Wang, W.X.; Yin, H. Chitosan oligosaccharide induces resistance to Tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signalling pathway. Sci. Rep. 2016, 6, 26144. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shan, C.L.; Ge, M.Y.; Wang, L.; Fang, Y.; Wang, Y.L.; Xie, G.L.; Sun, G.C. Antibacterial mechanism of chitosan and its applications in protection of plant from bacterial disease. Asian J. Chem. 2013, 25, 10033–10036. [Google Scholar] [CrossRef]
- Li, P.Q.; Linhardt, R.J.; Cao, Z.M. Structural characterization of oligochitosan elicitor from Fusarium sambucinum and its elicitation of defensive responses in Zanthoxylum bungeanum. Int. J. Mol. Sci. 2016, 17, 2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narula, K.; Elagamey, E.; Abdellatef, M.A.E.; Sinha, A.; Ghosh, S.; Chakraborty, N.; Chakraborty, S. Chitosan-triggered immunity to Fusarium in chickpea is associated with changes in the plant extracellular matrix architecture, stomatal closure and remodeling of the plant metabolome and proteome. Plant J. 2020, 103, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Povero, G.; Loreti, E.; Pucciariello, C.; Santaniello, A.; Di Tommaso, D.; Di Tommaso, G.; Kapetis, D.; Zolezzi, F.; Piaggesi, A.; Perata, P. Transcript profiling of chitosan-treated Arabidopsis seedlings. J. Plant Res. 2011, 124, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Aubel, G.; Cambier, P.; Dieu, M.; Van Cutsem, P. Plant immunity induced by COS-OGA elicitor is a cumulative process that involves salicylic acid. Plant Sci. 2016, 247, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Vanda, G.F.; Shabani, L.; Razavizadeh, R. Chitosan enhances rosmarinic acid production in shoot cultures of Melissa officinalis L. through the induction of methyl jasmonate. Bot. Stud. 2019, 60, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varlamov, V.P.; Mysyakina, I.S. Chitosan in biology, microbiology, medicine, and agriculture. Microbiology 2018, 87, 712–715. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Agullo, E.; Rodriguez, M.S.; Ramos, V.; Albertengo, L. Present and future role of chitin and chitosan in food. Macromol. Biosci. 2003, 3, 521–530. [Google Scholar] [CrossRef]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systematic study needs for food applications. Food Sci. Technol. Int. 2012, 18, 3–34. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Philibert, T.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohyd. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandia, M.D.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jimtaisong, A.; Saewan, N. Utilization of carboxymethyl chitosan in cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Li, K.X.; Ding, H.P.; Lv, J.; Zhang, J. Study on preparation of a chitosan/vitamin C complex and its properties in cosmetics. Nat. Prod. Commun. 2020, 15, 1–9. [Google Scholar]
- Sionkowska, A.; Kaczmarek, B.; Michalska, M.; Lewandowska, K.; Grabska, S. Preparation and characterization of collagen/chitosan/hyaluronic acid thin films for application in hair care cosmetics. Pure Appl. Chem. 2017, 89, 1829–1839. [Google Scholar] [CrossRef]
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zhang, Q. Study on pulmonary delivery of peptide drugs in rats: Effects of absorption enhancers on cellular membrane fluidity. Yao Xue Xue Bao 2003, 38, 957–961. [Google Scholar] [PubMed]
- Haque, T.; Chen, H.; Ouyang, W.; Martoni, C.; Lawuyi, B.; Urbanska, A.M.; Prakash, S. Superior cell delivery features of poly(ethylene glycol) incorporated alginate, chitosan, and poly-L-lysine microcapsules. Mol. Pharm. 2005, 2, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.R.; dos Santos, D.S., Jr.; Bosco, J.M.; Spolidorio, D.M.; Chierici Marcantonio, R.A. Evaluation of chitosan gel as antibiotic and photosensitizer delivery. Drug Deliv. 2008, 15, 417–422. [Google Scholar] [CrossRef]
- Alonso, M.J.; Coelho, D.; Engwer, C.; Fetzner, S.; Fuenzalida, J.; Goycoolea, F.; Hoffman, S.; Kollenbrock, S.; Menchicchi, B.; Moerschbacher, B.; et al. Chitosan-based nanomaterials for drug delivery and antibiotic-free bacterial control. NSTI-Nanotech. 2013, 3, 217–220. [Google Scholar]
- Ibrahim, H.; El-Bisi, M.; Taha, G.; El-Alfy, E. Chitosan nanoparticles loaded antibiotics as drug delivery biomaterial. J. Appl. Pharm. Sci. 2015, 5, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez Echazu, M.I.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for oral local delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Alexander, C.; Wells, C.M.; Bumgardner, J.D.; Carpenter, D.P.; Jennings, J.A. Chitosan for the delivery of antibiotics. In Chitosan Based Biomaterials; Woodhead Publishing: Cambridge, UK, 2017; Volume 2, pp. 147–173. [Google Scholar]
- Kiilll, C.P.; Barud, H.D.; Santagneli, S.H.; Ribeiro, S.J.L.; Silva, A.M.; Tercjak, A.; Gutierrez, J.; Pironi, A.M.; Gremiao, M.P.D. Synthesis and factorial design applied to a novel chitosan/sodium polyphosphate nanoparticles via ionotropic gelation as an RGD delivery system. Carbohyd. Polym. 2017, 157, 1695–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, B.; Aldawsari, H.M.; Badr-Eldin, S.M. Optimized chitosan/anion polyelectrolyte complex based inserts for vaginal delivery of fluconazole: In vitro/in vivo evaluation. Pharmaceutics 2018, 10, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, K.M.; Kumar, A.; Suneetha, M.; Han, S.S. pH and near-infrared active; chitosan-coated halloysite nanotubes loaded with curcumin-Au hybrid nanoparticles for cancer drug delivery. Int. J. Biol. Macromol. 2018, 112, 119–125. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kravanja, G.; Primozic, M.; Knez, Z.; Leitgeb, M. Chitosan-based (Nano) materials for novel biomedical applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, S.; Abbas, G.; Hanif, M.; Bernkop-Schnurch, A.; Jalil, A.; Yaqoob, M. Mucoadhesive micro-composites: Chitosan coated halloysite nanotubes for sustained drug delivery. Colloids Surf. B. Biointerfaces 2019, 184, 110527. [Google Scholar] [CrossRef]
- Sharma, P.K.; Halder, M.; Srivastava, U.; Singh, Y. Antibacterial PEG-chitosan hydrogels for controlled antibiotic/protein delivery. ACS Appl. Bio Mater. 2019, 2, 5313–5322. [Google Scholar] [CrossRef]
- Schlachet, I.; Moshe Halamish, H.; Sosnik, A. Mixed amphiphilic polymeric nanoparticles of chitosan, poly(vinyl alcohol) and poly(methyl methacrylate) for intranasal drug delivery: A preliminary in vivo study. Molecules 2020, 25, 4496. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Hsieh, C.M.; Tsai, T.; Yang, J.C.; Chen, C.T. Optimization and evaluation of a chitosan/hydroxypropyl methylcellulose hydrogel containing toluidine blue O for antimicrobial photodynamic inactivation. Int. J. Mol. Sci. 2015, 16, 20859–20872. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, P.; Santra, S. Hydrothermally treated chitosan hydrogel loaded with copper and zinc particles as a potential micronutrient-based antimicrobial feed additive. Front. Vet. Sci. 2015, 2, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romic, M.D.; Klaric, M.S.; Lovric, J.; Pepic, I.; Cetina-Cizmek, B.; Filipovic-Grcic, J.; Hafner, A. Melatonin-loaded chitosan/Pluronic (R) F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, P.; Lucero-Acuna, A.; Gutierrez-Valenzuela, C.A.; Moreno, R.; Esquivel, R. Systematic evaluation of pH and thermoresponsive poly(n-isopropylacrylamide-chitosan-fluorescein)microgel. e-Polymers 2017, 17, 399–408. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Chen, D. Preparation and characterization of chitosan based injectable hydrogels enhanced by chitin nano-whiskers. J. Mech. Behav. Biomed. Mater. 2017, 65, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Velazquez, J.C.; Rodriguez-Rodriguez, R.; Espinosa-Andrews, H.; Qui-Zapata, J.A.; Garcia-Morales, S.; Navarro-Lopez, D.E.; Luna-Barcenas, G.; Vassallo-Brigneti, E.C.; Garcia-Carvajal, Z.Y. Gelatin-chitosan-PVA hydrogels and their application in agriculture. J. Chem. Technol. Biotechnol. 2019, 94, 3495–3504. [Google Scholar] [CrossRef]
- Aksel, H.; Mahjour, F.; Bosaid, F.; Calamak, S.; Azim, A.A. Antimicrobial activity and biocompatibility of antibiotic-loaded chitosan hydrogels as a potential scaffold in regenerative endodontic treatment. J. Endod. 2020, 46, 1867–1875. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Shafiq, S.; Khan, S.M.; Ibrahim, S.M.; Abubshait, S.A.; Nazir, A.; Abbas, M.; Iqbal, M. Novel chitosan/guar gum/PVA hydrogel: Preparation, characterization and antimicrobial activity evaluation. Int. J. Biol. Macromol. 2020, 164, 499–509. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y.C. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Felt, O.; Carrel, A.; Baehni, P.; Buri, P.; Gurny, R. Chitosan as tear substitute: A wetting agent endowed with antimicrobial efficacy. J. Ocul. Pharmacol. Ther. 2000, 16, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.Y.; Liao, Y.T.; Tseng, Y.K.; Deng, F.S.; Lin, C.H. A potential antifungal effect of chitosan against Candida albicans is mediated via the inhibition of SAGA complex component expression and the subsequent alteration of cell surface integrity. Front. Microbiol. 2019, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.H.; Deng, F.S.; Chang, C.J.; Lin, C.H. Synergistic antifungal activity of chitosan with fluconazole against Candida albicans, Candida tropicalis, and fluconazole-resistant strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Avadi, M.R.; Sadeghi, A.M.M.; Tahzibi, A.; Bayati, K.; Pouladzadeh, M.; Zohuriaan-Mehr, M.J.; Rafiee-Tehrani, M. Diethylmethyl chitosan as an antimicrobial agent: Synthesis, characterization and antibacterial effects. Eur. Polym. J. 2004, 40, 1355–1361. [Google Scholar] [CrossRef]
- Wang, X.H.; Du, Y.M.; Fan, L.H.; Liu, H.; Hu, Y. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Y.M.; Wang, X.Y.; Feng, T. Self-aggregation of water-soluble chitosan and solubilization of thymol as an antimicrobial agent. J. Biomed. Mater. Res. A 2009, 90A, 874–881. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Estaca, J.; de Lacey, A.L.; Lopez-Caballero, M.E.; Gomez-Guillen, M.C.; Montero, P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ochoa, L.; Gonzales-Gonzales, A.; Gutierrez-Mendez, N.; Munoz-Castellanos, L.N.; Quintero-Ramos, A. Study of the antibacterial activity of chitosan-based films prepared with different molecular weights including spices essential oils and functional extracts as antimicrobial agents. Rev. Mex. Ing. Quim. 2011, 10, 455–463. [Google Scholar]
- Huang, L.Y.; Dai, T.H.; Xuan, Y.; Tegos, G.P.; Hamblin, M.R. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: Efficacy against bacterial burn infections. Antimicrob. Agents Chemother. 2011, 55, 3432–3438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I. Synthesis and structure-activity relationship of N-(cinnamyl) chitosan analogs as antimicrobial agents. Int. J. Biol. Macromol. 2013, 57, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J. Appl. Microbiol. 2013, 114, 956–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansilla, A.Y.; Albertengo, L.; Rodriguez, M.S.; Debbaudt, A.; Zuniga, A.; Casalongue, C.A. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Sabaa, M.W.; El-Ghandour, A.H.; Abdel-Aziz, M.M.; Abdel-Gawad, O.F. Quaternized N-substituted carboxymethyl chitosan derivatives as antimicrobial agents. Int. J. Biol. Macromol. 2013, 60, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Ma, R.; Lin, C.C.; Liu, Z.W.; Tang, T.T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, G.; Saoncella, O.; Kaner, P.; Altinkaya, S.A.; Figoli, A.; Bonchio, M.; Carraro, M. Chitosan-polyoxometalate nanocomposites: Synthesis, characterization and application as antimicrobial agents. J. Clust. Sci. 2014, 25, 839–854. [Google Scholar] [CrossRef] [Green Version]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.M.; Muraleedaran, K.; Mujeeb, V.M.A. Applications of chitosan powder with in situ synthesized nano ZnO particles as an antimicrobial agent. Int. J. Biol. Macromol. 2015, 77, 266–272. [Google Scholar]
- Arjunan, N.; Kurnari, H.L.J.; Singaravelu, C.M.; Kandasamy, R.; Kandasamy, J. Physicochemical investigations of biogenic chitosan-silver nanocomposite as antimicrobial and anticancer agent. Int. J. Biol. Macromol. 2016, 92, 77–87. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Hanpanich, O.; Wongkongkatep, P.; Pongtharangkul, T.; Wongkongkatep, J. Turning hydrophilic bacteria into biorenewable hydrophobic material with potential antimicrobial activity via interaction with chitosan. Bioresour. Technol. 2017, 230, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.X.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro and nanoparticles as antimicrobial agents: A review. Carbohyd. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Rivera Aguayo, P.; Bruna Larenas, T.; Alarcon Godoy, C.; Cayupe Rivas, B.; Gonzalez-Casanova, J.; Rojas-Gomez, D.; Caro Fuentes, N. Antimicrobial and antibiofilm capacity of chitosan nanoparticles against wild type strain of Pseudomonas sp. isolated from milk of cows diagnosed with bovine mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Felix, F.; Castillo-Ortega, M.M.; Yepiz-Gomez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohyd. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2005, 53, 3696–3701. [Google Scholar] [CrossRef] [PubMed]
- Kjm, K.M.; Son, J.H.; Kim, S.K.; Weller, C.L.; Hanna, M.A. Properties of chitosan films as a function of pH and solvent type. J. Food Sci. 2006, 71, e119–e124. [Google Scholar]
- Boy, R.; Maness, C.; Kotek, R. Properties of chitosan/soy protein blended films with added plasticizing agent as a function of solvent type at acidic pH. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 11–17. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J. Advances in chitin and chitosan modification through graft copolymerization: A comprehensive review. Iran. Polym. J. 2005, 14, 235–265. [Google Scholar]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P. Antibacterial effects of chitosan powder: Mechanisms of action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.C. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Kiakhani, M.; Arami, M.; Gharanjig, K. Application of a biopolymer chitosan-poly(propylene)imine dendrimer hybrid as an antimicrobial agent on the wool fabrics. Iran. Polym. J. 2013, 22, 931–940. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpaa, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, K.; Izumi, R.; Osaki, T.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Minami, S.; Okamoto, Y. Chitin, chitosan, and its derivatives for wound healing: Old and new materials. J. Funct. Biomater. 2015, 6, 104–142, Reprinted in J. Funct. Biomater. 2018, 9, 38. [Google Scholar]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Ke, C.L.; Liao, Y.T.; Lin, C.H. MSS2 maintains mitochondrial function and is required for chitosan resistance, invasive growth, biofilm formation and virulence in Candida albicans. Virulence 2021, 12, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef]
- Rohde, M. The gram-positive bacterial cell wall. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Gan, L.; Chen, S.; Jensen, G.J. Molecular organization of gram-negative peptidoglycan. Proc. Natl. Acad. Sci. USA 2008, 105, 18953–18957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, D.; Peschel, A. Molecular mechanisms of bacterial resistance to antimicrobial peptides. Curr. Top. Microbiol. Immunol. 2006, 306, 231–250. [Google Scholar] [PubMed]
- Raetz, C.R.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef] [Green Version]
- Alburquenque, C.; Bucarey, S.A.; Neira-Carrillo, A.; Urzua, B.; Hermosilla, G.; Tapia, C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.H.; Domard, A.; Muzzarelli, R.A.A.; Tokura, S.; Wang, D.M. Advances in chitin/chitosan science and their applications. Carbohyd. Polym. 2011, 84, 695. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.I.; Wu, J.C.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar] [PubMed]
- Muzzalupo, I.; Badolati, G.; Chiappetta, A.; Picci, N.; Muzzalupo, R. In vitro antifungal activity of Olive (Olea europaea) leaf extracts loaded in chitosan nanoparticles. Front. Bioeng. Biotechnol. 2020, 8, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, D.B.; de Camargo, L.E.A.; Khalil, N.M.; Auler, M.E.; Mainardes, R.M. Antifungal activity of chitosan-coated poly(lactic-co-glycolic) acid nanoparticles containing amphotericin B. Mycopathologia 2018, 183, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Guisbiers, G.; Mendoza, J.; Mimun, L.C.; Vincent, B.A.; Lopez-Ribot, J.L.; Nash, K.L. Synergistic antifungal effect of chitosan-stabilized selenium nanoparticles synthesized by pulsed laser ablation in liquids against Candida albicans biofilms. Int. J. Nanomed. 2018, 13, 2697–2708. [Google Scholar] [CrossRef] [Green Version]
- Kalagatur, N.K.; Nirmal Ghosh, O.S.; Sundararaj, N.; Mudili, V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018, 9, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, L.G.S.; Guedes, G.M.M.; da Silva, M.L.Q.; Castelo-Branco, D.; Sidrim, J.J.C.; Cordeiro, R.A.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Effect of the molecular weight of chitosan on its antifungal activity against Candida spp. in planktonic cells and biofilm. Carbohydr. Polym. 2018, 195, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Colom-Valiente, M.F.; Martinez-Peinado, P.; Martinez-Lopez, J.E.; Puelles, E.; Sempere-Ortells, J.M.; Lopez-Llorca, L.V. Carbon and nitrogen limitation increase chitosan antifungal activity in Neurospora crassa and fungal human pathogens. Fungal Biol. 2015, 119, 154–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, M.; Wu, C.; Ren, G.; Liang, X.; Wang, X.; Huang, J. Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chem. 2014, 155, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Viegas de Souza, R.H.; Takaki, M.; de Oliveira Pedro, R.; dos Santos Gabriel, J.; Tiera, M.J.; de Oliveira Tiera, V.A. Hydrophobic effect of amphiphilic derivatives of chitosan on the antifungal activity against Aspergillus flavus and Aspergillus parasiticus. Molecules 2013, 18, 4437–4450. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Sanchez, N.S.; Calahorra, M. Effects of chitosan on Candida albicans: Conditions for its antifungal activity. Biomed. Res. Int. 2013, 2013, 527549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Tian, W.; Li, B.; Wu, G.; Ibrahim, M.; Tao, Z.; Wang, Y.; Xie, G.; Li, H.; Sun, G. Antifungal effect and mechanism of chitosan against the rice sheath blight pathogen, Rhizoctonia solani. Biotechnol. Lett. 2012, 34, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.W.; Li, C.F.; Shih, D.Y.C. Antifungal activity of chitosan and its preservative effect on low-sugar candied kumquat. J. Food Prot. 1994, 57, 136–140. [Google Scholar] [CrossRef]
- Kendra, D.F.; Hadwiger, L.A. Relative effect of crab shell and fungal wall chitosan on Fusarium growth and induced resistance. Phytopathology 1985, 75, 1303. [Google Scholar]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Atai, Z.; Atai, M.; Amini, J.; Salehi, N. In vivo study of antifungal effects of low-molecular-weight chitosan against Candida albicans. J. Oral Sci. 2017, 59, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Perez-Berna, A.J.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Villalain, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef]
- Kumariya, R.; Sood, S.K.; Rajput, Y.S.; Saini, N.; Garsa, A.K. Increased membrane surface positive charge and altered membrane fluidity leads to cationic antimicrobial peptide resistance in Enterococcus faecalis. Biochim. Biophys. Aacta 2015, 1848, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- Ganan, M.; Lorentzen, S.B.; Aam, B.B.; Eijsink, V.G.H.; Gaustad, P.; Sorlie, M. Antibiotic saving effect of combination therapy through synergistic interactions between well-characterized chito-oligosaccharides and commercial antifungals against medically relevant yeasts. PLoS ONE 2019, 14, e0227098. [Google Scholar] [CrossRef] [PubMed]
- Work, E. Biochemistry of the bacterial cell wall. Nature 1957, 179, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [Green Version]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The Fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, H.; Acosta Gutierrez, S.; Bodrenko, I.; Malloci, G.; Scorciapino, M.A.; Winterhalter, M.; Ceccarelli, M. Bacterial outer membrane porins as electrostatic nanosieves: Exploring transport rules of small polar molecules. ACS Nano 2017, 11, 5465–5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gademann, K. Controlling protein transport by small molecules. Curr. Drug Targets 2011, 12, 1574–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgstaller, W. Transport of small lons and molecules through the plasma membrane of filamentous fungi. Crit. Rev. Microbiol. 1997, 23, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Demchick, P.; Koch, A.L. The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. J. Bacteriol. 1996, 178, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherrer, R.; Louden, L.; Gerhardt, P. Porosity of the yeast cell wall and membrane. J. Bacteriol. 1974, 118, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, R.D.; Hurd, A.F.; Cadby, A.; Hobbs, J.K.; Foster, S.J. Cell wall elongation mode in gram-negative bacteria is determined by peptidoglycan architecture. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, W.; Holtje, J.V. The architecture of the murein (peptidoglycan) in gram-negative bacteria: Vertical scaffold or horizontal layer(s)? J. Bacteriol. 2004, 186, 5978–5987. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.; Sood, P.; Lenardon, M.D.; Milne, G.; Olson, J.; Jensen, G.; Wolf, J.; Casadevall, A.; Adler-Moore, J.; Gow, N.A.R. The viscoelastic properties of the fungal cell wall allow traffic of AmBisome as intact liposome vesicles. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dmitriev, B.A.; Toukach, F.V.; Holst, O.; Rietschel, E.T.; Ehlers, S. Tertiary structure of Staphylococcus aureus cell wall murein. J. Bacteriol. 2004, 186, 7141–7148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulikov, S.N.; Tikhonov, V.E.; Bezrodnykh, E.A.; Lopatin, S.A.; Varlamov, V.P. Comparative evaluation of antimicrobial activity of oligochitosans against Klebsiella pneumoniae. Bioorg. Khim. 2015, 41, 67–73. [Google Scholar] [CrossRef]
- Denobel, J.G.; Dijkers, C.; Hooijberg, E.; Klis, F.M. Increased cell-wall porosity in Saccharomyces cerevisiae after treatment with dithiothreitol or EDTA. J. Gen. Microbiol. 1989, 135, 2077–2084. [Google Scholar]
- Denobel, J.G.; Klis, F.M.; Priem, J.; Munnik, T.; Vandenende, H. The gucanase-soluble mannoproteins limit cell-wall porosity in Saccharomyces cerevisiae. Yeast 1990, 6, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Stirke, A.; Celiesiute-Germaniene, R.; Zimkus, A.; Zurauskiene, N.; Simonis, P.; Dervinis, A.; Ramanavicius, A.; Balevicius, S. The link between yeast cell wall porosity and plasma membrane permeability after PEF treatment. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.J.; Youn, D.K.; Lee, S.H.; No, H.K.; Ha, J.G.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. Technol. 2010, 45, 676–682. [Google Scholar] [CrossRef]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marcal, H. Chitosan as a biomaterial: Influence of degree of deacetylation on its physiochemical, material and biological properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef] [Green Version]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial effect of a novel chitosan derivative and its synergistic effect with antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; Meng, Q.Y.; Li, Q.; Liu, J.B.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satitsri, S.; Muanprasat, C. Chitin and chitosan derivatives as biomaterial resources for biological and biomedical applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef] [PubMed]
- Piegat, A.; Zywicka, A.; Niemczyk, A.; Goszczynska, A. Antibacterial activity of N,O-acylated chitosan derivative. Polymers 2020, 13, 107. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Aotegen, B.; Xu, H. The influence of the different inductivity of acetyl phenyl-thiosemicarbazone-chitosan on antimicrobial activities. Int. J. Biol. Macromol. 2011, 48, 713–719. [Google Scholar] [CrossRef]
- Huang, H.F.; Peng, C.F. Antibacterial and antifungal activity of alkylsulfonated chitosan. Biomark. Genom. Med. 2015, 7, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, H.E.; Hassan, S.Y.; Yacout, G.A.; Mostafa, M.A.; El Sadek, M.M. Synthesis and biological evaluation of new imine- and amino-chitosan derivatives. Polymers 2015, 7, 2690–2700. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J.; Kim, H.; Gupta, M.K.; Dutta, P.K. A new chitosan-thymine conjugate: Synthesis, characterization and biological activity. Int. J. Biol. Macromol. 2012, 50, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.I.; Steurbaut, W.; Stevens, C.V. In vitro assessment of N-(benzyl)chitosan derivatives against some plant pathogenic bacteria and fungi. Eur. Polym. J. 2009, 45, 237–245. [Google Scholar] [CrossRef]
- Rabea, E.I.; El Badawy, M.; Rogge, T.M.; Stevens, C.V.; Hofte, M.; Steurbaut, W.; Smagghe, G. Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Manag. Sci. 2005, 61, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Patale, R.L.; Patravale, V.B. O, N-carboxymethyl chitosan-zinc complex: A novel chitosan complex with enhanced antimicrobial activity. Carbohyd. Polym. 2011, 85, 105–110. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Mellegard, H.; Kovacs, A.T.; Lindback, T.; Christensen, B.E.; Kuipers, O.P.; Granum, P.E. Transcriptional responses of Bacillus cereus towards challenges with the polysaccharide chitosan. PLoS ONE 2011, 6, e24304. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewska, A.; Boorsma, A.; Brul, S.; Hellingwerf, K.J.; Klis, F.M. Transcriptional response of Saccharomyces cerevisiae to the plasma membrane-perturbing compound chitosan. Eukaryot. Cell 2005, 4, 703–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaime, M.D.L.A.; Lopez-Llorca, L.V.; Conesa, A.; Lee, A.Y.; Proctor, M.; Heisler, L.E.; Gebbia, M.; Giaever, G.; Westwood, J.T.; Nislow, C. Identification of yeast genes that confer resistance to chitosan oligosaccharide (COS) using chemogenomics. BMC Genom. 2012, 13, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abarca, M.L.; Accensi, F.; Bragulat, M.R.; Cabanes, F.J. Current importance of ochratoxin A-producing Aspergillus spp. J. Food Prot. 2001, 64, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Garba, B.; Ren, Y.; Yao, M.; Xia, X.; Li, M.; Wang, Y. Antifungal activity of chitosan against Aspergillus ochraceus and its possible mechanisms of action. Int. J. Biol. Macromol. 2020, 158, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Kowbel, D.; Nueda, M.J.; Palma-Guerrero, J.; Glass, N.L.; Lopez-Llorca, L.V. Neurospora crassa transcriptomics reveals oxidative stress and plasma membrane homeostasis biology genes as key targets in response to chitosan. Mol. Biosyst. 2016, 12, 391–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez-Fernandez, M.; Sambles, C.; Lopez-Moya, F.; Nueda, M.J.; Studholme, D.J.; Lopez-Llorca, L.V. Chitosan modulates Pochonia chlamydosporia gene expression during nematode egg parasitism. Environ. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Forghani, F.; Hajihassani, A. Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci. 2020, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Palma-Guerrero, J.; Jansson, H.B.; Salinas, J.; Lopez-Llorca, L.V. Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J. Appl. Microbiol. 2008, 104, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibanez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan increases tomato root colonization by Pochonia chlamydosporia and their combination reduces root-knot nematode damage. Front. Plant Sci. 2017, 8, 1415. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Martinez, A.; Lenfant, N.; Escudero, N.; Zavala-Gonzalez, E.A.; Henrissat, B.; Lopez-Llorca, L.V. CAZyme content of Pochonia chlamydosporia reflects that chitin and chitosan modification are involved in nematode parasitism. Environ. Microbiol. 2016, 18, 4200–4215. [Google Scholar] [CrossRef] [Green Version]
- Palma-Guerrero, J.; Gomez-Vidal, S.; Tikhonov, V.E.; Salinas, J.; Jansson, H.B.; Lopez-Llorca, L.V. Comparative analysis of extracellular proteins from Pochonia chlamydosporia grown with chitosan or chitin as main carbon and nitrogen sources. Enzym. Microb. Technol. 2010, 46, 568–574. [Google Scholar] [CrossRef]
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullberg, B.J.; Arendrup, M.C. Invasive candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charway, G.N.A.; Park, S.; Yu, D.; Je, J.Y.; Kim, D.H.; Jung, W.K.; Kim, Y.M. In vitro antibacterial and synergistic effect of chitosan-phytochemical conjugates against antibiotic resistant fish pathogenic bacteria. Indian J. Microbiol. 2019, 59, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.A.; Yousuf, R.S.; Shoaib, M.H.; Asghar, M.A.; Ansar, S.; Zehravi, M.; Rehman, A.A. Synergistic combinations of chitosans and antibiotics in Staphylococcus aureus. Int. J. Nanomed. 2020, 13, 7841–7859. [Google Scholar] [CrossRef] [PubMed]
- Erman, A.; Hergouth, V.K.; Blango, M.G.; Kos, M.K.; Mulvey, M.A.; Veranic, P. Repeated treatments with chitosan in combination with antibiotics completely eradicate uropathogenic Escherichia coli from infected mouse urinary bladders. J. Infect. Dis. 2017, 216, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Yano, K.; Yamada, T.; Banno, Y.; Sekiya, T.; Nozawa, Y. Modification of lipid composition in a dimorphic fungus, Candida albicans during the yeast cell to hypha transformation. Jpn. J. Med. Mycol. 1982, 23, 159–165. [Google Scholar] [CrossRef]
- Brasil, M.; Filgueiras, A.; Campos, M.; Neves, M.; Eugênio, M.; Sena, L.; Sant’Anna, C.; da Silva, V.; Diniz, C.; Sant’Ana, A. Synergism in the antibacterial action of ternary mixtures involving silver nanoparticles, chitosan and antibiotics. J. Braz. Chem. Soc. 2018, 29, 2026–2033. [Google Scholar] [CrossRef]
- Seyam, S.; Nordin, N.A.; Alfatama, M. Recent progress of chitosan and chitosan derivatives-based nanoparticles: Pharmaceutical perspectives of oral insulin delivery. Pharmaceuticals 2020, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Ling, C.C.S.; Ambu, S.P.; Davamani, F. Cationic chitosan-propolis nanoparticles alter the zeta potential of S. epidermidis, inhibit biofilm by modulating gene expression and exibit synergism with antibiotics. PLoS ONE 2019, 14, e0213079. [Google Scholar] [CrossRef]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Goyal, A.K.; Rath, G. Formulation and evaluation of chitosan-based ampicillin trihydrate nanoparticles. Trop. J. Pharm. Res. 2010, 9, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Barakat, K.M.; Gohar, Y.M. Nanosilver-marine fungal chitosan as antibiotic synergizers against sepsis fish bacteria. Iran. J. Microbiol. 2015, 7, 324–332. [Google Scholar] [PubMed]
- Asghar, M.A.; Yousuf, R.I.; Shoaib, M.H.; Asghar, M.A.; Ansar, S.; Zehravi, M.; Abdul Rehman, A. Synergistic nanocomposites of different antibiotics coupled with green synthesized chitosan-based silver nanoparticles: Characterization, antibacterial, in vivo toxicological and biodistribution studies. Int. J. Nanomed. 2020, 15, 7841–7859. [Google Scholar] [CrossRef] [PubMed]
- Golmohamadi, M.; Ghorbani, H.R.; Otadi, M. Synthesis of chitosan nanoparticles loaded with antibiotics as drug carriers and the study of antibacterial activity. J. Nanoanal. 2019, 6, 72–79. [Google Scholar]
- Tin, S.; Sakharkar, K.R.; Lim, C.S.; Sakharkar, M.K. Activity of chitosans in combination with antibiotics in Pseudomonas aeruginosa. Int. J. Biol. Sci. 2009, 5, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahiwala, A.F.; Khan, G.A.; Bostanooei, N.M. Efficacy of levofloxacin, chitosan and EDTA combination against methicillin resistant Staphylococcus aureus skin infections: In vitro and in vivo evaluations. Int. J. Clin. Med. Microbiol. 2017, 2, 119. [Google Scholar] [CrossRef]
- Smith, J.K.; Bumgardner, J.D.; Courtney, H.S.; Smeltzer, M.S.; Haggard, W.O. Antibiotic-loaded chitosan film for infection prevention: A preliminary in vitro characterization. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Grimling, B.; Karolewicz, B.; Nawrot, U.; Wlodarczyk, K.; Gorniak, A. Physicochemical and antifungal properties of clotrimazole in combination with high-molecular weight chitosan as a multifunctional excipient. Mar. Drugs 2020, 18, 591. [Google Scholar] [CrossRef] [PubMed]
- Goller, S.; Turner, N.J. The antimicrobial effectiveness and cytotoxicity of the antibiotic-loaded chitosan: ECM scaffolds. Appl. Sci. 2020, 10, 3446. [Google Scholar] [CrossRef]
- Noel, S.P.; Courtney, H.; Bumgardner, J.D.; Haggard, W.O. Chitosan films: A potential local drug delivery system for antibiotics. Clin. Orthop. Relat. Res. 2008, 466, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Nimal, T.R.; Mohandas, A.; Riju, R.M.; Arun, S.M.; Raja, B.; Rangasamy, J. Ciprofloxacin- and fluconazole-containing fibrin-nanoparticle-incorporated chitosan bandages for the treatment of polymicrobial wound infections. ACS Appl. Bio Mater. 2018, 2, 243–254. [Google Scholar]
- Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan loaded into a hydrogel delivery system as a strategy to treat vaginal co-infection. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Norowski, P.A.; Courtney, H.S.; Babu, J.; Haggard, W.O.; Bumgardner, J.D. Chitosan coatings deliver antimicrobials from titanium implants: A preliminary study. Implant Dent. 2011, 20, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chunlin, H.; Bao, J.; Zhou, T.; Dong, Z. Antibiotic loaded chitosan bar. An in vitro, in vivo study of a possible treatment for osteomyelitis. Clin. Orthop. Relat. Res. 1998, 366, 239–247. [Google Scholar]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesaro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Bacteria | Functional Categories | Conclusions | Ref. | |
|---|---|---|---|---|
| S. aureus | Upregulation | 84 genes Membrane Bioenergetics Cell Division Metabolism of Carbohydrates Metabolism of Amino Acids Regulation of RNA Synthesis Protein Folding Adaptation to Atypical Conditions Phage-Related Functions | Chitosan binds to teichoic acids Chitosan increases membrane permeability and causes membrane depolarization Chitosan might be involved in energy metabolism | [107] |
| Downregulation | 82 genes Transport/Binding Proteins and Lipoproteins Metabolism of Nucleotides and Nucleic Acids Metabolism of Lipids | |||
| B. cereus | Upregulation | 57 genes Potassium Transport System Membrane Protein-Associated Genes | Chitosan caused the permeabilizing effect, resulting in the leakage of intracellular potassium Deletion of the kdp gene (an ATP-driven K+ transport system) exhibited no significant difference in the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) | [185] |
| Downregulation | 51 genes Chitin Binding Protein Metabolism of Amino Acids and Other Cellular Constituents Gluconeogenesis | |||
| Fungus | Functional Categories | Conclusion | Ref. | |
|---|---|---|---|---|
| S. cerevisiae | Upregulation | Treated for 15 min/46 genes ER Integral to Membrane Treated for 30 min/97 genes ER Integral to Membrane Cell Wall Cell Wall Organization and Biogenesis Vacuoles Plasma Membrane Treated for 60 min/97 genes ER Integral to Membrane Cell Wall Cell Wall Organization and Biogenesis Vacuoles Treated for 120 min/234 genes ER Integral to Membrane Cell Wall Cell Wall Organization and Biogenesis Vacuoles Stress Response Treated 180 min/432 genes ER Integral to Membrane Cell Wall Cell Wall Organization and Biogenesis Vacuoles Response to Stress | Chitosan may be representative of other plasma membrane-perturbing compounds Chitosan stress decreases translational activity Calcineurin-dependent pathway is involved Deletion of CIN5, CRZ1, or RIM1 exhibits high sensitivity to chitosan | [186] |
| Downregulation | Not available rRNA Processing Ribosomes | |||
| S. cerevisiae | Upregulation | 589 genes Transcription Cell Cycle Protein Modification Stress Response RAS Signal Transduction | COS does not have specific gene targets Membrane permeability is increased in the COS-treated budding yeast Synergistic antifungal activity between chitosan and fluconazole was found | [187] |
| Downregulation | 631 genes Protein Folding Protein Complex Assembly Mitochondrial Electron Transport | |||
| A. ochraceus | Upregulation | 309 genes Starch and Sucrose Metabolism Glycerophospholipid Metabolism Ether Lipid Metabolism Steroid Biosynthesis Mitochondrial Electron Transport | Chitosan damages the integrity of the cell surface architecture and affects membrane fluidity Chitosan affects protein biosynthesis Chitosan is an alternative compound to control fungal pathogens | [189] |
| Downregulation | 26 genes Ribosome Biogenesis in Eukaryotes Glycerophospholipid Metabolism Ether Lipid Metabolism Steroid Biosynthesis | |||
| N. crassa | Upregulation | 237 genes in total (4, 8, and 16 h of treatment) Peroxisome Organization, ROS Degradation and Fatty Acid Catabolism (4 h) Mitochondrial Function (4 h, 8 h and 16 h) Ribosome and Ribosome Biogenesis (16 h) Nucleolus (8 h and 16 h) Structural Molecule Activity (16 h) | A MFS transporter (NCU04534) and a glutathione transferase (NCU10521) are the targets of chitosan Chitosan treatment causes an increase in intracellular ROS Chitosan affects protein biosynthesis ΔNCU03639 (lipase), ΔNCU04537 (monosaccharide transporter), ΔNCU10521 (glutathione S-transferase), ΔNCU08907 Clock controller gene 13 (ccg-13) and ΔNCU07840 (plasma membrane protein with a het domain) are sensitive to chitosan The presence of Ca2+ increases chitosan tolerance | [190] |
| Downregulation | 291 genes in total (4, 8, and 16 h treatment) Peroxisome Organization, ROS Degradation and Fatty Acid Catabolism (16 h) Cell Cortex (4, 8, and 16 h) Vesicle Organization (8 and 16 h) Conjugation (4, 8, and 16 h) G Protein Receptor Signaling Pathway (16 h) Microtubule Organizing Center (4, 8, and 16 h) Ribosome and Ribosome Biogenesis (4 h) Nucleolus (4 h) Structural Molecule Activity (4 h) | |||
| P. chlamydosporia | Upregulation | 46 genes Redox Processes Carbohydrate Catabolism Proteolysis Carbohydrate Transport Cell Cycle Energetic Metabolism Lipid Metabolism Protein Synthesis and Modification Chitin and Chitosan Degradation Structural Constituent of Cell Wall | Chitosan activates the expression of cytochrome P450 ClCP1 and thioredoxin-like proteins P. chlamydosporia is more resistance to chitosan because it may contain more chitosanase genes Chitosan induces many monosaccharide transport genes Chitosan could be a non-toxic additive to reduce root-knot nematode parasitism | [191] |
| Downregulation | 90 Genes Oxidation–Reduction Metabolism Cellular Protein Metabolic Process Macromolecule Biosynthetic Process Small Molecule Metabolic Process Metal Transport | |||
| Chitosan/Antimicrobial Drug | Chitosan-Based Biomaterial | Findings | Microorganism(s) | Ref. |
|---|---|---|---|---|
| Chitosan/Sulfamethoxazole | Nanoparticle | Synergistic activity with sulfamethoxazole | P. aeruginosa | [213] |
| Chitosan/Amoxicillin Cefixime Levofloxacin | Nanoparticle | Significant antibacterial activity | P. aeruginosa E. coli S. aureus Salmonella typhi Klebsiella pneumoniae | [211] |
| Chitosan/Amikacin Rifampicin | Nanoparticle | Antibacterial activity against resistant strains | Aeromonas hydrophila Edwardsiella tarda Pasteurella piscicida P. aeruginosa Streptococcus faecium Streptococcus iniae Vibrio ordalli Yersinia ruckeri | [210] |
| Chitosan/Ciprofloxacin Chlortetracycline Hydrochloride Gentamycin sulfate | Nanoparticle | Inhibits the growth of gram-positive and gram-negative bacteria | E. coli S. aureus | [57] |
| Chitosan/Azithromycin Levofloxacin Tetracycline | Nanoparticle | Shows significant antibacterial effects | E. coli S. aureus | [203] |
| Chitosan/Rifampicin Ciprofloxacin Vancomycin Doxycycline Gentamicin | Nanoparticle | Inhibits bacterial biofilm and exhibits synergism with antibiotics | S. epidermidis | [206] |
| Chitosan/Clarithromycin | Nanoparticle | Shows antibacterial activity | S. aureus | [212] |
| Chitosan/Ciprofloxacin | Hydrogel | Inhibits bacterial growth | E. coli | [70] |
| Chitosan/Clindamycin | Hydrogel | Enhances the antibacterial properties | E. faecalis | [78] |
| Chitosan/Ciprofloxacin Fluconazole | Hydrogel(Bandage) | Shows significant antimicrobial activity | C. albicans E. coli S. aureus | [219] |
| Chitosan/Minocycline Rifampicin | Hydrogel | Provides bactericidal activity directly to the wound site | E. coli S. aureus | [217] |
| Chitosan/Tetracycline | Hydrogel | Has potential applications for antimicrobial action | S. aureus | [55] |
| Chitosan/Amikacin Daptomycin | Film | Effectively inhibits the growth of bacteria | S. aureus | [218] |
| Chitosan/Daptomycin Vancomycin | Film | Shows activity against gram-positive bacteria | S. aureus | [215] |
| Chitosan/Clotrimazole | Solid Mixtures | Acts synergistically with clotrimazole against non-albicans Candida strains | Candida glabrata | [216] |
| Chitosan/Levofloxacin | Hydroxypropyl methyl cellulose (HPMC) gel | Antibacterial activity against resistant strains | Methicillin-resistant S. aureus | [214] |
| Chitosan/Metronidazole | HPMC Gel | Anti-Candida activity | Candida species | [220] |
| Chitosan/Tetracycline Chlorhexidine | Chitosan-Coated Titanium Pins | Inhibits pathogen growth | A. actinomycetemcomitans S. epidermidis | [221] |
| Chitosan/Gentamicin | Chitosan Bar | Shows significant antibacterial activity | Microbes | [222] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. https://doi.org/10.3390/polym13060904
Ke C-L, Deng F-S, Chuang C-Y, Lin C-H. Antimicrobial Actions and Applications of Chitosan. Polymers. 2021; 13(6):904. https://doi.org/10.3390/polym13060904
Chicago/Turabian StyleKe, Cai-Ling, Fu-Sheng Deng, Chih-Yu Chuang, and Ching-Hsuan Lin. 2021. "Antimicrobial Actions and Applications of Chitosan" Polymers 13, no. 6: 904. https://doi.org/10.3390/polym13060904